Abstract
An emerging body of evidence supports a role for autophagy in the pathophysiology of type 1 and type 2 diabetes mellitus. Persistent high concentrations of glucose lead to imbalances in the antioxidant capacity within the cell resulting in oxidative stress-mediated injury in both disorders. An anticipated consequence of impaired autophagy is the accumulation of dysfunctional organelles such as mitochondria within the cell. Mitochondria are the primary site of the production of reactive oxygen species (ROS), and an imbalance in ROS production relative to the cytoprotective action of autophagy may lead to the accumulation of ROS. Impaired mitochondrial function associated with increased ROS levels have been proposed as mechanisms contributing to insulin resistance. In this article we review and interpret the literature that implicates a role for autophagy in the pathophysiology of type 1 and type 2 diabetes mellitus as it applies to β-cell dysfunction, and more broadly to organ systems involved in complications of diabetes including the cardiovascular, renal and nervous systems.
Key words: autophagy, diabetes mellitus, diabetes complications, oxidative stress, mitochondria dysfunction, autoimmunity
Introduction
Macroautophagy (hereafter referred to as autophagy) is a cellular process that sequesters senescent or damaged organelles/proteins in autophagosomes for recycling of their products.1 Autophagy is also involved in the removal of cells that have undergone classical type 1 programmed cell death or apoptosis. Hence, autophagy can be generally considered as a protector of cells against various stressors and a cellular response to routine wear-and-tear. Paradoxically, autophagy may also lead to a form of non-apoptotic cell death,2–5 which is called type 2 programmed cell death. Thus, autophagy can either protect cells or promote cell death, depending on the cellular and environmental context.
The life cycle of the autophagosome is a complex pathway which involves the coordinated activity of at least 18 gene products, characterized initially in yeast.6,7 The gene products are named progressively autophagy-related (Atg)1, Atg2, etc., and have mammalian functional orthologs which are the focus of an active body of research. A number of additional gene products identified in yeast are not required for autophagosome formation, such as Atg11, Atg19, Atg20, etc. The detection and characterization of several of these products have yielded valuable information regarding a potential role of autophagy in a variety of diseases including diabetes mellitus. For instance, Beclin 1, an ortholog of yeast Atg6, interacts with the class III phosphatidylinositol (PtdIns) 3-kinase complex in the nucleation steps of autophagosome formation, and also acts as a Bcl-2 binding protein.8 Another modulator of autophagy is Atg5, which plays a critical role in the expansion steps of autophagosome development, and also interacts with the Fas-activated death domain (FADD) complex, a component of the extrinsic apoptosis cascade.4 Additionally, the cleavage of Atg5 provokes apoptotic cell death, suggesting a potential dual role for this gene product in crosstalk between the autophagy and apoptosis pathways.9 As another example, Atg7, whose function involves the critical steps of Atg8 and Atg12 conjugation, plays a relevant role in animal transgene models of diabetes.10,11 Microtubule-associated protein 1 light chain 3 (LC3), a mammalian ortholog of Atg8, is critical for the conjugation and expansion steps of autophagosomal development, following its cleavage and lipidation into LC3-II.12 As a component of the mature autophagosome, LC3-II serves as a valuable molecular biomarker for the detection and assessment of autophagic activity.
A physiological role of autophagy in mammalian biology has been demonstrated in several in vivo animal models. Disruption of autophagy leads to failure of cavitation during embryogenesis, and accumulation of abnormal mitochondria in adult tissues.13,14 Dysfunctional autophagy probably plays a role in the pathophysiology of a variety of diseases including neurodegenerative disorders,15,16 cardiomyopathy17 and cancer.18 This probably involves the accumulation of damaged molecules and organelles. Autophagy also appears to play a role in the cellular changes associated with aging.19
Is there a Connection between Autophagy and Diabetes?
Diabetes mellitus is a metabolic disorder defined by persistent hyperglycemia. Most cases of diabetes are classified as either type 1 or type 2, although other categories are recognized, such as gestational diabetes. Abnormally elevated blood glucose levels may arise from a lack of insulin production (type 1 diabetes mellitus, T1D), or as a consequence of resistance to the cellular effects of insulin, as well as declining insulin production (type 2 diabetes mellitus, T2D).20,21 In both situations, it has become evident that persistent high concentrations of glucose lead to imbalances in the antioxidant capacity within the cell resulting in oxidative/nitrosative-mediated stress and injury. This scenario forms a metabolic common denominator for both types of diabetes.
An anticipated consequence of impaired autophagy is the accumulation of dysfunctional organelles, such as mitochondria, within the cell.22 Mitochondria are the primary site of the production of reactive oxygen species (ROS), and an imbalance in ROS production relative to the cytoprotective action of autophagy may lead to the accumulation of ROS.23 Impaired mitochondrial function associated with increased ROS levels has been implicated as mechanisms contributing to insulin resistance.24,25 Therefore, it is possible that impaired autophagy may contribute to insulin resistance.
Insulin and intracellular molecules such as mammalian target of rapamycin (mTOR) are well-known inhibitors of autophagy,26,27 whereas glucagon, a counter-regulatory hormone of insulin, induces autophagy.28 Cell organelles such as mitochondria29,30 and endoplasmic reticulum (ER)31,32 that play a crucial role in β-cell survival/death, insulin secretion and insulin action/sensitivity rely on autophagy to maintain normal cell function. These observations support the possibility that autophagy is involved in the natural history of diabetes via its role in influencing hormone action and organelle function. Other factors such as dyslipidemia and lipotoxicity may also be relevant in the induction of cell stress and the stimulation of autophagy.33
Aside from the oxidative-stress environment, there are likely significant differences in the modulation of autophagy in different tissues in diabetes. For instance, the pathways that regulate autophagy in the insulin-producing β-cells in type 1 diabetes may be quite different from the pathways associated with insulin resistance and promotion of autophagy in other known at-risk tissues, such as the retina, nervous system, cardiovascular system and kidney in type 2 diabetes. For example, the effects of remaining insulin secretory capacity in type 2 diabetes may blunt the autophagic response through the enhancement of nutrient uptake and the stimulation of transducing pathways and mediators, such as type1 PtdIns 3-kinase or mTOR.
In this review, we will examine current concepts regarding a potential role for autophagy in the natural history of diabetes mellitus. The core question to be addressed is: Does autophagy contribute to the pathophysiology in pancreatic β-cells, as well as other tissues known to be adversely affected during the natural history of diabetes including the cardiovascular, kidney and nervous systems?
Induction of Autophagy in Pancreatic β-cells
In this section we will review our current understanding regarding two relevant issues: (1) What are the conditions and molecular pathways that trigger autophagy in beta cells? (2) Does autophagy participate in a continuum of cellular responses to stress that ranges from an initial adaptive response to physiological stess and culminates in cell injury and death in diabetes?
To investigate a physiological role of autophagy, investigators have employed β cell-specific Atg7-deficient mice (Atg7f/f:RIP-Cre mice) by crossing mice carrying an Atg7-conditional allele (Atg7f/f) with transgenic mice expressing the Cre recombinase under the control of the β-cell-specific rat insulin promoter (RIP).11 The Atg7f/f:RIP-Cre mice demonstrate progressive degeneration of β-cells, characterized by cellular hypertrophy followed by depletion of insulin immunoreactivity. These β-cell degenerative changes are accompanied by accumulation of polyubiquitinated proteins and LC3-binding protein p62,34 with increased formation of large intracellular aggregates. Thus, autophagy appears to be involved in constitutive protein turnover in β-cells and defective autophagy is associated with formation of large inclusion bodies containing polyubiquitinated proteins, p62 and insulin. Glucose tolerance tests revealed that impairment of glucose homeostasis of Atg7f/f:RIP-Cre mice is caused by reduced glucose-stimulated insulin secretion. The accumulation of structurally and functionally defective mitochondria appeared to contribute to the reduced capacity to secrete insulin, based on the observation that glucose-stimulated ATP production is significantly reduced in islets isolated from Atg7f/f:RIP-Cre mice, compared to Atg7f/f mice.
Interestingly, lipopotoxicity may play an important role. Degenerative changes in β-cells of Atg7f/f:RIP-Cre mice become more evident when these mice are fed a high-fat diet, suggesting a higher susceptibility to lipotoxicity.33 In addition, the number of degradative vacuoles and p62 accumulation are enhanced. These results suggest that in the presence of insulin resistance, autophagy is increased and may play an essential role in eliminating senescent or damaged proteins and organelles in β-cells, thereby protecting these cells from death, and the animal from onset of diabetes.
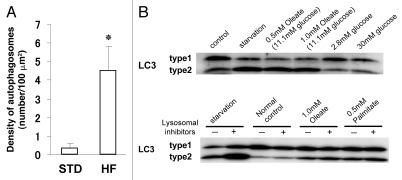
The potential importance of autophagy in pancreatic β-cell homeostasis is also suggested by the demonstration of upregulation of autophagy in secretory-deficient Rab3-/- β-cells,35 wherein intracellular insulin storage is maintained despite impaired insulin release because of increased activities of microautophagy and macroautophagy. Thus, autophagy may contribute to the maintenance of intracellular insulin content through acceleration of the insulin degradation rate in β-cells. Specifically, few autophagosomes are identified in β-cells in control C57BL/6 mice and in nondiabetic db/misty mice. In contrast, analysis of β-cells in diabetic db/db and in C57BL/6 mice fed a high-fat diet reveals active formation of autophagosomes. In contrast to what is observed in most other organs, starvation of C57BL/6 mice for 48 h does not result in any increase in autophagic vacuole formation in β-cells. On the other hand, ex vivo incubation of islets harvested from C57BL/6 mice in amino acid-starved medium results in increased formation of autophagosomes. In vitro studies indicate that exposure of INS-1β cells to low or high glucose (from 2.8 to 30 mM) does not induce autophagy. However, long-chain free fatty acids (FFA), such as oleate and palmitate, stimulate autophagy in INS-1β cells, when assessed by conversion of LC3-I to LC3-II (Fig. 1). Increased LC3-II levels in the presence of lysosomal inhibitors supports an increase in autophagy in response to FFA stimulation. This interpretation is supported by a recent report indicating enhanced formation of autophagosomes and autolysosomes following exposure of INS-1β-cells to palmitate for 12 h.37 This study also notes that FFA induce a gradual decrease in the level of phospho-mTOR, a signaling pathway that inhibits activation of autophagy. Since the increase in serum FFA level is observed under conditions with insulin resistance, stimulation of β-cell autophagy by FFA suggests its potential role in the regulation of β-cell function in diabetes and obesity-associated conditions.
Figure 1.
Autophagosome formation in pancreatic β-cells. Electron microscopy analysis reveals enhanced autophagosome formation in β-cells of 20-week-old mice fed a high-fat diet for 12 weeks (from 8 to 20 weeks of age). (A) Graph: enhanced formation of autophagosomes in β-cells of high-fat diet-fed C57BL/6 mice (HF) compared with standard diet-fed group (STD). Density of autophagosomes was calculated, and data are presented as mean ± SEM of three mice. *p < 0.01 versus controls. (B) FFA but not high glucose can induce autophagy in INS-1 cells. For control, cells were incubated in RPMI 1640 supplemented with FCS and amino acids. For starvation, cells were incubated for 2 h in Hank's buffer free of serum and amino acids. So that accumulation of LC3-II could be visualized, the lysosome inhibitors pepstatin A (10 µg/ml) and E-64-d (10 µg/ml) were added to the culture medium for the last 2 h period unless otherwise indicated. Adapted from reference 33, with permission.
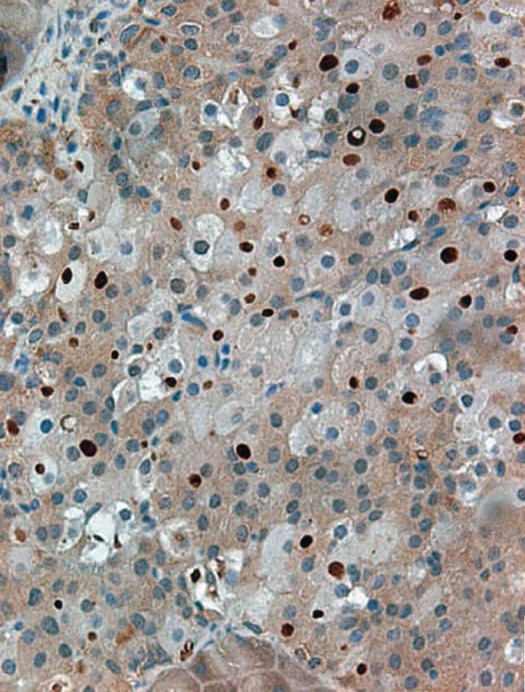
The increased presence of inclusion bodies in cells is a hallmark of degenerative conditions such as Parkinson, Alzheimer and alcoholic liver disease.37 Inclusion bodies in autophagydeficient β-cells contain large ubiquitin aggregates (Fig. 2).34,35 Accumulation of ubiquitin-containing inclusions is also observed in pancreatic islet cells of Zucker diabetic fatty rats and in insulinoma cells treated with high concentrations of glucose.38 Intriguingly, autophagy-competent pancreatic β-cells of transgenic mice expressing amyloidogenic human-type islet amyloid polypeptide contain ubiquitinated material as well.39 Both proteasomal and autophagy pathways play a role in the degradation of ubiquitinated proteins, supporting a complementary interaction between these pathways.40 Soluble or short-lived ubiquitinated proteins are more likely to be degraded by the proteasomal pathway, whereas long-lived or large ubiquitinated proteins appear to be degraded by the autophagy pathway.41
Figure 2.
Accumulation of ubiquitin aggregates in pancreatic β-cells of Atg7Δ β-cell mice. Pancreatic sections were subjected to immunohistochemistry using anti-ubiquitin antibodies. Several islet cells show accumulation of large protein aggregates containing ubiquitin (dark-brown spots). Adapted from reference 34, with permission.
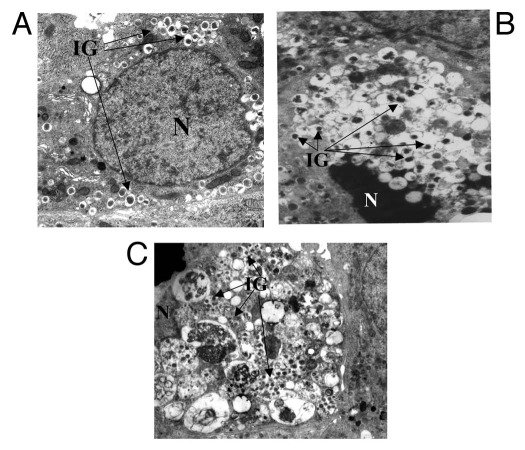
In a recent study, pancreatic samples from individuals with type 2 diabetes were examined using electron microscopy. The authors observed an increase in the number of dead β-cells in diabetic islets compared to nondiabetic cases.42 Many of the β-cells showed signs of apoptotic cell death (marked chromatin condensation and/or blebs). However, several others were characterized by massive vacuole overload without nuclear alterations, suggesting autophagy-associated cell death (Fig. 3). When transcription of molecules involved in the autophagic machinery was studied in isolated islets, unchanged expression of beclin 1 and Atg1/Ulk1 (involved in early steps of autophagy) and reduced transcription of lysosome-associated membrane protein (LAMP)-2 and cathepsin B and D (involved in later steps) were observed in the type 2 diabetic samples. In the same study, the authors exposed isolated nondiabetic islets to increased non-esterified fatty acids and observed a marked increase of vacuole accumulation, together with enhanced beta-cell death and decreased LAMP-2 expression. It was also noted that the alterations of autophagy in type 2 diabetic β-cells and those in nondiabetic islets pre-exposed to fatty acids could be corrected pharmacologically. Specifically, the anti-diabetic drug metformin is able to reduce the amount of autophagy-associated beta-cell death in isolated type 2 diabetic islets as well as in nondiabetic islets cultured with high levels of free fatty acids.42 Our current perspective regarding pathways involved in the induction of autophagy in beta cells in diabetes mellitus and the relationship of autophagy to programmed cell death is depicted in Figure 4.
Figure 3.
Electron microscopy of a normal human β-cell (A), an apoptotic type 2 diabetic β-cell with marked chromatin condensation (B), and a dead type 2 diabetic β-cell with evidence of massive vacuoles overload (C). N, nucleus; insulin granules. Magnification x10,000 (A and C), x7,000 (B). Adapted from reference 116, with permission.
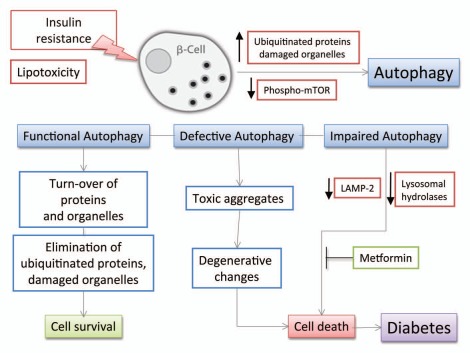
Figure 4.
Schematic diagram of the effects of insulin resistance on β-cell autophagy. Various stresses caused by peripheral insulin resistance can induce polyubiquitination of proteins and accumulation of damaged organelles as cytoplasmic aggregates in pancreatic β-cells. Ubiquitinated proteins and damaged organelles, which otherwise form toxic aggregates, are effectively cleared by autophagy. Thus, autophagy can be viewed as a defense mechanism against cellular damage in β-cells. Elevated free-fatty acids also stimulate β-cell autophagy by gradual decrease in the level of phospho-mTOR under the state of insulin resistance. Interestingly, lipotoxicity induces impaired autophagy in human β-cells with reduction of LAMP-2 and lysosomal hydrolases leading to vacuole accumulation and cell death.
In summary, an adaptive or cytoprotective role for autophagy has been postulated in lipoapoptosis in insulinoma cells that could be involved in the decrease of β-cell mass in T2D.36 However, other studies did not observe a cytoprotective role for autophagy in ER stress-induced death in fibroblasts.43 Decreased mTOR activation in the lipoapoptosis model also needs to be reconciled with increased mTOR activation in the insulin target tissues of obese animals.44 The relationship between autophagy and lipid-induced cell injury could be tissue-dependent, and the relationship between lipid-mediated injury, ER stress and activation of autophagy requires further study. A reduced level of β-cell autophagy could be a predisposing factor for type 2 diabetes. If confirmed, does a reduced level of β-cell autophagy play a pathogenic role in type 2 diabetic subjects? To address this question, a suitable method for monitoring the level of intracellular autophagy in prediabetic patients needs to be established.
Autoimmune Mechanisms in Type 1 Diabetes Mellitus and the Role of Autophagy
In contrast to T2D that develops as a consequence of insulin resistance and progressive pancreatic β-cell failure, type 1 diabetes involves the autoimmune-mediated destruction of pancreatic β-cells. Since pancreatic β-cell death is an integral part of the pathogenesis of T1D,45,46 and autophagy may be involved in the regulation of apoptosis and necrosis,47–49 it is possible that autophagy could contribute to the development of T1D at multiple stages of the disease. It is relevant that autophagy participates in antigen-presenting cell function by modulating macrophage survival/death,50 cytokine release from macrophages51 and antigen presentation by dendritic cells.52 Recent papers demonstrate an important role of autophagy in both class I MHC- and class II MHC-restricted pathways.53,54 Because both CD4+ T cells and CD8+ T cells are important effector cells in β-cell death,55,56 autophagy may affect the function of T cells and thereby, the development of T1D.
Interaction of Autophagy with Intracellular Signaling Pathways in Diabetes
There are diverging upstream pro-apoptotic signals in both T1D and T2D, for example, the transcription factors NFκB and STAT-1 in T1D and on other signaling molecules, still to be identified, in T2D. Activation of these upstream transcription factors may converge downstream into common apoptotic pathways, such as ER stress, activation of JNK and AMP-activated protein kinase (AMPK), mitochondrial dysfunction and production of reactive oxygen species (ROS).57
Elevation in ROS is essential for autophagy to proceed because their presence may control the activity of Atg4, a family of cysteine proteases that are necessary for autophagosome formation.58 ROS also induce the activation of poly(ADPribose) polymerase (PARP-1). PARP-1 plays an important role in repairing damaged DNA through an energetically expensive process, causing rapid depletion of cellular nicotinamide adenine dinucleotide (NAD+) and failure in ATP production.59 As mentioned previously, autophagy is inhibited by mTOR. One of the major upstream regulators of mTOR is AMPK, a critical energy sensor. A recent report demonstrates that ROS-induced PARP-1 activation promotes autophagy through AMPK activation, probably by suppression of mTOR.60
Production of ROS in beta cells is also induced by streptozotocin (STZ) treatment.61 STZ is an agent used widely to cause experimental diabetes because of its ability to selectively target and destroy insulin-producing pancreatic islet beta cells.62 The glucose moiety of STZ allows preferential uptake of the toxin into beta cells via the glucose transporter, GLUT-2. STZ-induced blocking of α-mannosidase activity and nitric oxide deficiency can cause accumulation of unfolded proteins, ER stress and activation of cellular signals leading to cell death.63 STZ is an alkylating agent that causes breakage of DNA strands. This leads to the activation of PARP-1 and a rapid energy depletion resulting in beta cell death.59 A recent report suggests that STZ is able to induce autophagy in beta cells and also activate the expression of the vacuole membrane protein 1 (VMP1).64 VMP1 is a trans-membrane protein whose expression triggers autophagosome formation through interacting with Beclin 1.65 VMP1 is not constitutively expressed in the pancreas but is highly activated in beta cells in response to experimental diabetes. These observations suggest that VMP1 expression is an early molecular event that switches on in beta cells in response to diabetic conditions. Therefore, VMP1 may participate in molecular pathways that activate autophagy in stressed beta cells.
A key factor related to beta cell function is its highly developed endoplasmic reticulum (ER) structure. Cytokines and gluco-lipotoxicity induce ER stress. Early changes in gene expression in beta cells have been described in response to ER stress in diabetes.66 ER stress decreases insulin gene expression, and this process is mediated by ATF6 (activating transcription factor 6).67 The molecular mechanism linking ER stress and autophagy remains to be clarified, but polyglutamine-induced LC3 conversion is reported to be mediated by ER stress-induced PERK/eIF2α phosphorylation. Therefore, under diabetic conditions beta cells may activate phenotypic changes associated with activation of autophagy.
There is a growing body of evidence that the hyperglycemic, dyslipidemic conditions observed in T1D is associated with enhanced production of proinflammatory cytokines and oxidative stress that has important consequences for β cell function.68 Several studies implicate a role for IL-1β in the activation of cell stress responses such as ER stress.69 The focus on the β cell is relevant because of its unique role in insulin production. The β cell also demonstrates a high rate of protein synthesis, which makes it susceptible to derangements such as the unfolded protein response.70 Hyperglycemia induces IL-1β production by the β cell, which is auto-stimulatory.71 This pathway may be an important transducer in the hyperglycemic environment in islets, aside from the known production of IL-1β by infiltrating immune cells in the pancreas. For example, a recent report indicates that the induction of IL-1β production by elevated glucose in β cells is observed only when basal levels of IL-1β mRNA are low.72 This supports a potentially important role for IL-1β acting as a metabolic sensor, as well as an inflammatory mediator. An interesting recent study suggests that loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production.73
There is a need for further studies to contrast and compare the tissue-specific regulation of autophagy by cytokines in pancreatic β cells and extra-pancreatic tissues. For instance, a recent report with EW7 cells indicates that the activation of autophagy by TNFα is inhibited by activation of NFκB.74 This potentially interesting observation needs to be studied in diabetic pancreatic tissue because of the relevant roles of both TNFα and NFκB in inducing apoptosis, as well as the modulation of cytoprotective cell responses. Since pancreatic β-cell death is an integral part of the pathogenesis of T1D75 and autophagy is an important regulator of apoptosis and necrosis,76,77 it is possible that autophagy could contribute to the development of diabetes at multiple stages of the disease. In studies related to Crohn disease, the deletion of the autophagy gene Atg16L1 leads to enhanced endotoxin-stimulated production of IL-1β, suggesting a role for autophagy in preventing the adverse effects of proinflammatory cytokines.51 It is also possible that Atg16L1 may have a non-autophagy-related role in Crohn disease.
Taken together, these observations suggest a potentially relevant role for autophagy in the pathophysiology of diabetes if the following criteria are met: (1) Diabetes is associated with an abnormal level of autophagy in pancreatic β cells that can be linked as part of a continuum to β cell death; (2) Autophagy is stimulated in resident macrophages by the presence of increased levels of proinflammatory cytokines; and (3) These events occur with concomitant stimulation of MHC II antigen presentation. If these criteria are confirmed in the laboratory and clinical setting, this framework could provide a critical link between pro-inflammatory oxidative stress, innate immunity and adaptive immunity. This would help shed light on a fundamental question in autoimmunity, namely, the process or processes through which the immune system in an organism loses tolerance to its own proteins and tissues.
Autophagy in Cardiovascular Complications in Diabetes
Coronary artery disease is a major health concern and the leading cause of death in individuals with diabetes. Endothelial dysfunction and alterations at the coronary vessel wall resulting in the formation of atherosclerotic plaques are at the foundation of this cardiovascular disease. Hyperlipidaemia, hypertension, hyperglycemia, obesity and other factors interact to induce the development and instability of atherosclerotic plaques.78 Enhanced oxidative stress appears to be a key factor in promoting high risk of atherosclerosis in diabetes.79 In nondiabetic models of atherosclerosis, mild oxidative stress seems to activate autophagy, which in turn facilitates the removal of damaged organelles. Under these conditions, autophagy appears to play a protective role against atherogenesis, in part due to a reduction in the number of macrophages in the atherosclerotic plaque.80,81 This process involves mTOR signaling pathways and probably other non-mTOR-related mechanisms, such as those mediated by TLR7 (JNK-p38-Erk).82,83 Autophagy in macrophages results in a more stable plaque, with a lower tendency to rupture.84 When autophagy is an insufficient response to pro-atherogenesis conditions (e.g., very high concentrations of ROS), leakage of mitochondrial proteins, such as cytochrome c, may promote apoptosis.85 Elevated levels of ROS also damage lysosomal membranes by interacting with lipids and proteins. As a consequence, leakage of lysosomal hydrolases into the cytoplasm may also induce apoptosis.
In atherosclerosis, autophagy is activated by several mechanisms: (1) Oxidized lipids (as 4-hydroxynonenal, malonyldialdehyde, oxidized LDL); (2) Endoplasmic reticulum stress (probably through chaperone proteins, such as glucose-regulated peptide GRP78/BiP); (3) Inflammation (at least in part through cytokine production); (4) Hypoxia and metabolic stress.86–89 All of these mechanisms are exacerbated in diabetes. Inflammatory cytokines such as TNFα increase the expression of LC3 and Beclin 1, promoting autophagy in plaque cells.90 Conversely, other chemokines, such as IL-4 and IL-13, appear to suppress autophagy through stimulation of type I PtdIns 3-kinase and the consequent activation of mTOR.91 Autophagy also may play a role in downregulating ApoB-rich apolipoprotein particles.92,93 Trapping of ApoB-containing lipoproteins at the arterial wall is an early event in atherosclerotic plaque development. The addition of polyunsaturated fatty acids to hepatocyte cultures increases the post-endoplasmic reticulum presecretory proteolysis of ApoB.93,94 That could result in a reduced circulation of ApoB-rich particles, possibly by an autophagic process.
Diabetic cardiomyopathy is another relevant chronic cardiovascular complication in diabetic patients. Cardiac-specific loss of Atg5 is associated with cardiac hypertrophy, left ventricular dilation and contractile dysfunction.95 In cardiac muscle, as in other tissues, insulin antagonizes AMPK, activating mTOR signaling pathways.96 Taken together, these observations suggest that constitutive autophagy represents a physiological cellular respose to damaged organelles, leading to cell survival and protecting myocardium from oxidative injury. Insufficient or impaired autophagy, as well as excessive autophagy, could result in cell death (by apoptosis or necrosis) with potential clinical relevance. The proportional contribution of autophagy and apoptosis to myocardial wall alterations in diabetic cardiomiopathy requires further research.
Autophagy in Diabetic Nephropathy
The streptozotocin (STZ)-induced diabetic rodent models demonstrate nephropathy affecting both proximal and distal tubular segments, and are characterized by the early inhibition of autophagy.97 The inhibition of autophagy in this model of early diabetic kidney injury can be reversed by insulin. Paradoxically, insulin seems to inhibit tubular cell autophagy in nondiabetic kidneys.97,98 This differential effect of insulin may be related to a dysfunction in the molecular mechanisms regulating autophagy in the presence of STZ and/or hyperglycemia. The effect of insulin in controlling renal autophagy in humans remains unclear.
In ischemia/reperfusion (I/R) models, overexpression of the anti-apoptotic protein Bcl-2 results in suppression of autophagosomal degradation, protecting tubular cells from I/R-induced death. Autophagy may be triggered by renal ischemia or reperfusion injury to eliminate damaged mitochondria and abort apoptosis.99 Moreover, mitochondrial disappearance is partially prevented by bafilomycin A1, an inhibitor of autophagosomal fusion with lysosomes, suggesting that autophagy is involved in this process. An increase of oxidative stress could be involved in ER damage. In diabetes, oxidative stress is clearly exacerbated and it has been considered a central factor for hyperglycemia-mediated damage.100 In addition, inhibition of poly(ADPribose) polymerase-1 (PARP-1) activity protects proximal tubular cells against ischemia-induced necrosis by preserving ATP levels.60 Thus, PARP-1 may participate in the mechanisms that regulate autophagy during diabetes-related renal damage (putatively through the LKB1-AMPK-mTor pathway). Therefore, it is possible that at early phases of nephropathy, a reduction of constitutive autophagy leads to the pathological changes described at this stage of disease. Further amplification of these pathological changes may be due to impaired or insufficient autophagic response. The actual involvement of impaired autophagy at end stages of the human disease requires further research. Similarly, establishment of a linkage between autophagy and apoptosis in diabetic nephropathy remains to be elucidated.
Autophagy in Diabetic Neuropathy
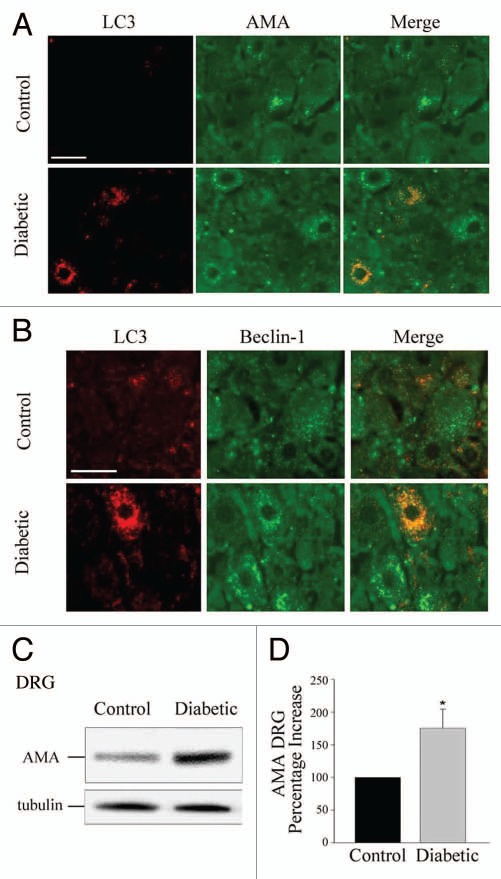
Numerous alterations have been described as part of the metabolic and cellular milieu in neural tissue in diabetes, including hyperglycemia, dyslipidemias and excess generation of reactive oxygen species and reactive nitrogen species (RNS).101–104 These conditions result in cytosolic and mitochondrial oxidative stress, generation of abnormally glycated proteins and dysfunctional mitochondria.105 These alterations have been the focus of a growing body of research that suggests enhanced autophagy is a cytoprotective response.7 In neural tissue, autophagy has been described as a cleansing mechanism that eliminates or ameliorates the damage of cellular stressors.106 Other studies indicate that deletion of Atg7 in Drosophila leads to accumulation of ubiquitin-positive aggregates in degenerating neurons,107 further substantiating the role of autophagy in the maintenance of health in neural tissues. Additionally, lack of insulin induces autophagic cell death in adult hippocampal neural cells.108 Numerous questions remain to be answered regarding the relative contribution of different stressors to autophagy and the interactions of the autophagy cascade with other cellular signals. For instance, insulinopenic, STZ-diabetic rats exhibit increased levels of autophagy in dorsal root ganglia (DRG) (Fig. 5) and interestingly, there is colocalization of LC3 with both mitochondria and also with the FAS (CD 95)-cascade mediator FADD.109 While the interaction of FADD with Atg5 has been previously described as necessary for autophagy,110 it is thought-provoking that there is crosstalk between the autophagic and the extrinsic apoptotic pathways in a model of diabetic neuropathy, suggesting the possibility that the stimulation of autophagy may lead to a reduction in the pool of FADD available to integrate the Fas-FADD-caspase-8 death-inducing signaling complex (DISC) necessary for surface membrane death-receptor stimulation of the extrinsic apoptosis cascade. The stimulation of autophagy in neural tissue may also be modulated by serum and autoimmune factors through membrane receptors. For instance, the presence of naturally occurring anti-Fas autoantibodies in humans has been reported in sera of normal donors.111 Studies with the human neuroblastoma SH-SY5Y cell line show that incubation of the cells with sera from subpopulations of patients with diabetes and neuropathy, leads to the activation of autophagy based on increased levels of LC3-II and colocalization with FADD, via a Fas-dependent mechanism.112 This observation leads to the provocative possibility that the humoral immune system can modulate autophagy, and that the metabolic and intracellular milieu present in diabetes elicits an autophagy response through membrane-based and intracellular pathways. Thus, there is mounting evidence indicating that autophagy plays a potentially significant role in the pathophysiology of diabetic neuropathy. Additional research is required to fully understand the induction of autophagy in diabetic nerve and its relationship to neuronal injury during the natural history of diabetic neuropathy, which typically requires years to become clinically evident.
Figure 5.
Diabetic rats demonstrate increased levels of anti-LC3 and anti-mitochondria (AMA) immunoreactivity that colocalize in dorsal root ganglion (DRG) neurons. (A) IHC detection of LC3 and colocalization with the mitochondrial AMA signal in DRG neurons from control and diabetic rats. (B) IHC detection of Beclin 1, LC3 and their colocalization in DRG neurons of normal, control and diabetic rats. (C) Representative immunoblot of mitochondrial (AMA) in DRG homogenates from normal (control) and diabetic rats. (D) Histogram of densitometric scannings of immunoblots of mitochondria (AMA) in DRG homogenates from control and diabetic rat DRG neurons. The normal control was set to 100%. Scale bar, 50 µm. *p < 0.05. Adapted from reference 109, with permission.
Therapeutic Implications
Rapamycin is a well-known agent that induces autophagy, and has been employed to suppress allograft rejection after islet transplantation in patients with T1D.113 However, rapamycin affects other aspects of cellular function besides autophagy, and it is premature to predict the potential of rapamycin or related compounds as therapeutic agents in diabetes. A recent paper reports that rapamycin ameliorates glucose intolerance in experimental animals fed a high-fat diet supplemented with branched chain amino acids but not those fed a high-fat diet alone,114 suggesting a potential role for rapamycin or related compounds in T2D. Several new modulators of autophagy are being developed and tested,115 which will hopefully lead to novel therapeutic opportunities involving diabetes, as well as other medical conditions.
Drugs already in clinical use could affect autophagy in diabetes. Metformin is a widely prescribed oral antidiabetic agent that reduces hepatic glucose output and enhances peripheral insulin sensitivity. Metformin seems to reduce autophagic vesicle accumulation and beta cell death in both beta cells from T2D patients and beta cells from nondiabetic controls exposed to nonesterified fatty acids (NEFA). These effects can be associated with restored LAMP2 expression.116 LAMP2 expression is reduced in cells from patients with diabetes and under high concentrations of NEFA. Metformin potentiates AMPK activity117 which inhibits mTOR.118 In addition, metformin may inhibit mTOR independent of AMPK.119 Since mTOR inhibition leads to increased removal of autophagic material, it is conceivable that metformin promotes the generation and subsequent elimination of autophagic vesicles by the inhibition of mTOR. Therefore, a link between LAMP2 and AMPK activity has been suggested, but it requires confirmation. It has been suggested that metformin may have some effect on preserving islet structure and beta cell mass in prediabetes and at early stages of the disease. This matter is still pending clarification, and could be an indirect result of the metformin action on insulin sensitivity.120 If this effect exists, the contribution of autophagy to this “islet protective effect” of metformin is unknown. New tissue-selective activators of AMPK hold potential as novel therapeutic agents in the metabolic field and several candidates are in the pipeline of several pharmaceutical companies.121 Further exploration of the effects of these agents on autophagy is justified. Long-acting fatty acid analogues also can increase AMPK activity and, by this mechanism, act to modify autophagy in diabetes.122 Hypothetically, nutritional interventions may also have some impact on autophagy rates in dysglycemic patients.
Summary
There is ample evidence supporting an active role for autophagy in the pathophysiology of diabetes mellitus. Elucidation of the specific extracellualar and intracellular conditions that stimulate autophagy and the linkage of these conditions to either cell survival or cell injury and death in different cell types is a rapidly evolving and fruitful field of research. The development of therapies to take advantage of the potential cytoprotective effect of autophagy in diabetes is a potentially promising avenue of investigation.
Acknowledgements
C.D.G. and M.I.V. thank ANPCyT (PICT 20555); CONICET (PIP 1607) and UBA (M076), Buenos Aires, Argentina for their support; M.P. is supported in part by the European Community, project IMIDIA, N. 115005; J.W.W. is supported in part by the following grants from the National Institutes of Health: R01-056997, R01-052387, M01-RR-00042 and ULIRR024986.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/13044
References
- 1.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–6324. doi: 10.1158/0008-5472.CAN-06-4217. [DOI] [PubMed] [Google Scholar]
- 3.Park KJ, Lee SH, Kim TI, Lee HW, Lee CH, Kim EH, et al. 3: A human scfv antibody against trail receptor 2 induces autophagic cell death in both trail-sensitive and trail-resistant cancer cells. Cancer Res. 2008;67:7317–7334. doi: 10.1158/0008-5472.CAN-06-4766. [DOI] [PubMed] [Google Scholar]
- 4.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of atg5 and FADD in autophagic cell death: Dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2007;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 5.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Klionsky D. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–95. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;1:137–148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 10.Fujitani Y, Kawamori R, Watada H. The role of autophagy in pancreatic beta-cell and diabetes. Autophagy. 2009;5:280–282. doi: 10.4161/auto.5.2.7656. [DOI] [PubMed] [Google Scholar]
- 11.Jung HS, Lee MS. Macroautophagy in homeostasis of pancreatic β-cell. Autophagy. 2009;5:241–243. doi: 10.4161/auto.5.2.7518. [DOI] [PubMed] [Google Scholar]
- 12.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation induced and constitutive autophagy in atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 17.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 18.Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793:1516–1523. doi: 10.1016/j.bbamcr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36:2392–2404. doi: 10.1016/j.biocel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ. Modern-day clinical course of type 1 diabetes mellitus after 30 years'/lpage> duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomgarden ZT. The 6th Annual World Congress on the Insulin Resistance Syndrome. Diabetes Care. 2009;32:127–133. doi: 10.2337/dc09-zb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 25.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Eng J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein s6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 27.Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 29.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucoseinduced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernales S, Schuck S, Walter P. ER-phagy: Selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 32.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 33.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine β-cells. Mol Endocrinol. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 36.Choi SE, Lee SM, Lee YJ, Li LJ, Lee SJ, Lee JH, et al. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 2009;150:126–134. doi: 10.1210/en.2008-0483. [DOI] [PubMed] [Google Scholar]
- 37.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy: therapeutic implications. Curr Top Dev Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 38.Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH. Ubiquitinated protein aggregates form in pancreatic β-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 39.Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol. 2007;293:1656–1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- 40.Pandey UB, Nie Z, Batlevi Y, McCary BA, Ritson GP, Nedelsky NB, et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu M, Waguri S, Chiba T, Murata S, Iwata JI, Tanida I, et al. Loss of autophagy in the central nervous system causes neudodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 42.Marchetti P, Masini M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy. 2009;5:1055–1056. doi: 10.4161/auto.5.7.9511. [DOI] [PubMed] [Google Scholar]
- 43.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Mizushima N, Kimchi A. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 44.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 45.Kim YH, Kim S, Kim KA, Yagita H, Kayagaki N, Kim KW, Lee MS. Apoptosis of pancreatic β-cells detected in accelerated diabetes of nod mice: No role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol. 1999;29:455–465. doi: 10.1002/(SICI)1521-4141(199902)29:02<455::AID-IMMU455>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 46.Mathis D, Vence L, Benoist C. β-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 47.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 49.Wu YT, Tan HL, Huang Q, Kim YS, Pan N, Ong WY, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy. 2008;4:457–466. doi: 10.4161/auto.5662. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281:19179–19187. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 51.Saitoh T, Fujita N, Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein atg16l1 enhances endotoxin-induced il-1β/lpage> production. Nature. 2008;456:264–269. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 52.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 53.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Pontesilli O, Gill RD, La Rosa FG, Lafferty KJ. The role of CD4+ and CD8+ T cells in the destruction of islet grafts by spontaneously diabetic mice. Proc Natl Acad Sci USA. 1991;88:527–531. doi: 10.1073/pnas.88.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 58.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, et al. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- 60.Huang Q, Wu YT, Tan HL, Ong CN, Shen HM. A novel function of poly(ADP-ribose)polymerase-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Differ. 2009;16:264–277. doi: 10.1038/cdd.2008.151. [DOI] [PubMed] [Google Scholar]
- 61.Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu XQ, Wu L, Guo XJ. Effect of Bu-Zhong-Yi-Qi-Tang on deficiency of N-glycan/nitric oxide and islet damage induced by streptozotocin in diabetic rats. World J Gastroenterol. 2009;15:1730–1737. doi: 10.3748/wjg.15.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grasso D, Sacchetti ML, Bruno L, Lo Ré/lpage> A, Iovanna JL, Gonzalez CD, Vaccaro MI. Autophagy and VMP1 expression are early cellular events in experimental diabetes. Pancreatology. 2009;9:81–88. doi: 10.1159/000178878. [DOI] [PubMed] [Google Scholar]
- 65.Ropolo A, Grasso D, Pardo R, Sacchetti ML, Archange C, Lo Re A, et al. The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem. 2007;282:37124–37133. doi: 10.1074/jbc.M706956200. [DOI] [PubMed] [Google Scholar]
- 66.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 67.Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, et al. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via upregulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008;149:3832–3841. doi: 10.1210/en.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venieratos PD, Drossopoulou GI, Kapodistria KD, Tsilibary EC, Kitsiou PV. High glucose induces suppression of insulin signaling and apoptosis via upregulation of endogenous IL-1β/lpage> and suppressor of cytokine signaling-1 in mouse pancreatic beta cells. Cell Signal. 2010;22:791–800. doi: 10.1016/j.cellsig.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Gurzov EN, Ortis F, Cunha DA, Gosset G, Li M, Cardozo AK, Eizirik DL. Signaling by IL-1β/lpage> + IFNγ and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic β-cell apoptosis. Cell Death Differ. 2009;16:1539–1550. doi: 10.1038/cdd.2009.99. [DOI] [PubMed] [Google Scholar]
- 70.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Böni-Schnetzler M, Ehses JA, Faulenbach M, Donath MY. Insulitis in type 2 diabetes. Diabetes Obes Metab. 2008;10:201–204. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 72.Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 74.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Souquère S, et al. NFκB activation represses tumor necrosis factor-α-induced autophagy. J Biol Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- 75.Kim YH, Kim S, Kim KA, Yagita H, Kayagaki N, Kim KW, Lee MS. Apoptosis of pancreatic β-cells detected in accelerated diabetes of nod mice: No role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol. 1999;29:455–465. doi: 10.1002/(SICI)1521-4141(199902)29:02<455::AID-IMMU455>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 76.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 78.Cheng AY, Leiter LA. Diabetes and cardiovascular disease: the role of glycemic control. Curr Diab Rep. 2009;9:65–72. doi: 10.1007/s11892-009-0012-y. [DOI] [PubMed] [Google Scholar]
- 79.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 80.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 81.Schrijvers DM, De Meyer GRY, Herman AG, Martinet W. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP, et al. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol. 2007;49:706–715. doi: 10.1016/j.jacc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 83.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 85.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2000;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 86.Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and celular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- 87.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2000;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 88.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heymann D. Autophagy: A protective mechanism in response to stress and inflammation. Curr Opin Investig Drugs. 2006;7:443–450. [PMC free article] [PubMed] [Google Scholar]
- 90.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNFα/lpage> regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;8:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 91.Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr Opin Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.A Fisher EA, Williams KJ. Autophagy of an oxidized, aggregated protein beyond the ER: a pathway for remarkably late-stage quality control. Autophagy. 2008;4:721–723. doi: 10.4161/auto.6346. [DOI] [PubMed] [Google Scholar]
- 93.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinet W, De Meyer RY. Autophagy in Atherosclerosis. A cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 95.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomycytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 96.Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- 97.Barbosa Júnior Ade A, Zhou H, Hültenschmidt D, Totovic V, Jurilj N, Pfeifer U. Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:359–366. doi: 10.1007/BF02890439. [DOI] [PubMed] [Google Scholar]
- 98.Han K, Lehringer-Polzin M, Zhou H, Pfeifer U. Cellular autophagy in proximal tubules of early diabetic rats following insulin treatment and islet transplantation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:367–373. doi: 10.1007/BF02890440. [DOI] [PubMed] [Google Scholar]
- 99.Isaka Y, Suzuki C, Abe T, Okumi M, Ichimaru N, Imamura R, et al. Bcl-2 protects tubular epithelial cells from ischemia/reperfusion injury by dual mechanisms. Transplant Proc. 2009;41:52–54. doi: 10.1016/j.transproceed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 100.Brownlee M. Preventing kidney cell suicide. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1107-1284. [DOI] [PubMed] [Google Scholar]
- 101.Burul-Bozkurt N, Pekiner C, Kelicen P. Diabetes Alters Aromatase Enzyme levels in sciatic nerve and hippocanpus tissues of rats. Cell Mol Neurobiol. 2010;30:445–451. doi: 10.1007/s10571-009-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vincent AM, Hinder LM, Pop-Busi R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Negi G, Kumar A, Sharma SS. Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochmical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;391:102–106. doi: 10.1016/j.bbrc.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Askwith T, Zeng W, Effo MC, Stevens MJ. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: implications for pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab. 2009;297:620–628. doi: 10.1152/ajpendo.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007;6:352–361. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- 107.Juhasz G, Neufeld TP. Drosophila Atg7: required for stress resistance, longevity and neuronal homeostasis, but not for metamorphosis. Autophagy. 2008;4:357–358. doi: 10.4161/auto.5572. [DOI] [PubMed] [Google Scholar]
- 108.Baek SH, Kim EK, Goudreau JL, Lookingland KJ, Kim Sw, Yu SW. Insulin withdrawal-induced cell death in adult hippocampal neural stem cells as a model of autopahgic cell death. Autophagy. 2009;5:277–279. doi: 10.4161/auto.5.2.7641. [DOI] [PubMed] [Google Scholar]
- 109.Towns R, Kabeya Y, Yoshimori T, Guo C, Shangguan Y, Hong S, et al. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitocondria in SY5Y cells. Autophagy. 2005;1:163–170. doi: 10.4161/auto.1.3.2068. [DOI] [PubMed] [Google Scholar]
- 110.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 111.von Gunten S, Schaub A, Vogel M, Stadler BM, Miescher S, Simon HU. Immunological and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin (IVIg) preparations. Blood. 2006;108:4255–4259. doi: 10.1182/blood-2006-05-021568. [DOI] [PubMed] [Google Scholar]
- 112.Towns R, Guo C, Shangguan Y, Hong S, Wiley JW. Type 2 diabetes with neuropathy: autoantibody stimulation of autophagy via Fas. Neuroreport. 2008;19:265–269. doi: 10.1097/WNR.0b013e3282f4cb50. [DOI] [PubMed] [Google Scholar]
- 113.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Eng J Med. 2003;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 114.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martinet W, Verheye S, De Meyer GR. Everolimusinduced mTOR inhibition selectively depletes macrophages in atherosclerotic plaques by autophagy. Autophagy. 2007;3:241–244. doi: 10.4161/auto.3711. [DOI] [PubMed] [Google Scholar]
- 116.Masini M, Bugliani M, Lupi R, Del Guerra S, Boggi U, Filipponi F, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 117.Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 118.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signaling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi SH, Zhao ZS, Lee YJ, Kim SK, Kim DJ, Ahn CW, et al. The different mechanisms of insulin sensitizers to prevent type 2 diabetes in OLETF rats. Diabetes Metab Res Rev. 2007;23:411–418. doi: 10.1002/dmrr.756. [DOI] [PubMed] [Google Scholar]
- 121.Zhou G, Sebhat IK, Zhang BB. AMPK activators-potential therapeutics for metabolic and other diseases. Acta Physiol (Oxf) 2009;196:175–190. doi: 10.1111/j.1748-1716.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 122.Za'tara G, Bar-Tana J, Kalderon B, Suter M, Morad E, Samovski D, et al. AMPK activation by long chain fatty acyl analogs. Biochem Pharmacol. 2008;76:1263–1275. doi: 10.1016/j.bcp.2008.08.028. [DOI] [PubMed] [Google Scholar]