Abstract
The serine/threonine kinase ULK1 is a mammalian homolog of Atg1, part of the Atg1 kinase complex, which is the most upstream component of the core autophagy machinery conserved from yeast to mammals. In budding yeast, activity of the Atg1 kinase complex is inhibited by TORC1 (target of rapamycin complex 1), but how the counterpart ULK1 complex in mammalian cells is regulated has been unknown. Our laboratories recently discovered that AMPK associates with, and directly phosphorylates, ULK1 on several sites and this modification is required for ULK1 activation after glucose deprivation. In contrast, when nutrients are plentiful, the mTORC1 complex phosphorylates ULK1, preventing its association and activation by AMPK. These studies have revealed a molecular mechanism of ULK1 regulation by nutrient signals via the actions of AMPK and mTORC1.
Key words: autophagy, ULK1, AMPK, mTOR, 14-3-3
Genetic studies in Saccharomyces cerevisiae defined the core components of the autophagy machinery. A serine/threonine kinase, Atg1 and its regulatory subunits Atg13 and Atg17 are some of the most upstream components of the autophagy pathway. Their well-conserved mammalian counterparts are ULK1/2, mATG13 and FIP200, respectively (Fig. 1).
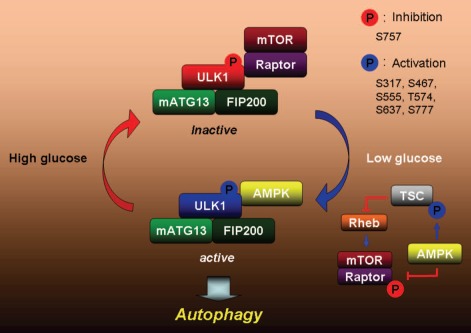
Figure 1.
A proposed model for ULK1 regulation by AMPK and mTORC1. When nutrients are plentiful, mTORC1 is active and it inhibits ULK1 by phosphorylating ULK1 and preventing its interaction with AMPK. When nutrients are scarce, AMPK is activated. The active AMPK inhibits mTORC1 by phosphorylating TSC2 and raptor, and stimulates ULK1 by directly phosphorylating it on multiple sites in ULK1. The active ULK1 complex then initiates autophagy.
As the autophagy-initiating kinase, decoding ULK1 regulation is critical to understanding autophagy regulation. The question as to how the autophagy pathway integrates cellular nutrient and energy cues is a key issue in the field. Both mTORC1 and AMPK sense and integrate cellular nutrition and energy signals to maintain cellular homeostasis by altering both anabolic and catabolic processes, including autophagy. Overwhelming evidence supports a key inhibitory role of mTORC1 in autophagy. Recent studies from our laboratories have made an important step forward in the understanding of ULK1 regulation and autophagy induction in response to nutrient signals.
In the present studies, genetic analysis in mouse liver as well as the nematode Caenorhabditis elegans demonstrated that both AMPK and ULK1 are required for proper autophagic flux in both contexts. Enforced expression of an activated form of AMPK in worm hypodermal cells is sufficient to promote autophagy, which is reduced by RNAi to ULK1 in this setting. These genetic data also support the idea that AMPK lies upstream of ULK1 and that AMPK regulation of ULK1 is required for proper autophagy.
Our two laboratories, independently studying ULK1 regulation and performing a screen for new AMPK substrates, both identified ULK1 as a direct substrate of AMPK. Collectively, we found six different AMPK sites (S317, S467, S555, T575, S637 and S777) in ULK1 in vivo using mass spectrometry and antibodies directed against four of these sites. We observed that the phosphorylation of these residues is dependent on AMPK and mirrors the kinetics of the known AMPK substrates ACC and raptor. Two of the AMPK phosphorylation sites in ULK1 (S555 and T575) also appear to be 14-3-3 binding sites, which parallels the two AMPK phosphorylation sites in raptor that serve as 14-3-3 binding sites. Notably, ULK1 kinase activity increases in an AMPK-dependent manner following glucose deprivation. Moreover, ULK1 can be directly activated in vitro by AMPK, demonstrating a direct role of AMPK in ULK1 regulation. Interestingly, two of the sites, S317 and S777, do not match the AMPK consensus motif, but they contribute to AMPK-dependent ULK1 kinase activation. Furthermore, a few additional sites bearing elements of the well-defined AMPK consensus motif also exist in ULK1, so future studies will be needed to fully quantitatively annotate all the phosphorylation sites in ULK1.
Both studies found that mutation of AMPK phosphorylation sites in ULK1 resulted in loss of ULK1 function, reflecting the fact that both AMPK and ULK1 act to stimulate autophagy in many different contexts. Reconstitution of ULK1-knockout cells with the AMPK nonphosphorylatable ULK1 mutants resulted in defective autophagy, altered mitochondrial homeostasis and reduced cell survival upon starvation, which phenocopied ULK1 deletion or RNAi for ATG5, a core component of the downstream autophagy machinery. These observations demonstrate the functional importance of ULK1 phosphorylation by AMPK in autophagy induction.
Interestingly, our studies, along with additional recent reports, have found that the AMPK kinase complex is tightly bound to the ULK1 kinase complex in cells. Notably, rapamycin treatment increases co-immunoprecipitation of AMPK and ULK1, suggesting that mTORC1 activity may regulate their association. Indeed, we determined that mTORC1 phosphorylates ULK1 on S757 and this phosphorylation disrupts the interaction between ULK1 and AMPK. Consistently, phosphorylation of the AMPK sites and mTORC1 site in ULK1 are inversely regulated under various conditions. Phosphorylation of the two AMPK sites, S317 and S777, is suppressed in TSC1-/- MEFs, or by RHEB co-transfection, conditions that have elevated mTORC1 activity. Taken together, these data suggest that under nutrient-rich conditions, active mTORC1 phosphorylates ULK1 to disrupt ULK1-AMPK interaction, thus keeping ULK1 inactive. Once cellular energy is depleted, such as during glucose deprivation, AMPK is activated and then inhibits mTORC1 via phosphorylation of both TSC2 and raptor, therefore, reducing S757 phosphorylation on ULK1. The S757 unphosphorylated ULK1 is able to associate with, and be activated by, AMPK (see Fig. 1). This coordinated phosphorylation of ULK1 by mTORC1 and AMPK may provide a mechanism by which cells can properly respond to a wide range of stimuli. Therefore, a balance of the activity of these three kinase complexes serves to modulate many of the cellular decisions about autophagy induction, growth and survival.
Given the concerted actions of the AMPK, ULK1 and mTORC1 complexes, each of which contain multiple regulatory subunits, it is likely that additional layers of biochemical complexity are embedded in the crosstalk of these kinase complexes and their control of growth, autophagy and cell survival. Given the derangement of autophagy in a number of human pathologies, it will be of interest to determine whether ULK1 regulation is abnormally altered in these conditions. Cells have adapted diverse uses for autophagy to custom tailor intracellular bioenergetics and homeostasis, which are just now beginning to be decoded. With a deeper understanding of the critical connections governing autophagy, future therapeutic options utilizing this pathway will no doubt arise.
Punctum to: Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. and Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152.