Abstract
Hepatitis C virus (HCV) infects approximately 130 million people worldwide. The clinical sequelae of this chronic disease include cirrhosis, functional failure and carcinoma of the liver. HCV induces autophagy, a fundamental cellular process for maintaining homeostasis and mediating innate immune response, and also inhibits autophagic protein degradation and suppresses antiviral immunity. In addition to this ploy, the HCV serine protease composed of the viral nonstructural proteins 3/4A (NS3/4A) can enzymatically digest two cellular proteins, mitochondria-associated antiviral signaling protein (MAVS) and toll/interleukin-1 receptor domain containing adaptor inducing IFNβ (TRIF). Since these two proteins are the adaptor molecules in the retinoic acid-inducible gene I (RIG-I) and TLR3 pathways, respectively, their cleavage has been suggested as a pivotal mechanism by which HCV blunts the IFNα/β signaling and antiviral responses. Thus far, how HCV perturbs autophagy and copes with IFNα/β in the liver remains unclear.
Keywords: autophagy, hepatitis C virus, liver disease, NS3/4A protease, RIG-I, Type I interferon, TLR3, transgenic mouse
To tackle these questions, we generated transgenic mice with hepatocyte-specific expression of HCV NS3/4A proteins. Immunohistochemistry shows the presence of NS3 protein in about 89% of the hepatocytes. The viral serine protease indeed cleaves MAVS, and only 21% hepatocytes in transgenic mice show the intact protein. Does this MAVS cleavage block the IFNα/β pathways and the innate immunity in the liver? We examined this question with an i.v. bolus of vesicular stomatitis virus (VSV), which elicits a rapid, RIG-I-dependent IFNα/β response in mice. To our surprise, NS3 transgenic mice mount strong IFNβ and IFN-stimulated gene (ISG) responses and promptly clear VSV in the liver. We also noticed that transgenic mice, when challenged, show strong ISG activation, which isn't necessarily proportional to their relative IFNβ induction. We speculate that the involvement of the IFN-positive feedback loop in the neighboring bystander hepatocytes and hepatic immune cells could partially compensate for NS3-mediated MAVS cleavage in the liver. With respect to HCV in patients, the hepatic distribution of HCV RNA has been reported to be focal, whereas ISG induction appears all across the liver.
The HCV genome contains the pathogen-associated molecular pattern (PAMP) sequence for RIG-I recognition. To further define the function of HCV-mediated MAVS cleavage, we hydrodynamically delivered HCV genomic RNA to the liver of our transgenic mice. Similar to VSV, HCV RNA results in a potent induction of IFNβ and ISG responses in the liver. Unexpectedly, however, the HCV RNA injection renders the NS3 protein undetectable. After ruling out the possibilities of a blockage in transcription and de novo protein synthesis, as well as immunoproteasome-mediated degradation, we hypothesized that an accelerated NS3 protein turnover is linked to the type I IFN response in the liver. To test this notion, we injected transgenic mice with recombinant IFNα or -β. IFNα infusion does not change the levels of NS3 expression, whereas IFNβ leads to a complete sequestration of NS3 in the liver. Protein blot analyses of LC3/Atg8 led us to the possibility that IFNα challenge induces an upregulation of autophagy. IFNβ injection results in decreased levels of both LC3-I and -II, indicative of enhanced autophagy as well as autolysosomal proteolysis. The blockade of this proteolysis by chloroquine prevents NS3 loss in the liver. Intriguingly, IFNβ or HCV RNA injection to a different strain of transgenic mice expressing the HCV structural proteins also renders the viral core protein undetectable; the same treatment, however, does not affect the levels of the Cre recombinase, a cytosolic transgenic protein, in the liver of the HCV mice. These results indicate that IFNβ targets HCV proteins to autophagic pathways, possibly due to their close association with the mitochondria or ER membranes.
HCV survives in an environment that is under constant immune pressure. To understand the mechanistic aspects of the virus-host interaction in such a hostile setting, we examined autophagic trafficking of HCV NS3 in response to IFN injection. Analysis of gradient centrifugation-derived vesicles from liver cells indicates that only the IFNβ-induced autophagosomes are directed to the lysosome for maturation and removal, whereas IFNα-induced subcellular vesicles are trafficked to the late endosomes. Interestingly, although HCV RNA can induce the lipidation of LC3 in the absence of MAVS, IFNβ cannot. These results indicate that IFNβ induced autophagy via a MAVS-dependent manner, which is different from that induced by HCV. Since MAVS is associated with mitochondria, IFNβ-induced autophagy likely involves mitochondria. Previously, we have shown that HCV induces incomplete autophagy via an unfolded protein response. The current data suggest possible mechanisms of IFNα/β in delivering viral components to the TLR3-containing endosomes, setting off a second wave of TLR3/TRIF-mediated antiviral response in vivo (Fig. 1).
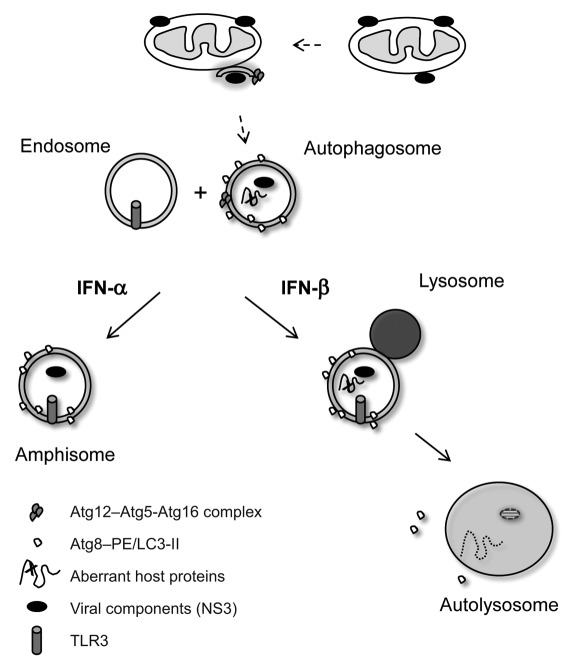
Figure 1.
IFNα and -β have differential effects on autophagic trafficking of HCV proteins. HCV NS3/4A proteins are associated with the outer mitochondrial membrane. It is possible that these viral proteins are delivered to the autophagic pathways via the vesicles originating from the mitochondria (dashed lines). We showed that exogenous IFNβ, as well as conditions favoring strong IFNβ production (e.g., VSV or HCV RNA injection), promotes NS3 trafficking to autophagosomes, maturation, lysosomal fusion and viral protein degradation. This IFNβ-mediated NS3 proteolysis in the liver of transgenic mice could be blocked by chloroquine. In contrast, IFNα also trafficked the subcellular vesicles to late endosomes, but not lysosomes. These data suggested a differential trafficking of viral components by IFNα/β and a mechanism for their delivery to the TLR3-containing endosomes, setting off a second wave of TLR3/TRIF-mediated antiviral response in vivo.
In summary, our results demonstrate that the MAVS cleavage alone does not compromise global type I IFN and ISG responses in the liver. While HCV induces an incomplete autophagy for its replication and immune evasion, the IFNβ and -α responses target viral components to differential autophagic compartments for removal by lysosomal hydrolases and/or perhaps TLR3-mediated immune recognition. While several reports have revealed that HCV can subvert autophagy for its evolutionary advantage, our data have underscored a role of innate immune responses in wrestling this machinery and hampering viral replication. From this study, the autophagic apparatus appears to be a focal point for virus-host interaction in the liver. Viral replication clearly generates intracellular stress, which is detected by the host and has pathophysiological consequences. Short-term stress of the normal cellular processes can lead to production of chemokines (e.g., CXCL10) and cytokines (e.g., IL-7), as well as increased co-stimulatory molecule expression and antigen presentation; sustained strain and compensatory mechanisms, however, may result in fundamental deviations, leading to metabolic abnormalities and carcinogenesis. Additional studies focusing on the virus-autophagy interface may lead to new antivirals or novel therapeutics that shift the balance and result in viral clearance.
Acknowledgements
This work was supported by National Institutes of Health Grants AI069142 and AI043003. M.M.D. is a James W. McLaughlin Postdoctoral Fellow and a Hans Popper Postdoctoral Fellow of the American Liver Foundation. We thank Ms. Mardelle Susman for her editorial help.
Abbreviations
- ER
endoplasmic reticulum
- HCV
hepatitis C virus
- IFN
interferon
- ISG
IFN-stimulated gene
- LC3
microtubule-associated protein 1 light chain 3
- RIG-I
retinoic acid-inducible gene I
- MAVS
mitochondria-associated antiviral signaling protein
- PAMP
pathogen-associated molecular pattern
- TLR3
toll-like receptor 3
- TRIF
toll/interleukin-1 receptor domain containing adaptor-inducing IFNβ
- VSV
vesicular stomatitis virus
Punctum to: Desai MM, Gong B, Chan T, Davey RA, Soong L, Kolokoltsov AA, Sun J. Differential, Type I Interferon-Mediated Autophagic Trafficking of Hepatitis C Virus Proteins in Mouse Liver. Gastroenterology. 2011;141:674–685. doi: 10.1053/j.gastro.2011.04.060.