Abstract
In this review we put the spotlight on crosslinked polymer nanogels, a promising platform that has the characteristics of an “ideal” drug delivery vehicle. Some of the key aspects of drug delivery vehicle design like stability, response to biologically relevant stimuli, passive targeting, active targeting, toxicity and ease of synthesis are discussed. We discuss several delivery systems in this light and highlight some examples of systems, which satisfy some or all of these design requirements. In particular, we point to the advantages that crosslinked polymeric systems bring to drug delivery. We review some of the synthetic methods of nanogel synthesis and conclude with the diverse applications in drug delivery where nanogels have been fruitfully employed.
Keywords: Nanomaterial, Ligand display, Encapsulation stability, Stimuli responsive
1. Introduction
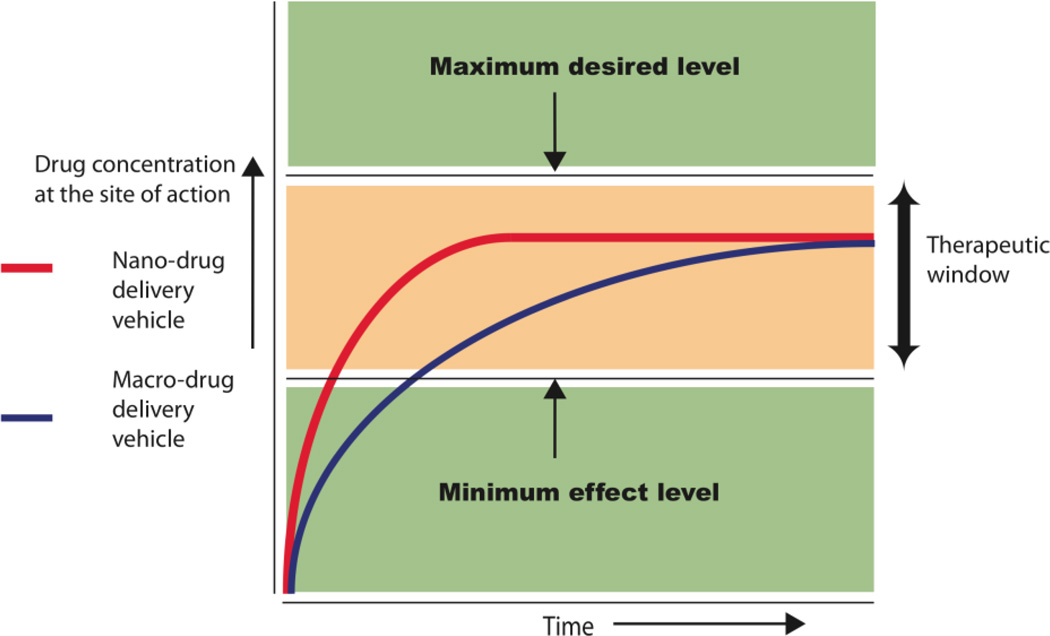
Nanogels have found applications in several fields such as sensing [1],diagnostics [2, 3] and bioengineering [4], but its greatest impact has been in the area of drug delivery. Nanogels and other nanosized drug delivery systems have several advantages over macro-sized delivery systems. For example, one of the key aspects in drug delivery is controlled release of drugs. Figure 1 shows release profiles of “ideal” drug delivery vehicles highlighting the therapeutic window: at concentrations above this window the drug would cause toxic side effects, while below this limit the drug would be ineffective as a drug [5]. Though such control can be achieved in macro-sized systems, if appropriately engineered nano-sized delivery systems can achieve finer temporal control over drug release rates due to their large surface area. Nanogels can also be inherently useful in systems that require a burst release. Nanosystems, unlike bulk drug delivery systems, can enter cells to deliver drugs and can be designed to respond to intracellular cues. Further, since nanomaterials can circulate in the body after being injected they have the ability to target diseases at the site of disorder. This feature of nanomaterials is especially useful in cancer therapy, where the size of the delivery system is key to target cancers through the enhanced permeability and retention effect (EPR) [6]. Diseased cells can also be targeted by attaching ligands or antibodies to the surface of nano-drug delivery systems. Targeting allows nanocarriers to hone into diseased cells by targeting specific features of a disease phenotype, such as an over expressed protein or enzyme. Another important aspect of delivery that is now being given the importance it deserves is the drug encapsulation stability in these carriers. This is especially relevant because it is increasingly realized that the thermodynamic parameters like percent (%) loading do not adequately describe how stable the delivery vehicle would be during circulation in blood, since these vehicles could potentially leak out drugs into hydrophobic sites in surrounding tissue and blood components. Delivery vehicles, based on a single platform, that can satisfy all basic requirements of a versatile nanoscopic delivery vehicle are quite rare. These features however are the foundations of a good delivery vehicle and are fundamental design requirements. Thus there are key aspects of a delivery vehicle design that we describe as the basic anatomy of a drug delivery vehicle (see Figure 2). Each of these aspects will be addressed individually in this review and are briefly introduced below.
Fig. 1.
Release profile of nano drug delivery systems when compared to bulk delivery systems.
Fig. 2.
The anatomy of a drug delivery vehicle.
The Anatomy of a Drug Delivery Vehicle
Encapsulation stability: Drug molecules should be stably encapsulated such that these do not prematurely leak during circulation. This is essential to insure maximal therapeutic efficacy with minimal side effects.
Response to stimuli: Stability during encapsulation is desirable during circulation and once at a target site the drug should be released. Thus response to stimuli is an essential feature of a drug delivery vehicle.
Passive targeting: This design aspect is key to targeting several diseases especially, cancer and arthritis. This aspect of design, being controlled by size, also determines circulation time or clearance times form the body.
Active targeting: This strategy is used to target specific disease phenotypes and so reduce side effects. A delivery system should allow in its design, handles to attach active targeting ligands.
Toxicity: The delivery system should be non-toxic and ideally it should be biodegradable with non-toxic degradation products, which get cleared from the body.
Ease of synthesis: A less important aspect for laboratory investigations, but crucial for industrial scale production and ultimate approval by regulatory agencies such as the FDA.
With these desirable features of an “ideal” drug delivery vehicle stated we now describe how self-assembling polymeric nanogels from this viewpoint provide a versatile platform that performs well in these drug delivery design features. Following this discussion, we talk about the methods of synthesis of nanogels and end with describing some examples of their applications in drug delivery.
2. Anatomy of a Drug Delivery Vehicle
2.1. A Case for Encapsulation Stability: Does Loading Percent Matter?
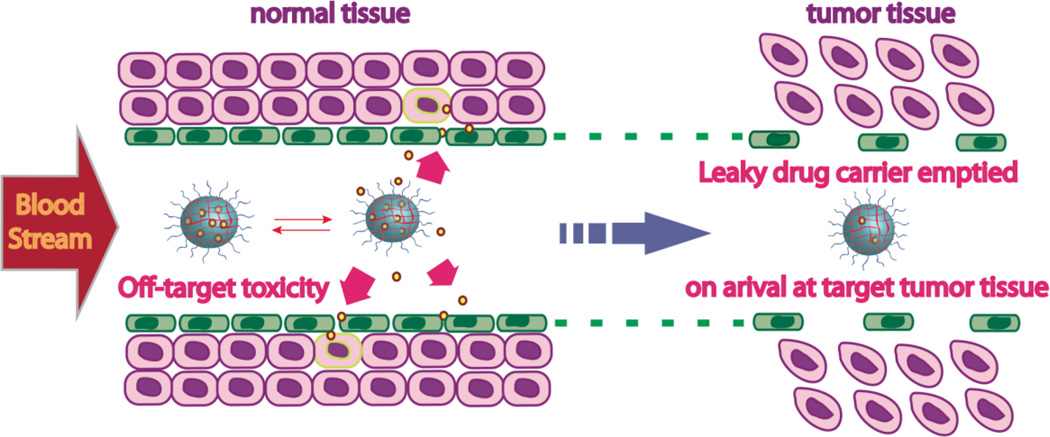
Encapsulation stability is an important feature that needs to be addressed when designing a drug delivery vehicle. Accidental leakage can result in the supply of drug molecules to healthy cells as well as a nearly empty vehicle upon arrival at the disease site, as illustrated in Figure 3.
Fig. 3.
Problems with unstable leaky delivery carriers.
Encapsulation of a delivery system is often reported in terms of its percent drug loading capacity i.e. the weight percent of the drug loaded per unit weight of the delivery vehicle. Note however that percent loading is simply the thermodynamic distribution coefficient of the drug molecule between the interior of the nanocarrier and the bulk solvent (aqueous phase), as illustrated in Figure 4. For most delivery carriers, this value has been suggested to be reasonable between 5 and 25% [7]. High loading of drug is a desirable feature in drug delivery, as this greatly reduces the amount of the nanocarrier used during medication.
Fig. 4.
Loading capacity measurements carried out in isolated aqueous environment.
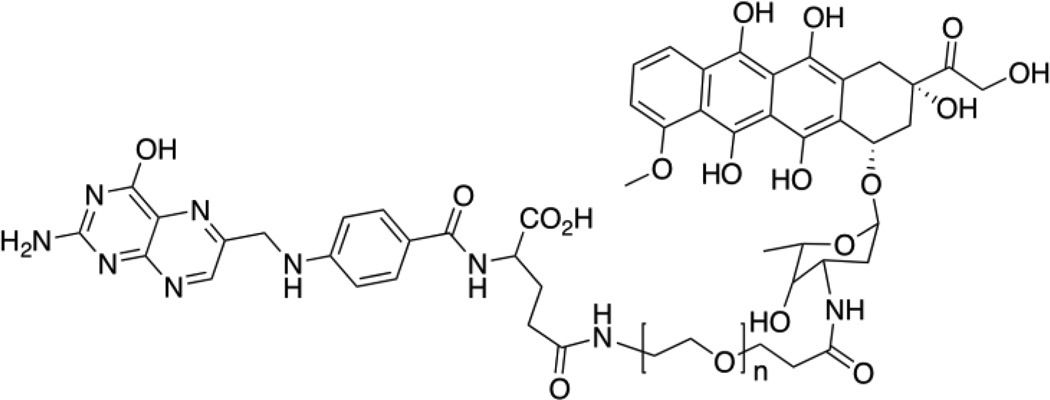
Typically high reported loading capacities are in the range of 10 to 60% [8–13]. In one such report, a loading capacity of 26 wt% of an anti-cancer drug was obtained with a relatively simple design, where the hydrophobic drug doxorubicin (Dox) was conjugated on one end of a bi-functional PEG chain with amine and carboxylic acid functionalities (see Figure 5).
Fig. 5.
Structure of self-assembling Dox modified PEG micelle with folic acid as a targeting ligand.
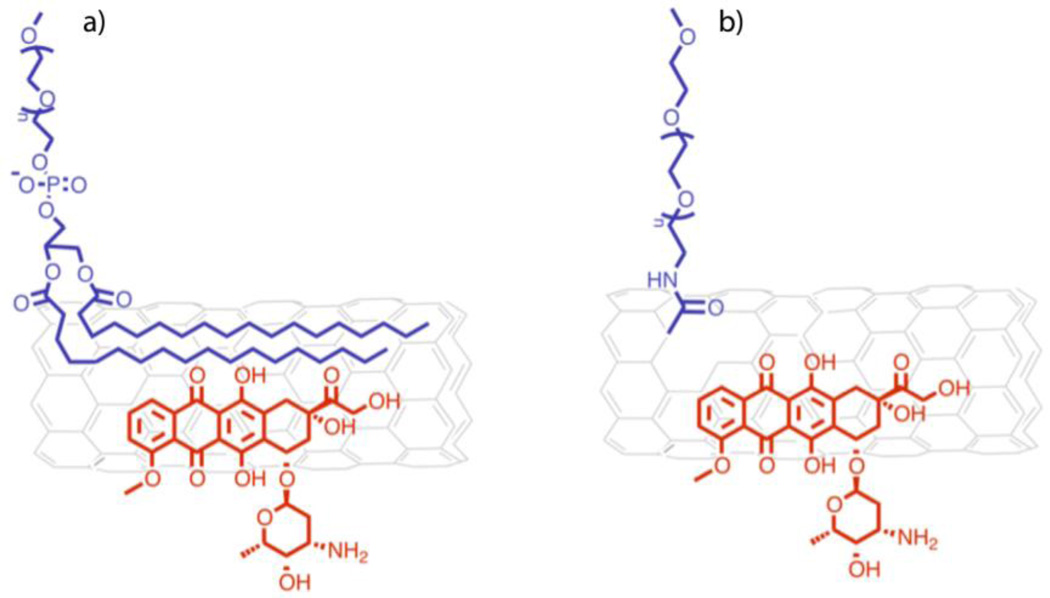
The amine functionality on doxorubicin was coupled with the carboxylic acid functionality on PEG and the amine functionality on the PEG was coupled with a targeting folic acid moiety by NHS/DCC coupling [10]. The hydrophilic-lipophilic balance (HLB) of this system is sufficient to achieve a stable micellar assembly. Free dox was also subsequently loaded into the vehicle by precipitating the drug in the presence of the micelle. This system was reported to show promise in human tumor xenograft animal model. Similarly, a single walled carbon nanotube SWCNT based delivery system using non-covalent supramolecular methods of drug loading was reported to have exceptionally high loading capacity at 50 to 60% of Dox [13] (see Figure 6). Aqueous dispersion of SWCNTs was made possible by the attachment of surface PEG groups by covalent (carboxylic acid functionalization and amide coupling chemistry) and non-covalent (supramolecular association of PEG-phospholipids with SWCNTs via hydrophobic interactions) means.
Fig. 6.
Dox loaded on large hydrophobic surface area on CNT. Carbon nanotubes a) non-covalently or b) covalently modified with PEG.
This system made use of pi-pi interactions in addition to hydrophobic interactions between doxorubicin and the carbon nanotubes. Interestingly, it was shown that the binding energy of doxorubicin (Dox) to SWCNTs depended on the diameter of the nanotube, with larger diameter (1.9 nm) nanotubes binding Dox with a binding affinity of 59 kJ/mol compared to the smaller diameter (1.3 nm) nanotubes at 48 kJ/mol. Animal studies using the same system showed that SWNT were localized into tumors, but were also taken up by the reticuloendothelial system (RES) [14]. Clearance of these delivery systems from the body is also a concern that must be addressed.
Percent loading is an important measure of the ability of the carrier to hold large amounts of drug which usually also determines drug dosage requirements during administration and consequently also its side effects. Percent loading alone, however, is not a sufficient indicator of delivery vehicle stability and additional studies that determine encapsulation stability are necessary [7]. This is because percent loading is representative of a thermodynamic distribution coefficient of the drug in the delivery carrier in preference to the surrounding aqueous environment, while encapsulation stability in a complex environment like the body, is determined by equilibrium rates of drug exchange with its environment [15]. During circulation in turbulent flow conditions in the blood, delivery vehicles encounter blood cells, serum, lipid-membranes and several potential hydrophobic components where drugs could be leaked. Studies that report a lack of correlation between in vitro and in vivo studies highlight the need to measure stability in more quantifiable and universal terms. Burt et al. synthesized block copolymers consisting of a MePEG block and a block of either PDLLA, copolymers of poly(d,l-lactide-co-caprolactone) (PDLLACL) or poly(glycolide-co-caprolactone) (PGACL) [16]. Stability of the block-co-polymer micelles was evaluated in terms of visual evidence for carrier instability, i.e. appearance of turbidity in solution over time. In this study, it was reported that the MePEG-PDLLA copolymer had comparatively the best stability with paclitaxel compared to PDLLACL–MePEG and PGACL–MePEG copolymers. The biodistribution of paclitaxel and the delivery vehicle in Sprague-Dawley rats were monitored using tritiated paclitaxel and 14C labeled MePEG-PDLLA copolymers. It was found that while paclitaxel was distributed throughout the rat body, the MePEG-PDLLA was rapidly eliminated in the urine. It was concluded that there was no correlation between in vitro loading stability of paclitaxel in polymer micelles and their encapsulation stability in blood.
Thus an in vitro technique to monitor encapsulation stability is useful to guide development of drug delivery vehicles. In one such report, stability of polymer micelles was tested by co-encapsulating a FRET pair, DiO/DiI, and studying the leakage of dye into model DOPC supported bilayers [17]. Leakage of the dyes from the micelle led to separation between the FRET donor and acceptor to distances greater than the Föster radius and consequently loss of FRET. Using a similar experimental design, in vitro studies to monitor dye leakage into cells to study the mechanism of cell uptake was also carried out. These studies demonstrated that while PEG-PDLLA polymer micelles encapsulated the DiO/DiI pair stably, the dye pair was internalized into the cell faster than the polymer itself. These observations were also made with PEG-PCL micelles. The authors emphasized the need for a crosslinked system that prevents such dye leakage if targeted therapy were to be effective.
A simple FRET based technique was recently described to compare drug stability in various delivery systems [18] (see Figure 7). In this method, two lipophilic FRET pair dyes (DiO/DiI) are first independently sequestered in drug carriers and then the carriers are mixed.
Fig. 7.
Structure of lipophilic FRET pair dyes and the FRET based mixing experiment for determining delivery vehicle stability.
Two limiting experimental outcomes are possible. If the dye molecules are stably encapsulated they will never find hydrophobic pockets of neighboring carriers and we will not observe any FRET upon exciting the donor dye. However, if the vehicle is leaky, it should allow for exchange of dye molecules between the two containers leading to an enhancement in FRET. This principle has been applied to quantify leakage using what is defined as the leakage coefficient (∧). Leakage coefficients of various surfactants, including commercial Pluronic-P85s, were also compared and are tabulated below. Leakage coefficient is reported as the slope of the linear fit of the plot of FRET ratio, Ia/(Id + Ia), where Ia and Id are the fluorescence intensities of the acceptor (DiI) and the donor (DiO) respectively, with time. These studies demonstrated that crosslinked polymer nanogels give exceptionally good and tunable encapsulation stability, compared to uncrosslinked systems such as Pluronics®. Experimentally determined leakage coefficients are tabulated in Table 1. The composition of the delivery vehicle, and ultimately its supramolecular structure in solution, controls its encapsulation stability. Designing a self-assembling stable delivery vehicle involves choosing the right materials to give an appropriate hydrophilic/lipophilic balance (HLB) yielding a stable supramolecular assembly with good drug encapsulation. Leakage coefficient values tabulated below testify to the robustness of nanogels as stable drug delivery carriers.
Table 1.
Experimentally reported leakage coefficient (∧) values for various micellar systems
| Drug delivery system | Stability (∧) values reported (h−1) |
|---|---|
| Pluronic-P85s | 0.501 |
| CTAB | 0.850 |
| Tween80 | 0.501 |
| Polymer precursor to nanogel | 0.739 |
| Nanogel 6% crosslinked | 0.124 |
| Nanogel 13% crosslinked | 0.011 |
| Nanogel 25% crosslinked | 0.005 |
2.2. Response to stimuli
While stability of encapsulation in a delivery carrier is desirable during circulation, drug delivery will only be effective if the drug is released once it reaches its intended target. This can be achieved by incorporating chemical moieties into the design that make the carrier responsive to stimuli relevant to the disease being targeted.
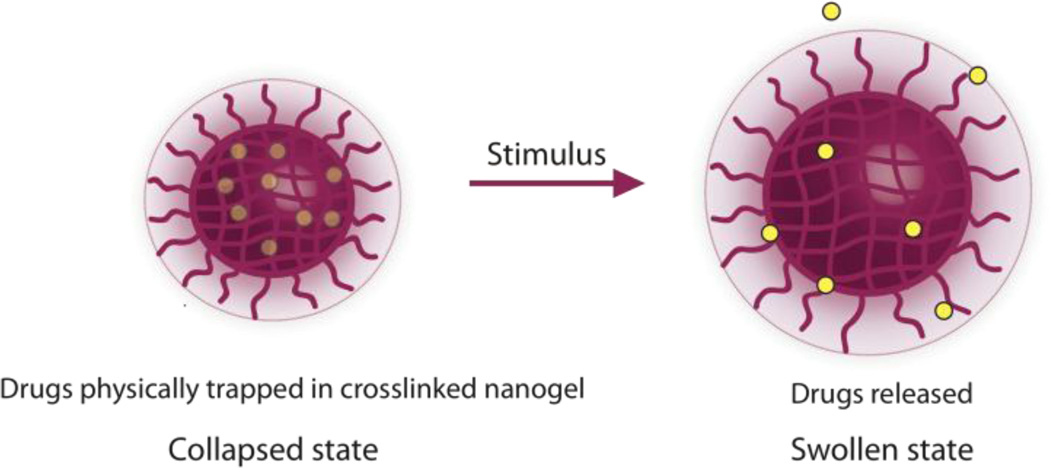
There are two kinds of stimuli, broadly defined, that can be engineered into delivery systems: chemical and physical. When drugs are covalently bound to the delivery carrier they necessarily require a chemical response to cleave the linking bond between the carrier and the drug. This chemical response may be triggered by an enzymatic reaction, a reaction with biological chemicals, changes in pH, redox, or even by an external stimulus such as light. However, if the drug is encapsulated through a physical or supramolecular interactions, the delivery vehicle could be engineered to release the drug due to a chemical response, such as those described above, as well as a physical change in the structure of the vehicle, such as swelling, which could be triggered by temperature, pH, etc (see Figure 8).
Fig. 8.
Drugs encapsulated through non-covalent means can be released from the delivery vehicle that responds to stimuli by a physical change in structure.
Physical entrapment of the drug also adds versatility to a drug carrier since a variety of different drugs could be encapsulated into the same type of vehicle without the need for a change in the drug attachment chemistry, which can often cause the drug to loose its therapeutic activity. On the other hand, covalently bound systems have the advantage of greater drug stability compared to conventional non-covalent encapsulation techniques. In the following subsections, we discuss both these approaches to drug encapsulation in detail. We begin with methods of covalent attachment of drugs and follow this discussion with non-covalent drug encapsulation methods.
2.2.1. Engineering release of covalently attached drugs by response to stimuli
Covalent tethering of drugs to the delivery carriers has the advantage that there are no equilibrium rate constants that determine stability. However, the drug could be prematurely released if the drug were attached through bonds that are either easily hydrolysable or are cleaved by ubiquitous enzymes like esterases. A well known example of a polymer drug that uses covalent attachment is the SMANCS system where antitumor drug neocarzinostatin (NCS) is covalently attached to a poly(styrene-co-maleic acid/anhydride) (SMA) carrier through amide bonds [19]. This system was the first demonstration of the tumoritropic nature of polymer drug conjugates, where the polymer drug was shown to localize into tumor tissue by the enhanced permeation and retention (EPR) effect [6, 20–23]. The amide bond has its advantages in that it is stable during circulation, but it limits the delivery carrier because of the fact that it is hard to cleave. Thus, for the most part a carrier’s delivery of drug if conjugated by an amide bond is expected to be sluggish even at its intended target.
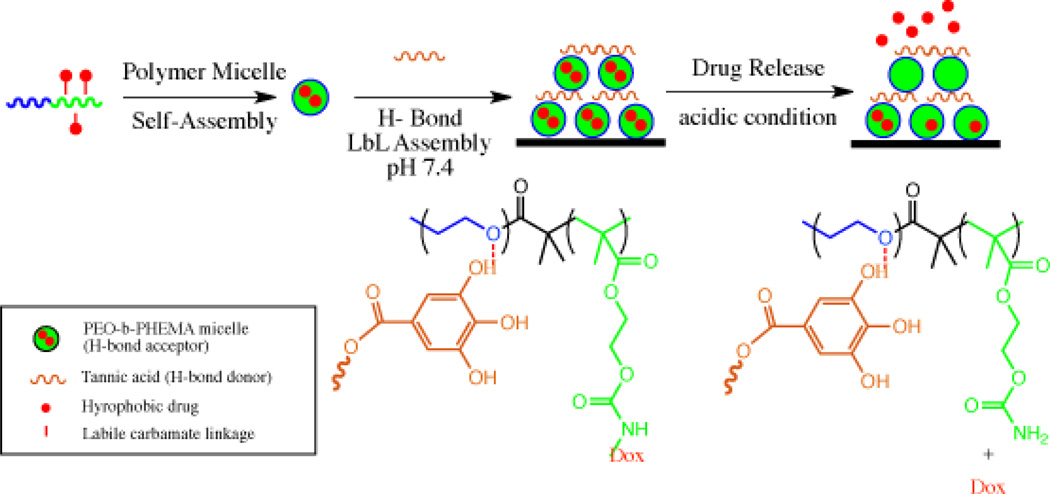
A way around this is to employ linkers that are sensitive to either the disease environment or are sensitive to intracellular conditions that are very different from the extracellular conditions encountered during circulation. Acid sensitive linkers can sense intracellular environments, because acidification of endosomes takes place upon uptake of the carrier by endocytosis. Further, the environment surrounding tumors are also known to be acidic giving an additional mechanism for targeting cancer. Hammond et al., engineered a polymeric delivery vehicle using a layer by layer assembly approach [24]. In this system, poly(ethylene oxide)-block-poly(2-hydroxylethyl methacrylate) (PEO-b-PHEMA) was conjugated with doxorubicin using acid-sensitive carbamate bonds and was coated with biologically compatible hydrogen bond donor –tannic acid which is a polyacid (see Figure 9). The PEO block served as a hydrogen bond acceptor for the polyacid. Tannic acid is also known to have antitumor, antibacterial and antioxidant properties. Multi-layer thin films were assembled on silicon or glass slides with Dox micelles and tannic acid and Dox release was shown to be dependent on the pH with significantly greater release of Dox observed at pH 4 and 5 compared to pH 7.4.
Fig. 9.
Scheme of drug release and structure of PEO-b-PHEMA polymer attached to Dox through acid cleavable carbamate bond before and after acid treatment.
Hydrazone functionality based linkers are also commonly employed as acid sensitive tethers for drugs. Other linkers that are specific to biological stimuli include enzyme cleavable bonds, disulfides, and light sensitive linkers. Langer et al. synthesized dextran polymers conjugated to an anticancer drug methotrexate (MTX) through an MMP2/MMP9 sensitive peptide linker [25]. The polymer drug conjugate MTX-PVGLIG-dextran was shown to release 60 to 90% of MTX in the presence of the MMPs; no release was observed when controls with a scrambled peptide and polymer drug conjugate without peptide linker were used. Notably it was also reported that enzyme kinetics of MMP2 were dictated by the surface charge of the assemblies, with negatively charged polymer conjugates showing lower MMP activity. This was attributed to the possible repulsion between negatively charged MMP2 and negatively charged polymer conjugates. Toxicity of the polymer conjugate was compared in HT-1080 cell line that over expressed MMP enzymes and BT-20 cell line, which was used as a control due to its very low level of MMP enzyme expression. It was found that, in the absence of serum proteins, 14% of the peptide linkers were cleaved in HT-1080 culture and none were cleaved in the control cell line. However, cytotoxicity assays in the two cell lines showed that the polymer-conjugated drug had the same toxicity in both. In tumor xenograft model in mice, these conjugates showed localization in tumors by the EPR effect and better anti-tumor effects compared to free MTX [26]. It was found that even though the polymer conjugates were localized in tumors, the amount of peptide linkers cleaved was just sufficient for antitumor activity. Linkers that are not specific to intracellular environments or disease phenotypes but are biodegradable include carbonates, anhydrides, esters, orthoesters and amides. In the next section we will discuss non-covalent encapsulation of drugs into assemblies.
2.2.2.Engineering release of non-covalently entrapped drugs by response to stimuli
Non-covalent methods of encapsulation essentially require a supra-molecular assembly in the form of polymeric micelles, liposomes or nanogels. Crosslinked assemblies such as nanogels provide additional stability to the encapsulated drug, when compared to uncrosslinked systems. Similar to covalent drug conjugation strategies, specific functionalities need to be incorporated into the delivery carrier so one can trigger release of an encapsulated drug in response to biologically relevant stimuli or a stimulus like light that can be remotely triggered. Disulfide crosslinking is a popular strategy due the ease of synthetic incorporation into the delivery carrier and because disulfide bonds are readily cleaved inside cells which contain millimolar concentrations of glutathione as compared to micromolar concentrations in extracellular environments. Li et al. reported a series of nanogels based on oligo(ethylene glycol) acrylate (OEGA) and 2-(5,5-dimethyl-1,3-dioxan-2-yloxy) ethyl acrylate (DMDEA), which were crosslinked using microemulsion polymerization with disulfide crosslinkers [27] (see Figure 10). The nanogel with the highest loading capacity was found to have 4% loading with Dox and 7% loading with paclitaxel (PTX). In addition to the redox sensitivity, imparted by the disulfide linker, the nanogel was also temperature and pH sensitive owing to the OEGA and DMDEA, respectively. The release was found to be faster at pH 5.0 with Dox loaded nanogels reaching about 90% release over 6 hours compared to these nanogels at pH 7.4 reaching about 20% release over 8 hours. Similarly, PTX loaded nanogels showed about 70% release in 7–8 hours at pH 5.0 and about 20% release over 12 hours at pH 7.4. Moreover, by using pH and redox stimuli, the rate was found to increase further. Nile red encapsulated nanogels at pH 5.0 with reducing agent Dithiothreitol (DTT) showed the highest release at about 80% over 5 hours compared to 20% release over 5 hours at pH 7.4 with DTT. Inducing disassembly using multiple stimuli gives additional control over release rates. For example, a multi-stimuli responsive micelle forming block copolymer that responds to pH, redox and temperature shows faster release rates, when any two stimuli were applied compared to the application of a single stimulus [28]. This system was designed using a block copolymer of temperature sensitive poly(N-isopropylacrylamide) (NIPAM) and an acid sensitive tetrahydropyran (THP) derivative monomer connected by a redox sensitive disulphide bond. This approach has important implications, because in some cases to achieve a therapeutic effect one needs to reach the therapeutic window as fast as possible. For example, MDR cell lines that pump out drugs can become resistant to chemotherapy unless the targeted cancer cell is swamped with the therapeutic drug before it gets a chance to pump it out [29, 30].
Fig. 10.
pH and redox sensitive OEGA-co-DMDEA disulfide nanogel.
An alternate technique for attaining controlled release is layer-by-layer (LBL) functionalization of drug delivery vehicles through which other properties like cellular uptake of nano-carriers can also be tailored and specific tissues can be targeted. Using LBL methods, the surface of a polymethacrylic acid co ethyl acrylate nanogel was coated with cationic poly(allylamine hydrochloride) and anionic poly(sodium 4-styrenesulfonate). These coatings altered the drug (Procaine hydrochloride) diffusion rates from these nanogels such that the time of diffusion of the drug was slower when the number of layers of coating was higher. The colloidal stability of coated nanogels was dictated by the final layer coating such that a PAH coating was stable up to a pH 8, while PSS coatings were stable at all pH ranges [31].
2.3. Passive targeting and size control
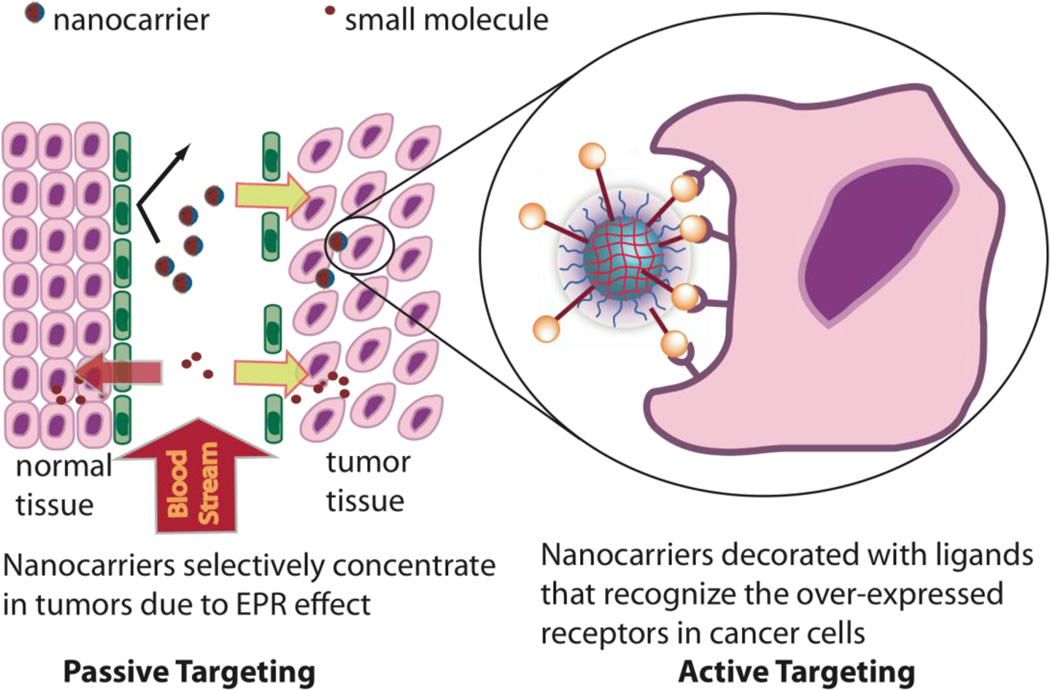
The concept of passive targeting of a delivery vehicle was first demonstrated by Maeda using the SMANCS system [22, 23]. Passive targeting is based on the enhanced permeation retention effect (EPR) (see Figure 11). The vasculature around cancer cells are poorly formed, which leads to large gaps between the cells and consequently to enhanced permeation of large macromolecular delivery systems in the range of 20–200 nm.
Fig. 11.
The concept of passive targeting through the EPR effect and active targeting through ligand display.
Also, due to the rapid growth of the tumor, lymphatic drainage is also poorly formed which leads to nanomaterials like drug delivery vehicles being trapped in the vicinity of tumor tissue. Thus the EPR phenomenon in cancers gives the opportunity to target diseased cells simply by controlling the size of the delivery system. In this regard, supramolecular systems with control over size have distinct advantages for therapies targeting cancer and arthritis. Furthermore, the size of a delivery vehicle also dictates how readily a cell takes it up and which pathway it uses. For example, caveolin mediated endocytosis, which avoids the lysosomal degradation pathway altogether, puts size restrictions on the delivery carrier that can be efficiently taken up into caveole [32, 33]. A nanogel system demonstrating size control was synthesized by copolymerization of N-isopropylacrylamide or N-isopropylmethacrylamide, with acrylic acid or 4-acrylamidofluorescein using N,N′-methylenebis(acrylamide) as the cross-linker. Functional groups such as N-isopropylacrylamide (NIPAM) and polyethylene glycol (PEG) show the lower critical solution temperature (LCST) which is the temperature at which these groups become desolvated due to loss of hydrogen bonding with water and aggregate. Using the LCST behavior of the nanogel, Lyon et al. were able to make a core/shell type nanogel by heating the core thus making it denser. Control over the size and monodispersity of the core/shell nanogel was systematically achieved by either varying the surfactant or initiator concentrations. The authors demonstrated that the sizes of these nanogels could be controlled from 40 nm to 140 nm, a range suitable to tailor to any of the above-mentioned applications [34].
2.4. Active targeting – Importance of design in the display of ligands
Active targeting refers to the incorporation of ligands intended to specifically target disease phenotypes. These ligands can range from large antibodies to small molecule ligands for cell surface receptors. Active targeting strategies are most effective when ligands are surface functionalized and are available readily for cell surface recognition. This often requires the design of a delivery vehicle to allow for attachment of active targeting ligands after assembly of the drug carrier. The importance of this strategy was demonstrated in a DNA delivery system where the sequence of attachment of a cell penetrating peptide made significant differences on DNA delivery efficiency [35] (see Figure 12 and 13). It was observed that if the TAT peptide was attached to the delivery vehicle before complexation with DNA, the delivery transfection efficiency was 25% lower than when TAT was attached to the preformed assembly. Clearly this study demonstrated the importance of post-assembly surface modification of a drug loaded delivery vehicle. Similarly in another example of this strategy, 2-(diethylamino)ethyl methacrylate (EAMA) and α-vinylbenzyl-ω-carboxy-PEG were copolymerized by emulsion polymerization with ethylene glycol dimethacrylate (EDMA) as the crosslinker (see Figure 14). The PEG monomer itself was used as the emulsion stabilizer and sizes of nanogels were controlled in the range of 50 to 680 nm by varying the ratio of PEG to EAMA with lower PEG ratios giving larger diameter particles. After nanogel synthesis the ω-carboxy functionality on the PEG groups were demonstrated to be available for further functionalization by carrying out a carbodiimide mediated coupling of the carboxylic acid with the fluorophore 6,7-dimethoxy-1-methyl-2(1H)-quinoxalinone-3-propionylcarboxylic acid hydrazide (DMEQ-hydrazide) [36]. Davis et.al. reported the first siRNA delivery in humans using a systemically administered delivery vehicle [37, 38]. The delivery vehicle is a self-assembling system consisting of cyclodextrincontaining polymer (CDP) which is easily surface functionalized with adamantine-PEG and adamantine-PEG-human transferrin (Tf) conjugates which form inclusion complexes with cyclodextrin (see Figure 15). For tumor visualization 5 nm gold nanoparticles capped with thiolated PEG and adamantine at the distal end were also attached to the delivery vehicle. It was shown that these siRNA delivery vehicles could cause RNAi mediated silencing in humans. Such systems have been reported to be assembled by first complexing cyclodextrin polycation conjugates with nucleic acids followed by addition of the surface targeting ligands functionalized with adamantine to the preformed polyplex demonstrating that these systems allow for post assembly functionalization with targeting ligands [39].
Fig. 12.
Post assembly surface functionalization strategy.
Fig. 13.
Post assembly surface modification is important for display of ligands.
Fig. 14.
Structure of a) reactive ω-carboxy PEG monomer, b) 2-(diethylamino)ethyl methacrylate (EAMA) and c) ethylene glycol dimethacrylate (EDMA)
Fig. 15.
Structure of cationic polymer used to complex nucleic acids and ligand/PEG functionalized adamantine which can be attached post polyplex formation via host guest interactions with cyclodextrin.
2.5. Toxicity of the delivery system
For practical application of drug delivery, even if all of the above design criteria are met, the inherent toxicity of drug delivery vehicles will ultimately decide its usefulness. Designing a non-toxic delivery vehicle involves choosing materials which are either non-toxic to begin with or which become metabolized into non-toxic components before any harm can be done in the body. For example, methacrylate- and acrylate-based polymeric systems can be easily hydrolyzed into small molecule alcohols and relatively non-toxic poly-methacrylic acid and poly-acrylic acid components [40, 41]. In such systems toxicity of the vehicle would be dictated mainly by its hydrolyzed small molecule components. A low toxicity nanogel system was synthesized by crosslinking a self-assembling block copolymer of a block of poly(oligoethylene glycol) (POEG) and a block of randomly co-polymerized vinyl benzyl chloride (VBC) and pentafluorophenyl acrylate (PFP-A) (see Figure 16) [42]. VBC groups on the polymer backbone were activated by treatment with sodium methanethiosulfonate that activated this functionality for thiol disulfide exchange reactions. Once this block copolymer self assembled in water, it was crosslinked using diamine crosslinkers incorporating acid sensitive ketal functionality. Release of dyes in the presence of various stimuli such as pH and redox was demonstrated and the nanogels were to be taken up by NIH-3T3 cells over a period of 48 hours. These nanogels made up of acrylate and methacrylate backbone showed 90% cell viability up to a concentration of 8 mg/ml, indicating very low toxicity.
Fig. 16.
Structure of polymer with PEG, PFP and activated disulfide as pendant groups.
2.6. Ease of synthesis
For real world applications, synthesis and purification of a delivery system should be easy and scalable. Several, nanogel systems reported employ emulsion polymerization conditions and require the use of surfactants, which need to be removed after synthesis or other complex setups for synthesis. Park et al reported an emulsion free synthesis of heparin nanogels [43]. In this system thiolated heparin was prepared by reaction with sodium periodate followed by cysteamine and finally reduced using NaBH4. Thiolated heparin was then mixed with PEG and sonicated to give disulfide crosslinked heparin nanogels. Sizes of nanogels were varied by varying weight ratios of PEG to heparin-SH and since heparin is known to show anti-cancer properties these nanogels were tested against mouse melanoma cells. Release of heparin was shown to depend on the concentration of glutathione and caspase activity indicative of apoptotic cell death was shown to be higher than that caused by heparin alone. DeSimone et. al fabricated PEG nanogels for drug delivery applications using the PRINT (Particle Replication In Non-wetting Templates) methodology which unlike other imprinting techniques uses nonwetting templates which eliminate the formation of residual interconnecting films between molded objects [44]. In this top-down approach a non-wetting perfluoropolyether mold is prepared on a master silicon template. This is followed by molding a solution mixture of PEG triacrylate, PEG monomethyl ether monomethacrylate, 2,2-diethoxyacetophenone, and para-hydroxystyrene [45]. The mold was finally UV cured to give the PEG nanogels, which were harvested by physical means.
2.7. Outlook
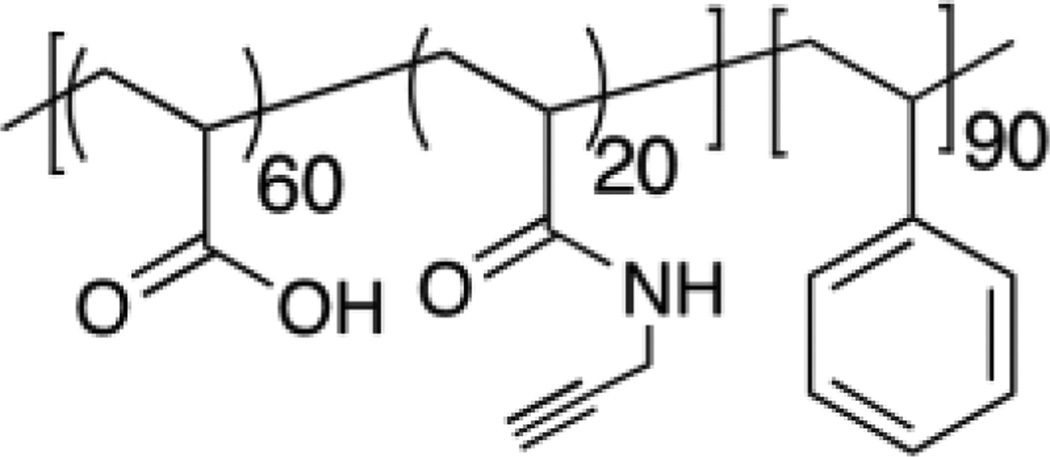
Ideally, a versatile delivery vehicle will possess combination of several, if not all the, characteristics mentioned above. We exemplify the versatility of one such system with an example from our group. Recently, we reported on a simple PEG-PDS polymer precursor derived nanogel system with disulfide crosslinks, the synthesis of which will be described in section 3.1.1 [46] (see Figure 17). The PEG chains on the polymer backbone give it biocompatibility, water solubility and are known to prolong circulation time and decrease uptake by the reticuloendothelial system (RES) [47, 48]. PDS units on the other hand provide the lipophilic functionality to attain the right hydrophilic lipophilic balance (HLB) in solution to form the assembly in addition to allowing hydrophobic drug encapsulation and crosslinking. As described above in section 2.1, we were able to stably encapsulate hydrophobic drugs with good loading capacity up to 20 wt% and control leakage of the nanogel by tuning crosslinking density. FRET based methods were used to quantify the rates of dye exchange from the nanogel to its surroundings [18]. We also showed that these results hold true in cell culture studies as well [49]. Additionally, we were able to control the size of the nanogel by varying the molecular weight, composition of the nanogel and exploiting the lower critical solution temperature (LCST) behavior of the PEG component of this system. Moreover, the sizes of these nanogels are dictated by the size of the uncrosslinked precursor polymer assembly irrespective of the crosslinking density. This gives a fine handle to tune sizes of nanogels in the range of 20 to 200 nm reproducibly, which is useful for targeting diseases like cancer, arthritis [50], choroidal neovascularization [51] and myocardial infarction [52], since these diseases are characterized by highly permeable vasculature and poor lymphatic drainage. Further, these nanogels allow for easy surface functionalization with thiol modified ligands and it was shown when cell targeting peptides like TAT were attached to its surface, the nanogels were taken up quickly into cells indicating that these surface functionalized ligands are available for cell surface recognition and active targeting. Also, noteworthy is that this self-assembled system is made from a synthetically accessible, simple random copolymer compared to several systems in the literature making it a versatile candidate for drug delivery applications. Thus crosslinked nanogel systems in particular hold great promise as drug delivery vehicles.
Fig. 17.
Schematic for PEG-PDS nanogel assembly.
3. Methods of Synthesis of Nanogels
The methods to prepare nanogels will be discussed in this section by dividing them into two major approaches: 1) preparing nanogels from polymer precursors; 2) fabricating nanogel networks via heterogeneous polymerization of monomers. These two approaches are illustrated in Figure 18.
Fig. 18.
Methods of nanogel synthesis: The polymer precursor method and the emulsion method.
3.1. Preparation of nanogels from polymer precursors
Amphiphilic copolymers are known to self-assemble in solution to form various nanoscopic structures, thus providing a versatile platform to synthesize nanogels by simply locking the assembly. In this section, several examples of nanogel preparation through this methodology will be given and details discussed based on cross-linking reaction types employed to fix the self-assembled polymer.
3.1.1.Disulfide based cross-linking
In the outlook section, we highlighted the nanogel system prepared by self-cross-linking amphiphilic random copolymers [18, 46, 49]. These polymers contain polyethylene glycol as a hydrophilic unit and pyridyl disulfide (PDS) as the hydrophobic and crosslinkable unit, and form nanoscale assemblies in aqueous solutions. The addition of deficient amounts of dithiothreitol (DTT) reduces controlled amount of PDS groups to thiols, which further exchange with remaining PDS groups affording a cross-linked nanogel. Nanogels with different sizes can be easily obtained by cross-linking polymer assemblies whose sizes are tuned by varying polymer concentration and utilizing the LCST behavior of polymers. Nanogels based on thiol-exchange using lipoic acid containing dextran (Dex-LAs) were synthesized in a similar manner (see Figure 19) [53]. Nanogels loaded with doxorubicin (DOX) were prepared from the assembly of Dex-LAs, which were readily cross-linked with a catalytic amount of DTT.
Fig. 19.
Structure of Dex-LA precursor polymer.
3.1.2.Amine based cross-linking
Amine groups also commonly used in preparation of nanogels due to their reactivity toward carboxylic acids, activated esters, isocynates, iodides and others. The Wooley group developed a methodology to prepare shell-crosslinked knedel-like structures (SCKs) using amine crosslinkers. A variety of amphiphilic block copolymers in which poly(acrylic acid) were employed as the hydrophilic and cross-linkable block were synthesized (see Figure 20) [54–56].
Fig. 20.
Poly(acrylic acid) bearing block copolymers crosslinked by diamine crosslinkers.
The amidation of carboxylic acid with diamine crosslinkers, following the self assembly of the block copolymers, cross-linked the micellar assemblies. The remaining carboxylic groups can be converted to other functionalities for orthogonal surface modification. As seen in Figure 21, activated esters such as N-acryloxysuccinimide (NAS), pentafluorophenylacrylate (PFPA) and p-nitrophenylacrylate were incorporated into copolymers as cross-linkable units to make cross-linked micelles [42, 57, 58].
Fig. 21.
Structures of a) N-acryloxysuccinimide, b) p-nitrophenylacrylate and c) pentafluorophenyl acrylate containing precursor polymers.
Additionally, reactions with isocyanate provide another cross-linking approach to make nanogels. pH Responsive cross-linked micelles were obtained by the addition of excess amounts of 1,8-diaminooctane to micellar aggregates of 3-isopropenyl-α,α-dimethylbenzyl isocyanate bearing copolymers (see Figure 22) [59].
Fig. 22.
Precursor polymer with activated isocyanate pendant functionality.
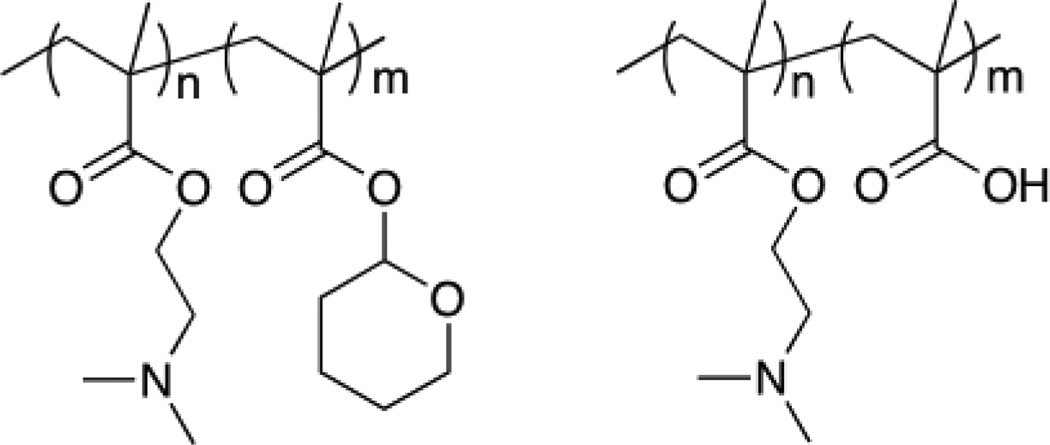
The Armes group reported the synthesis of zwitterionic shell cross-linked micelles through the amine quarternization of 2-(dimethylamino)ethyl methacrylate-2-tertrahydropyranyl methacrylate (DMAEMA-THPMA) diblock copolymer precursors (shown in Figure 23) with 1,2-bis-(2-iodoethoxy)ethane (BIEE) and hydrolysis of THP. By varying the sequence of cross-linking and THP hydrolysis, the charge of the core and the shell can be alternated [60].
Fig. 23.
DMAEMA block precursor copolymers crosslinked by quaternization of amines to give core or shell crosslinked nanogels.
3.1.3.Click chemistry based cross-linking
Wooley and Hawker groups also reported a nanogel synthesis method utilizing Click chemistry [61]. Amino bearing alkynyl groups were immobilized to the corona of assembled micelles formed from amphiphilic diblock copolymers of poly(acrylic acid)-b-poly(styrene) via amidation of acrylic acid groups (see Figure 24).
Fig. 24.
Alkynyl polymer precursor for crosslinking using Click chemistry.
The following Click reactions, between Click-readied micelles and azido dendrimers, covalently cross-linked the micelles resulting in nanogel networks. Liu and coworkers utilized Click chemistry to prepare core-cross-linked polyion complex micelles with thermo responsive coronas with high stability against pH and salt stimuli (see Figure 25) [62].
Fig. 25.
Azido polymer precursors used to prepare core-cross-linked micelles.
3.1.4.Photo-induced cross-linking
As an alternative to the above cross-linking techniques, photo-induced cross-linking has been utilized to stabilize polymer assemblies which are functionalized with polymerizable or dimerizable units (see Figure 26) [63].
Fig. 26.
Polymer precursor with photopolymerizable functionality.
For instance, double hydrophilic block copolymers containing coumarin (shown in Figure 27), which is known to dimerize when treated with UV light >310 nm, were assembled to micelles and subsequently photo-cross-linked [64]. The synthesized nanogels show intra-particular LCST and inter-particular upper critical solution temperature (UCST) behaviors. UCST is the critical temperature above which the components of a mixture are miscible.
Fig. 27.
Coumarin containing polymers can form reversibly crosslinkable nanogels.
Light sensitive functionality have been incorporated into dendrimer structures to induce drug release in response to light stimulation [65]. Our group introduced coumarin into dendrimers as a reversible cross-linker to control the accessibility of substrate in the dendrimer assembly (see Figure 28) [66]. When the assembly solution was exposed to UV light (365 nm), the enzymatic degradation of substrate was highly suppressed since the ester groups were confined in the interior of assembly. Treating the crosslinked assembly by UV light (250 nm) recovers the enzymatic action due to the decrosslinking of the coumarin dimer, which exposes the substrate to enzymes.
Fig. 28.
Enzymatic access to crosslinked dendrimer particles can be restricted by crosslinking coumarin functionality.
3.1.5.Physical cross-linking
There are also a variety of reported nanogels synthesized by physical cross-linking. In 1993, Akiyoshi reported the preparation of monodisperse nanogels using cholesterol-modified polysaccharides where the hydrophobic interactions among cholesterol groups afforded a physical crosslinking [67]. Driving forces, other than hydrophobic interactions, to form physically cross-linked nanogels include host-guest interactions and electrostatics. A review regarding nanogel preparation from these associating polymers has been published recently [68].
3.2. Fabricating nanogel networks via heterogeneous polymerization of monomers
Emulsion polymerization can be divided into two categories based on the continuous phase, namely, emulsion and inverse emulsion polymerization. Nanogels can be chemically synthesized when bifunctional monomers are incorporated and polymerization is initiated in these heterogeneous colloidal systems. Matyjaszewski group recently combined Atom Transfer Radical Polymerization (ATRP) and emulsion polymerization to achieve biodegradable nanogels introducing a disulfide bridged bifunctional monomer [69]. They also covalently incorporate proteins into the nanogels to form protein-nanogel hybrids using ATRP in water in oil miniemulsions or inverse miniemulsions. In particular, protein bearing an ATRP functionality was used as a co-initiator to initiate the polymerization of the monomers stably dispersed in an inverse miniemulsion system [70].
4. Applications of Nanogels
Nanogels show a lot of promise as delivery systems in particular due to their encapsulation stability, in addition to water solubility and biocompatibility. These nanocarriers have been utilized in a variety of fields including cancer drug delivery. The following examples from the literature demonstrate the diversity in applications and the versatility of these delivery systems. In 2010, Du et al. designed a pH-responsive charge-conversional nanogel for promoted tumoral-cell uptake and doxorubicin (DOX) delivery [71]. These nanogels were prepared from poly(2-aminoethyl methacrylate hydrochloride) (PAMA) and subsequently were treated with 2,3-dimethylmaleic anhydride (DMMA) to produce a negatively charged nanogel (see Figure 29).
Fig. 29.
Hydrolysis of PAMA-DMMA nanogels at pH 6.8.
Zeta potential of these nanogels showed that they posses a significant negative charge (−17 mV) which increased within 35 minutes to 0 mV under acidic conditions. PAMA-DMMA nanogels loaded with DOX showed an increase in the release rate as the pH value decreased. Furthermore, cell viability studies of MDA-MB-435s cells incubated with DOX-loaded nanogels were shown to possess higher cytotoxicity at pH 6.8 relative to that at pH 7.4 and also compared to free DOX.
In another application, delivery of therapeutic molecules to treat inflammatory disorders such as rheumatoid arthritis was investigated. Macrophage cells, of the immune system, are the targeted for photodynamic therapy as a treatment for this disorder. Schmitt et al. and colleagues developed chitosan-based nanogels decorated with hyaluronate to target macrophages, which were then loaded with one of three different photosensitizers, i.e. tetra-phenyl-porphyrin-tetra-sulfonate (TPPS4), tetra-phenyl-chlorin-tetra-carboxylate (TPCC4) and chlorin e6 (Ce6) (see Figure 30) [50].
Fig. 30.
Chemical structures of the photosensitizers: (a) Tetra-phenyl-porphyrin-tetra-sulfonate (TPPS4). (b) Tetra-phenyl-chlorin-tetra-carboxylic acid (TPCC4). (c) Chlorin e6 (Ce6).
It was found that TPPS4-nanogels and TPCC4-nanogels were not toxic, when exposed to murine RAW 264.7 and human THP-1 macrophages in the absence of light exposure. Ce6-nanogels was found to be fairly toxic which was attributed to the known intrinsic toxicity of this photosensitizer. Further studies demonstrated that these PS-nanogels were rapidly taken up (<4 h) by the target cells and accumulated in their cytoplasm and organelles. In vivo studies using a mouse model of antigen-induced arthritis (AIA) demonstrated that after injection, nanogel-encapsulated photosensitizer had longer retention times in the inflamed joints when compared to free photosensitizers. It was suggested that photodynamic therapy using this hyaluronate-chitosan nanogels are effective in the treatment of inflamed articular joints.
Nanogels have also been utilized for the delivery of local anesthetic drugs. Yin et al. designed a biodegradable delivery system where lidocaine was encapsulated into poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL or PCEC) nanoparticles (see Figure 31) [72]. Carriers were coated with hydrophilic thermo-sensitive Pluronic F-127 hydrogels to form the composite carrier nanogel system. Infiltration anaesthesia was quantified for lidocaine-loaded PCEC nanoparticles (lido-nano), lidocaine in thermo-sensitive Pluronic hydrogels (lido-gel) and lidocaine-loaded PCEC nanoparticles in Pluronic hydrogels (lido-nano gel). It was found that lido-nano gel produced long-lasting infiltration anaesthesia of about 360 mins when compared with lido-gel (150 mins), lido-nano (180 mins) and lidocaine solution (110 mins). There were no observations of systemic toxicities or skin irritations due to the biocompatibility of the components of the carriers. The degradation of PCEC nanoparticles results in the low-molecular weight PEG and PCL, which are known to be biocompatible, and Pluronic F-127 is approved by the FDA for use in humans. Nanogels thus effectively infiltrate into the wound for prolonging effects of these anesthetics, which is useful for treatment during post-operative periods.
Fig. 31.
Chemical structure of poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC).
Physically crosslinked nanogels composed of hexadecyl groups-bearing cationic cycloamylose have been developed for delivery of pDNA (see Figure 32) [73]. Cycloamylose, a large cationic cyclodextrin ring, was employed for complexation of pDNA. Phospholipase A2 was co-delivered with pDNA to help in endosome disruption by catalyzing the hydrolysis of membrane phospholipids triggering the release of the pDNA in the cytoplasm. It was found that the levels of protein expression due to delivery of pDNA were enhanced with co-delivery of PLA2 using the C16-catCA nanogels/PLA2 system. Hemolysis studies using sheep red blood cells showed that the activity for the native PLA2 was maintained when co-delivered with pDNA into the nanogels. These studies suggest that the co-delivery of phospholipases could be an effective technique for enhancing endosomal escape of drug delivery vehicles.
Fig. 32.
Chemical structure of C16-catCA derivate.
Tamura et al. studied the delivery of siRNA using a polyion complex based on PEGylated polyamine nanogels containing a chemically cross-linked core (see Figure 33) [74]. In nucleic acid delivery complexes between amine based carriers and nucleic acid drugs are defined in terms of the ratio of amines on the carrier to the number of phosphates on the nucleic acid backbone. This ratio is referred to as the N/P ratio with a higher N/P ratio implying greater number of amine based complexing agents per nucleic acid. Gene silencing activity against firefly luciferase in HuH-7 cells showed that these nanogel/ siRNA complexes possess high transfection efficiencies at low nitrogen to phosphates (N/P) ratios when compared with the uncrosslinked complexes.
Fig. 33.
Schematic illustration of the nanogel siRNA polyion complex.
However, cellular uptake of the nanogels/siRNA complexes was found to be lower than that of oligofectamine/siRNA complexes. This was suggested to be due to steric hindrance between PEG chains and the cell membrane. The chemically cross-linked nanogel was composed of poly[2-(N,N-diethylaminoethyl)methacrylate] (PDEAMA) (see Figure 34) which served as a siRNA complexing functionality and an aid for endosomal escape, bifunctional ethylene glycol dimethacrylate as the crosslinker and hetrobifunctional PEG macromonomer. Most drug delivery systems due to their size, are taken up into cells by endocytosis. However, once inside the endosomes, the drugs are either ineffective because their sites of action are at the cytosol or are eventually degraded in the lysozomes. Thus in most cases in order to achieve efficacious drug delivery a vehicle should not only internalize into the cell but also be able to escape the endosome effectively. To achieve endosomal escape, proton sponge functionality was introduced into the nanogel so that during internalization and subsequent acidification of the endosome these functionalities buffer the endosomal pH eventually causing disruption of the endosome. This is presumably due to the relative increase in osmotic pressure inside the endosome. Thus, it was reasoned that the polyamine core of this nanogel was protonated as a result of the decrease in pH in the endosomal compartment facilitating the escape of the nanocarriers into the cytoplasm through the proton sponge effect. Thus PEGylated polyamine siRNA nanogels have potential applications in cancer therapy and for the treatment of genetic disorders.
Fig. 34.
Chemical structure of acetal-PEG-PDEAMA nanogel.
Conclusions
There are several components of a drug delivery vehicle that need to be fine tuned and refined to be effective. We emphasize on the need to test the encapsulation stability of a delivery system. Polymeric nanogels have stood up to this task and have shown that compared to several other delivery systems have exceptional stability, respond to biologically relevant stimuli, allow for convenient functionalization with cell targeting ligands and are easy to synthesize. Their sizes can also be controlled for various applications in drug delivery and can be tailored for low cytotoxicity. With these foundations in place nanogels, have opened up opportunities for further development for a variety of biomedical applications, especially drug delivery.
Acknowledgements
We would like to thank NIGMS of the NIH (GM-065255), NSF-NSEC and NSF-MRSEC for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peng HS, Stolwijk JA, Sun LN, Wegener J, Wolfbeis OS. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew. Chem. Int. Ed. 2010;49:4246–4249. doi: 10.1002/anie.200906926. [DOI] [PubMed] [Google Scholar]
- 2.Oishi M, Sumitani S, Nagasaki Y. On-off regulation of 19F magnetic resonance signals based on pH-sensitive PEGylated nanogels for potential tumor-specific smart 19F MRI probes. Bioconjugate Chem. 2007;18:1379–1382. doi: 10.1021/bc7002154. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa U, Nomura SM, Kaul SC, Hirano T, Akiyoshi K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem. Biophys. Res. Commun. 2005;331:917–921. doi: 10.1016/j.bbrc.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi C, Hasegawa U, Saita Y, Hemmi H, Hayata T, Nakashima K, Ezura Y, Amagasa T, Akiyoshi K, Noda M. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J. Cell. Physiol. 2009;220:1–7. doi: 10.1002/jcp.21760. [DOI] [PubMed] [Google Scholar]
- 5.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 8.Kwona G, Naitob M, Yokoyamac M, Okanoc T, Sakuraic Y, Kataoka K. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J. Control. Release. 1997;48:195–201. [Google Scholar]
- 9.Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J. Control. Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Control. Release. 2004;100:247–256. doi: 10.1016/j.jconrel.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, Shebuskib RJ, Levy RJ. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Control. Release. 1997;43:197–212. [Google Scholar]
- 12.Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control. Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 15.Bae Y, Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Delivery Rev. 2009;61:768–784. doi: 10.1016/j.addr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv. Drug Delivery Rev. 2002;54:191–202. doi: 10.1016/s0169-409x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiwpanich S, Ryu JH, Bickerton S, Thayumanavan S. Noncovalent encapsulation stabilities in supramolecular nanoassemblies. J. Am. Chem. Soc. 2010;132:10683–10685. doi: 10.1021/ja105059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv. Drug Delivery Rev. 2001;46:169–185. doi: 10.1016/s0169-409x(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 22.Maeda H, Ueda M, Morinaga T, Matsumoto T. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J. Med. Chem. 1985;28:455–461. doi: 10.1021/jm00382a012. [DOI] [PubMed] [Google Scholar]
- 23.Maeda H, Takeshita J, Kanamaru R. A lipophilic derivative of neocarzinostatin. A polymer conjugation of an antitumor protein antibiotic. Int. J. Pept. Protein Res. 1979;14:81–87. doi: 10.1111/j.1399-3011.1979.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim BS, Lee HI, Min Y, Poon Z, Hammond PT. Hydrogen-bonded multilayer of pH-responsive polymeric micelles with tannic acid for surface drug delivery. Chem. Commun. 2009;28:4194–4196. doi: 10.1039/b908688a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chau Y, Tan FE, Langer R. Synthesis and characterization of dextran-peptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjugate Chem. 2004;15:931–941. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- 26.Chau Y, Dang NM, Tan FE, Langer R. Investigation of targeting mechanism of new dextran-peptide-methotrexate conjugates using biodistribution study in matrix-metalloproteinase-overexpressing tumor xenograft model. J. Pharm. Sci. 2006;95:542–551. doi: 10.1002/jps.20548. [DOI] [PubMed] [Google Scholar]
- 27.Qiao ZY, Zhang R, Du FS, Liang DH, Li ZC. Multi-responsive nanogels containing motifs of ortho ester, oligo(ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release. 2011;152:57–66. doi: 10.1016/j.jconrel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Klaikherd A, Nagamani C, Thayumanavan S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J. Am. Chem. Soc. 2009;131:4830–4838. doi: 10.1021/ja809475a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 2009;109:2989–3011. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 30.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 31.Tan JP, Wang Q, Tam KC. Control of burst release from nanogels via layer by layer assembly. J. Control. Release. 2008;128:248–254. doi: 10.1016/j.jconrel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Tiruppathi C, Minshall RD, Malik AB. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3:4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blackburn WH, Lyon LA. Size controlled synthesis of monodispersed, core/shell nanogels. Colloid Polym. Sci. 2008;286:563–569. doi: 10.1007/s00396-007-1805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy R, Jerry DJ, Thayumanavan S. Virus-inspired approach to nonviral gene delivery vehicles. Biomacromolecules. 2009;10:2189–2193. doi: 10.1021/bm900370p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi H, Iijima M, Kataoka K, Nagasaki Y. pH-sensitive nanogel possessing reactive PEG tethered chains on the surface. Macromolecules. 2004;37:5389–5396. [Google Scholar]
- 37.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, Kornbrust DJ, Davis ME. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pun SH, Davis ME. Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate Chem. 2002;13:630–639. doi: 10.1021/bc0155768. [DOI] [PubMed] [Google Scholar]
- 40.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 41.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 42.Duong HTT, Marquis CP, Whittaker M, Davis TP, Boyer C. Acid degradable and biocompatible polymeric nanoparticles for the potential codelivery of therapeutic agents. Macromolecules. 2011;44:8008–8019. [Google Scholar]
- 43.Bae KH, Mok H, Park TG. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials. 2008;29:3376–3383. doi: 10.1016/j.biomaterials.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 44.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 45.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, Desimone JM. Nanofabricated particles for engineered drug therapies: a preliminary biodistribution study of PRINT nanoparticles. J. Control. Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu JH, Jiwpanich S, Chacko R, Bickerton S, Thayumanavan S. Surface-functionalizable polymer nanogels with facile hydrophobic guest encapsulation capabilities. J. Am. Chem. Soc. 2010;132:8246–8247. doi: 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- 47.Blume G, Cevc G, Crommelin MD, Bakker-Woudenberg IA, Kluft C, Storm G. Specific targeting with poly(ethylene glycol)-modified liposomes: coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim. Biophys. Acta. 1993;1149:180–184. doi: 10.1016/0005-2736(93)90039-3. [DOI] [PubMed] [Google Scholar]
- 48.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 49.Ryu JH, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. Self-cross-linked polymer nanogels: a versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2010;132:17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt F, Lagopoulos L, Kauper P, Rossi N, Busso N, Barge J, Wagnieres G, Laue C, Wandrey C, Juillerat-Jeanneret L. Chitosan-based nanogels for selective delivery of photosensitizers to macrophages and improved retention in and therapy of articular joints. J. Control. Release. 2010;144:242–250. doi: 10.1016/j.jconrel.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Yasukawa T, Tabata Y, Kimura H, Ogura Y. Recent advances in intraocular drug delivery systems. Recent Pat. Drug Deliv. Formul. 2011;5:1–10. doi: 10.2174/187221111794109529. [DOI] [PubMed] [Google Scholar]
- 52.Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J. Control. Release. 2004;94:187–193. doi: 10.1016/j.jconrel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Li Yl, Zhu L, Liu Z, Cheng R, Meng F, Cui JH, Ji SJ, Zhong Z. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew. Chem. Int. Ed. 2009;48:9144–9918. doi: 10.1002/anie.200904260. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, Remsen EE, Wooley KL. Amphiphilic core–shell nanospheres obtained by intramicellar shell crosslinking of polymer micelles with poly(ethylene oxide) linkers. Chem. Commun. 1998:1415–1416. [Google Scholar]
- 55.Joralemon MJ, Smith NL, Holowka D, Baird B, Wooley KL. Antigen-decorated shell cross-linked nanoparticles: synthesis, characterization, and antibody interactions. Bioconjugate Chem. 2005;16:1246–1256. doi: 10.1021/bc0501505. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Du W, Sun G, Wooley KL. pH-responsive shell cross-linked nanoparticles with hydrolytically labile cross-links. Macromolecules. 2008;41:6605–6607. [Google Scholar]
- 57.Li Y, Lokitz BS, Armes SP, McCormick CL. Synthesis of reversible shell cross-linked micelles for controlled release of bioactive agents. Macromolecules. 2006;39:2726–2728. [Google Scholar]
- 58.Hu YC, Pan CY. Bioaffinitive and nanosized polymeric micelles based on a reactive block copolymer. Macromol. Rapid Commun. 2005;26:4288–4289. [Google Scholar]
- 59.Kim Y, Pourgholami MH, Morris DL, Stenzel MH. Triggering the fast release of drugs from crosslinked micelles in an acidic environment. J. Mater. Chem. 2011;21:12777–12783. [Google Scholar]
- 60.Butu V, Lowe AB, Billingham NC, Armes SP. Synthesis of zwitterionic shell cross-linked micelles. J. Am. Chem. Soc. 1999;121:4288–4289. [Google Scholar]
- 61.Joralemon MJ, O'Reilly RK, Hawker CJ, Wooley KL. Shell Click-crosslinked (SCC) nanoparticles: a new methodology for synthesis and orthogonal functionalization. J. Am. Chem. Soc. 2005;127:16899–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Zhou Y, Zhu Z, Ge Z, Liu S. Polyion complex micelles possessing thermoresponsive coronas and their covalent core stabilization via “Click” chemistry. Macromolecules. 2008;41:1444–1454. [Google Scholar]
- 63.Pioge S, Nesterenko A, Brotons G, Pascual S, Fontaine L, Gaillard C, Nicol E. Core cross-linking of dynamic diblock copolymer micelles: quantitative study of photopolymerization efficiency and micelle structure. Macromolecules. 2011;44:594–603. [Google Scholar]
- 64.He J, Yan B, Tremblay L, Zhao Y. Both core- and shell-cross-linked nanogels: photoinduced size change, intraparticle LCST, and interparticle UCST thermal behaviors. Langmuir. 2011;27:436–444. doi: 10.1021/la1040322. [DOI] [PubMed] [Google Scholar]
- 65.Yesilyurt V, Ramireddy R, Thayumanavan S. Photoregulated release of noncovalent guests from dendritic amphiphilic nanocontainers. Angew. Chem. Int. Ed. 2011;50:3038–3042. doi: 10.1002/anie.201006193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raghupathi KR, Azagarsamy MA, Thayumanavan S. Guest-release control in enzyme-sensitive, amphiphilic-dendrimer-based nanoparticles through photochemical crosslinking. Chem. Eur. J. 2011;17:11752–11760. doi: 10.1002/chem.201101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules. 1993;26:3062–3068. [Google Scholar]
- 68.Sasaki Y, Akiyoshi K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem. Rec. 2010;10:366–376. doi: 10.1002/tcr.201000008. [DOI] [PubMed] [Google Scholar]
- 69.Oh JK, Tang C, Gao H, Tsarevsky NV, Matyjaszewski K. Inverse miniemulsion ATRP: a new method for synthesis and functionalization of well-defined water-soluble/cross-linked polymeric particles. J. Am. Chem. Soc. 2006;128:5578–5584. doi: 10.1021/ja060586a. [DOI] [PubMed] [Google Scholar]
- 70.Averick SE, Magenau AJD, Simakova A, Woodman BF, Seong A, Mehl RA, Matyjaszewski K. Covalently incorporated protein–nanogels using AGET ATRP in an inverse miniemulsion. Polym. Chem. 2011;2:1476–1478. [Google Scholar]
- 71.Du JZ, Sun TM, Song WJ, Wu J, Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. Int. Ed. 2010;49:3621–3626. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- 72.Yin QQ, Wu L, Gou ML, Qian ZY, Zhang WS, Liu J. Long-lasting infiltration anaesthesia by lidocaine-loaded biodegradable nanoparticles in hydrogel in rats. Acta Anaesthesiol. Scand. 2009;53:1207–1213. doi: 10.1111/j.1399-6576.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 73.Toita S, Sawada S, Akiyoshi K. Polysaccharide nanogel gene delivery system with endosome-escaping function: co-delivery of plasmid DNA and phospholipase A2. J. Control. Release. 2011;155:54–59. doi: 10.1016/j.jconrel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Tamura A, Oishi M, Nagasaki Y. Enhanced cytoplasmic delivery of siRNA using a stabilized polyion complex based on PEGylated nanogels with a cross-linked polyamine structure. Biomacromolecules. 2009;10:1818–1827. doi: 10.1021/bm900252d. [DOI] [PubMed] [Google Scholar]