Abstract
Effective treatment of cancer cells with chemotherapeutic drugs relies on their ability to induce cell death, making the discovery of their mechanisms of action crucial. Arsenic trioxide (As2O3), used in the treatment of promyelocytic leukemia (PML), triggers cell death in several solid tumor cell lines including ovarian carcinomas. While As2O3 is remarkably cytotoxic in human ovarian cancer cells, its mechanism of action is poorly understood. We recently investigated the effects of As2O3 on several transforming growth factor-β (TGFβ) signaling mediators to better understand its cell death mechanism. Indeed, dysregulated (TGFβ) signaling is typical of ovarian cancers. Based on our findings, we propose that As2O3 induces a Beclin 1-independent autophagic pathway in ovarian carcinoma cells by modulating SnoN/SkiL expression, implicating SnoN as a novel therapeutic target for ovarian cancers.
Key words: autophagy, TGFβ, SnoN/SkiL, arsenic trioxide, Beclin 1
The treatment of ovarian carcinomas has faced numerous challenges due to developed resistance to chemotherapeutic drugs. Thus, the identification of novel therapeutic targets for the treatment of ovarian cancer remains an ongoing challenge. One potential signaling pathway to target is the TGFβ signaling pathway, wherein some of its mediators are dysregulated in ovarian carcinomas including EVI1 and SnoN (located at the 3q26.2 locus). As2O3 is an example of a chemotherapeutic drug for treatment of PML, which elicits cell death. This drug has been previously reported to degrade EVI1 in leukemia cells. Since the mechanisms by which As2O3 mediates its cell death effects have not been clearly elucidated, we thus investigated the effects of As2O3 on TGFβ signaling mediators to better understand its mechanism of action.
We observed that increasing doses of As2O3 induces autophagy and apoptosis by modulating expression of TGFβ signaling mediators in ovarian carcinoma cells (HEY and OVCA429). Notably, we observed decreased expression of EVI1 forms (EVI1, MDS1/EVI1 and EVI1Del190–515) with increasing doses of As2O3. When the As2O3 treated cells are treated with a proteasome inhibitor, MG132, the expression of EVI1 is recovered suggesting that EVI1 degradation occurs via the proteasome. In addition, As2O3 attenuates the expression of several other TGFβ signaling components such as TAK1, TGFβRII, SMAD2/3 and AKT. In contrast, the expression of SnoN is augmented with increasing doses of As2O3. Although TAK1 has the ability to phosphorylate SnoN which targets it for degradation, TAK1 does not appear to be responsible for the reduction of SnoN we observed in our cells (assessed using TAK1 siRNA). When cells are treated with proteasome inhibitors (i.e., MG132 or PS-341), we observed a further increase in SnoN expression suggesting that SnoN is further stabilized.
Normal and carcinoma cells may react distinctively to the same stimuli. Indeed, we observed that normal immortalized ovarian epithelial T80 ovarian cells are more sensitive to As2O3 compared to HEY and SKOV3 cells. Based on cell morphology (assessed by light microscopy) and measurements of apoptosis conducted in response to As2O3, T80 cells are significantly more apoptotic compared to both HEY and SKOV3. In addition, As2O3 treated T80 cells show a greater reduction of glutathione (GSH) levels compared to HEY and SKOV3. By western analysis, we determined that both HEY and SKOV3 cells are high expressing multi-drug resistance protein 1 (MRP1) cell lines whose expression could potentially contribute to the cells' resistance to As2O3 in the following manner: GSH binds to As2O3, which is then expelled from the cell through MRP1 channels thus leading to resistance to As2O3-mediated cell death.
A combination of western analysis of microtubule-associated protein 1 light chain 3 (LC3), fluorescence microscopy of EGFP-LC3 and transmission electron microscopy (TEM) were performed to positively identify the induction of autophagy upon As2O3 treatment. In all our cell lines analyzed (T80, HEY and SKOV3), the increased expression of SnoN (with increasing doses of As2O3) correlated with both LC3-I (cytosolic form) and LC3-II (membrane bound) expression. Using TEM, we positively identified the formation of autophagosomes with As2O3 treatment beginning at 3 hours. Furthermore, an As2O3 time course treatment profile demonstrates a peak increase in both SnoN and LC3 expression at 9 hours. By 18 hours, a reduction in the LC3-II/LC3-I ratio is associated with an increase in cleaved PARP levels indicating that the cells are undergoing apoptosis.
In order to demonstrate that As2O3 alters the expression of TGFβ signaling mediators through the generation of reactive oxygen species (ROS), we initially assessed changes in cell morphology by light microscopy, which revealed that cells treated with As2O3 alone are more apoptotic relative to cells co-treated with As2O3 and N-acetyl-L-cysteine (NAC). To confirm this observation, we performed apoptosis assays using propidium iodide (PI) and Annexin-V staining, which demonstrated that NAC opposes the effects of As2O3 leading to increased cell viability. This correlated with attenuated expression of SnoN with a corresponding recovery of several other TGFβ signaling mediators such as EVI1.
Several autophagy and apoptotic inhibitors were used to further define the effects of As2O3 on autophagy and apoptosis, specifically to determine whether autophagy arises to protect cells from apoptosis as a cell survival mechanism. We observed a reduced percentage of autophagosomes in cells treated with As2O3 in combination with 3-methyladenine (3-MA), an early stage autophagy inhibitor, compared to As2O3 alone. In addition, we observed reduced expression of LC3 with increased PARP cleavage, suggesting that a reduction in autophagy leads to increased levels of apoptosis. In contrast to cells cotreated with As2O3 and 3-MA, wherein the expression of Beclin 1 is unchanged, cells treated with resveratrol in combination with 3-MA do not show any marked changes in LC3 (or Beclin 1). We did, however, observe both elevated LC3 and Beclin 1 expression upon nutrient starvation with EBSS. We also performed knockdown studies of critical ATG genes involved in the autophagy pathway. Although knockdown of beclin 1 does not affect LC3 expression or the percentage of autophagosomes upon As2O3 treatment, knockdown of beclin 1 leads to a reduction in LC3 levels upon nutrient starvation. In contrast, knockdown of other ATG genes such as ATG5, ATG7 and hVPS34 does markedly affect the ratio of LC3-II/LC3-I and the percentage of autophagosomes upon As2O3 treatment. Interestingly, knockdown of hVPS34 affects the expression of LC3 only with As2O3 but not with resveratrol treatment. Therefore, we concluded that Beclin 1 does not appear to play a role in As2O3-induced autophagy. Furthermore, As2O3-induced autophagy is hVps34-dependent in contrast to resveratrol-induced autophagy, which is both Beclin 1- and hVps34-independent.
Since we observed that SnoN expression correlates with LC3 expression, we investigated whether modulation of SnoN levels could alter As2O3-mediated induction of autophagy. In As2O3-treated HEY cells, siRNA targeting SnoN increases apoptosis (evident by light micrographs, western analysis of PARP cleavage and cell viability assays including apoptosis assays) while decreasing the LC3-II/LC3-I ratio and the percentage of EGFP-LC3 puncta. The increased sensitivity to cell death upon treatment with As2O3 in cells with reduced SnoN expression suggests that this TGFβ transcriptional mediator could be involved in regulating the autophagic and apoptotic pathways.
Our studies suggest that SnoN/SkiL, a TGFβ signaling mediator, can modulate As2O3-induced autophagy. Furthermore, we have demonstrated that As2O3-induced autophagy in the ovarian cell lines occurs via a Beclin 1-independent but hVPS34-dependent mechanism (Fig. 1). Research is presently ongoing to further our understanding of these TGFβ mediators in regulating drug-induced autophagy, which will more clearly define the mechanism by which these mediators regulate drug-induced cell death for future development of therapeutic targeting strategies.
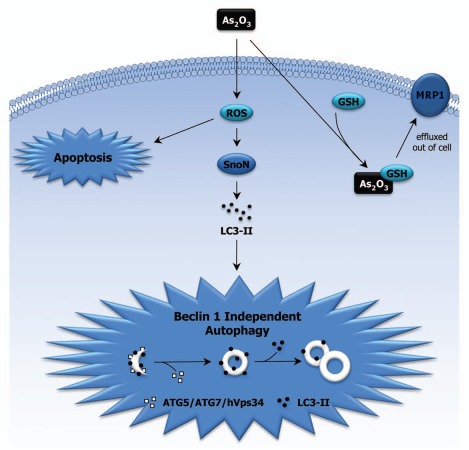
Figure 1.
Involvement of SnoN in As2O3-induced Autophagy. As2O3 alters the expression of TGFβ signaling mediators via the generation of reactive oxygen species (ROS). Our results suggest that the increase in SnoN expression leads to changes in LC3-II levels, implicating a role for SnoN in As2O3-induced autophagy, which could potentially bypass the apoptotic pathway. In addition, As2O3 bound to GSH can be effluxed out of the cell via MRP1 channels, which leads to cellular protection towards As2O3 in cell lines with elevated MRP1 levels.
Punctum to: Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1 independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Diff. 2010 doi: 10.1038/cdd.2010.53. In press.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13041