Abstract
Efficient synaptic transmission at the neuromuscular junction (NMJ) requires the topological maturation of the postsynaptic apparatus from an oval acetylcholine receptor (AChR)-rich plaque into a complex pretzel-shaped array of branches. However, compared to NMJ formation very little is known about the mechanisms that regulate NMJ maturation. Recently the process of in vivo transformation from plaque into pretzel has been reproduced in vitro by culturing myotubes aneurally on laminin-coated substrate. It was proposed that the formation of complex AChR clusters is regulated by a MuSK-dependent muscle intrinsic program. To elucidate the structure–function role of MuSK in the aneural maturation of AChR pretzels, we used muscle cell lines expressing MuSK mutant and chimeric proteins. Here we report, that besides its role during agrin-induced AChR clustering, MuSK kinase activity is also necessary for substrate-dependent cluster formation. Constitutive-active MuSK induces larger AChR clusters, a faster cluster maturation on laminin and increases the anchorage of AChRs to the cytoskeleton compared to MuSK wild-type. In addition, we find that the juxtamembrane region of MuSK, which has previously been shown to regulate agrin-induced AChR clustering, is unable to induce complex AChR clusters on laminin substrate. Most interestingly, MuSK kinase activity is not sufficient for laminin-dependent AChR cluster formation since the MuSK ectodomain is also required suggesting a so far undiscovered instructive role for the extracellular domain of MuSK.
Abbreviations: AChR, acetylcholine receptor; BGT, bungarotoxin; MuSK, muscle-specific kinase; NMJ, neuromuscular junction; PF, post fusion; RTK, receptor tyrosine kinase
Keywords: MuSK, Neuromuscular junction (NMJ), Nicotinic acetylcholine receptor (AChR), Receptor tyrosine kinase (RTK), Skeletal muscle, Synapses
Introduction
Pre- and postsynaptic elements coordinate the differentiation of one another as synaptogenesis proceeds, leading to precise opposition of pre- and postsynaptic specializations. How these interactions elicit postsynaptic differentiation is derived in large part from studies of the NMJ (Sanes and Lichtman, 2001).
It has long been thought that motor axons direct differentiation of the underlying postsynaptic membrane. In particular, the nerve-derived proteoglycan agrin has been implicated as central synaptic organizer (Ngo et al., 2007). It binds to the lipoprotein receptor Lrp4, which induces subterminal activation of MuSK, a receptor tyrosine kinase (RTK) that is expressed selectively in skeletal muscle and localized to NMJs (Kim et al., 2008; Zhang et al., 2008). Activated MuSK induces co-clustering of rapsyn and AChRs via intracellular pathways (Ghazanfari et al., 2011).
During postnatal maturation of NMJs, an initial, small oval-shaped AChR cluster is transformed into an array of pretzel-shaped branches that mirror the branching pattern of the motor nerve terminal, forming precisely matched pre- and postsynaptic branches (Sanes and Lichtman, 2001). Invagination of the muscle membrane leads to the formation of primary and secondary folds and growth of the muscle increases the size of the synapse. At the same time different proteins are expressed in different areas along the postjunctional folds resulting in a more complex molecular architecture of the maturing synapse (Sanes and Lichtman, 2001). These maturational changes are thought to be required for efficient neuromuscular transmission and normal motor function (Slater, 2008). Very little is known about molecular mechanisms regulating the maturation process. Laminins found in the basal lamina of the synaptic cleft have been shown to regulate postsynaptic maturation by recruiting the dystrophin–glycoprotein complex (DGC) (Nishimune et al., 2008). DGC is a cytoskeletal protein complex important for the stabilization of AChR clusters and the structural maintenance of the NMJ (Adams et al., 2004; Grady et al., 2000; Jacobson et al., 2001). In particular, anchorage of AChRs to the postsynaptic actin cytoskeleton is critical for regulating cluster stability (Mitsui et al., 2000). In that respect, it appears of specific interest that podosomes have been implicated in cluster maturation (Proszynski et al., 2009). Podosomes are dynamic actin-rich organelles that are involved in matrix remodeling (Gimona et al., 2008). More recently, another cytoskeletal regulator namely the Rho guanine nucleotide exchange factor ephexin1 has been demonstrated as a crucial factor for postsynaptic maturation of NMJs (Shi et al., 2010). Ephexin1-deficient mice fail to form mature pretzel-like NMJs and display muscle weakness as well as impaired neuromuscular transmission.
For a long time it was generally assumed that the branching pattern of the motor axon dictates the topology of the mature postsynaptic apparatus. Mainly because the topologically complex pretzel-like arrays typical of the mature synapse were not observed to form in the absence of innervation, either in vitro or in vivo (Moss and Schuetze, 1987; Slater, 1982). More recently, studies on aneural AChR cluster formation in cultured muscle cells have revealed that complex AChR clusters occur in the absence of nerve when cells are attached to a matrix-coated substrate like laminin (Kummer et al., 2004). Aneural pretzels share remarkable similarities to mature NMJs: they are oblong, broken at one side, elaborately branched and approximately the size of adult NMJs. Numerous postsynaptic markers are associated with these structures, including clusters of Syne-1-rich myonuclei. Pretzels develop in vitro from simpler structures through a series of transitions mirroring those that occur in association with axons in vivo. These morphological changes require the postsynaptic proteins MuSK and rapsyn suggesting that a muscle intrinsic program is able to shape the NMJ without input from the nerve.
MuSK activation via agrin and Lrp4 is characterized by an induced autophosphorylation of MuSK and an increased kinase activity (Glass et al., 1996; Watty et al., 2000). A downstream signaling cascade then results in the aggregation of AChRs at synaptic sites (Ghazanfari et al., 2011; Wang et al., 2006). A series of studies have shown that MuSK kinase activity and subsequent downstream signaling are crucial for AChR clustering and NMJ formation (Fuhrer et al., 1997; Herbst and Burden, 2000; Zhou et al., 1999). The role of MuSK during later aspects of NMJ development is less well understood. Conditional knock-out of MuSK and RNAi-mediated knock-down of MuSK expression have shown that NMJs disassemble upon MuSK elimination supporting a role for MuSK during NMJ maintenance (Hesser et al., 2006; Kong et al., 2004). However, MuSK function during NMJ maturation is still unclear.
We set out to study the molecular mechanisms involved in the nerve-independent formation of AChRs pretzel by dissecting the role of MuSK. We used muscle cell lines expressing MuSK mutant proteins to examine their ability to form aneural AChR pretzels on laminin. The analysis of AChR cluster morphology, size and number as well as the analysis of the temporal events revealed that MuSK kinase activity is required but not sufficient for aneural cluster formation. Furthermore, the temporal sequence of cluster maturation and the size of mature AChR clusters are altered in muscle cells expressing a constitutively active form of MuSK. We show that MuSK specific signaling is required for substrate-dependent AChR clustering and that the juxtamembrane region of MuSK in the context of the different kinase is unable to induce the formation of complex AChR clusters. Furthermore, we find that the extracellular domain of MuSK is crucial for AChR cluster formation on laminin substrate. Taken together, our study suggests a diverse role of MuSK during substrate-dependent AChR clustering, which involves kinase activity, MuSK-specific downstream signaling and structural information from the extracellular domain.
Results
Expression of wild-type MuSK in MuSK−/− muscle cells restores complex AChR cluster formation
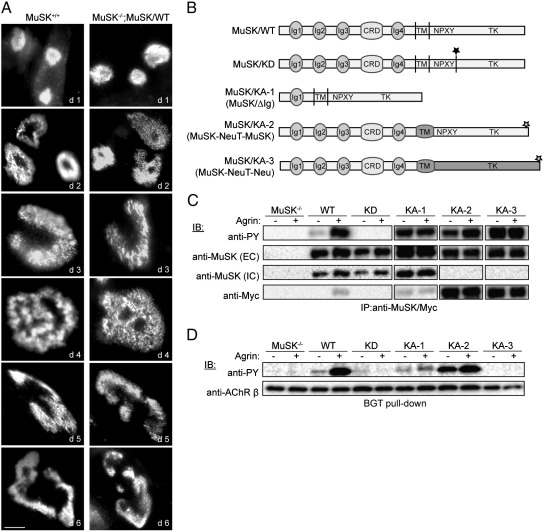
Kummer et al. have reported that muscle cells lacking MuSK expression are unable to form complex AChR clusters on laminin (Kummer et al., 2004). Transient expression of MuSK-GFP was able to rescue complex cluster formation. In order to learn more about the exact function of MuSK during aneural AChR clustering we sought to identify structural and functional domains in MuSK that are required for the formation of complex AChR clusters. For that we have employed a previously established cell culture system that is based on MuSK−/− muscle cell lines where MuSK mutants are expressed and AChR clustering is used as a read-out for MuSK function (Herbst and Burden, 2000). To test whether these cells represent a useful system to study complex cluster formation, MuSK−/− muscle cells expressing MuSK wild-type were differentiated and cultured on laminin substrate. AChR clusters were stained with Alexa 594-conjugated α-bungarotoxin (BGT) starting from post fusion (PF) day 1 to day 6. As shown in Fig. 1A, forced expression of MuSK wild-type restores laminin-induced complex AChR cluster formation. MuSK−/− muscle cells without ectopic MuSK expression are unable to form AChR clusters on laminin (Supplemental Fig. S1A). Taken together, like wild-type muscle cells, rescued muscle cells are able to recapitulate the morphological changes from plaque to pretzel.
Fig. 1.
Expression of wild-type and mutant MuSK in MuSK−/− muscle cells. (A) MuSK+/+ and MuSK−/− myotubes expressing MuSK wild-type (WT) were cultured on laminin and stained with α-BGT. Forced expression of MuSK wild-type in MuSK−/− muscle cells restores complex AChR cluster formation on laminin. Representative images of clusters are shown. Scale bar, 10 μm. (B) Cartoon showing MuSK wild-type and the different MuSK mutant constructs. Black asterisk marks K608A mutation. Gray asterisks mark Myc tag. (C) MuSK−/− muscle cells expressing MuSK wild-type and mutant proteins were differentiated for 4 days on gelatin-coated plates. Fully mature myotubes were stimulated with agrin (+, A4B8; −, A0B0), and MuSK was immunoprecipitated from cell lysates and assayed by immunoblotting. Wild-type MuSK is phosphorylated in response to neural agrin. Kinase-dead MuSK (KD) is unresponsive to agrin treatment whereas kinase-active MuSK constructs are phosphorylated independent of agrin. (D) AChRs were isolated from MuSK−/− muscle cells expressing wild-type and mutant MuSK differentiated on gelatin. Phosphorylation was detected by immunoblotting. AChR β phosphorylation is induced by neural agrin in cells expressing MuSK wild-type but not in cells expressing MuSK/KD or MuSK/KA-3. MuSK/KA-1 and KA-2 stimulate AChR β phosphorylation independent of agrin. PY, phosphotyrosine; EC, extracellular epitope; IC, intracellular epitope; IB, immunoblot.
MuSK kinase activity is required but not sufficient for complex AChR cluster formation
MuSK kinase activity and subsequent downstream signaling are essential for agrin-induced AChR clustering. To determine the role of MuSK kinase activity during the formation of substrate-induced complex AChR clusters we generated MuSK−/− muscle cells expressing different MuSK kinase mutants (Fig. 1B). To test the requirement of MuSK kinase activity we used a MuSK kinase-dead (MuSK/KD) mutant, which has lysine 608 in the autoactivation loop substituted by an alanine leading to a deficit in kinase activation (Glass et al., 1997). To dissect the role of MuSK kinase activity, in particular ligand-independent kinase activity, we used three different kinase-active mutants: (1) MuSK kinase-active-1 (MuSK/KA-1) lacks most of the extracellular domain including Ig2–4. This mutant is phosphorylated independent of agrin (Zhou et al., 1999). (2) MuSK/KA-2 (MuSK-NeuT-MuSK) has the MuSK transmembrane region replaced by the Neu transmembrane region (NeuT) (Jones et al., 1999). A mutation in the NeuT region leads to a constitutive dimerization and consequent activation of the receptor. (3) MuSK/KA-3 (MuSK-NeuT-Neu) has the MuSK extracellular domain fused to the transmembrane and intracellular domains of Neu (Jones et al., 1999). Expression of these constructs in HEK 293T cells confirmed their proposed activation status (Supplemental Fig. S1B). To examine the activation of the MuSK mutant proteins in response to agrin, we treated MuSK−/− myotubes expressing MuSK wild-type or one of the MuSK mutant proteins with soluble neural (A4B8) and muscle (A0B0) agrin, immunoprecipitated MuSK and probed immunoblots with anti-phosphotyrosine antibodies. Fig. 1C shows that MuSK wild-type is activated in response to neural agrin, whereas MuSK/KD lacks phosphorylation in the absence and presence of neural agrin. In contrast, the kinase-active MuSK proteins (KA-1, KA-2, KA-3) are phosphorylated and therefore active independent of neural agrin. To study downstream signaling we isolated AChRs using α-BGT from myotubes and probed immunoblots with anti-phosphotyrosine (Fig. 1D). AChR β phosphorylation is induced upon agrin-stimulated activation of MuSK wild-type. In muscle cells expressing MuSK/KD, AChR β phosphorylation is absent due to a lack of MuSK kinase activity. Constitutive-active MuSK/KA-1 and MuSK/KA-2 are able to induce AChR β phosphorylation in the absence of neural agrin, albeit weakly in the case of MuSK/KA-1. In contrast, MuSK/KA-3 lacks the ability to stimulate AChR β phosphorylation.
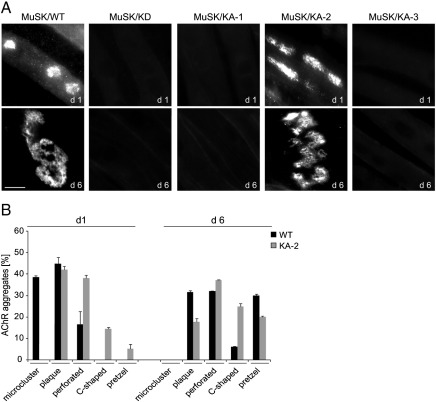
We next asked whether MuSK kinase activity is necessary for the laminin-induced formation of complex AChR clusters. As described above MuSK−/− myotubes expressing either MuSK wild-type or the different MuSK mutant proteins were cultured on laminin and AChRs were labeled with α-BGT. In order to determine the effect on cluster formation, cells at PF day 1 and PF day 6 were analyzed. As shown in Fig. 2A, MuSK−/− myotubes expressing MuSK wild-type form plaque-shaped AChR clusters at day 1 and pretzel-shaped clusters at day 6. A similar change in AChR cluster morphology is seen in muscle cells expressing MuSK/KA-2. In contrast, neither MuSK−/− muscle cells expressing MuSK/KD nor MuSK/KA-1 or MuSK/KA-3 are able to form AChR clusters on laminin. A quantification of the different morphologies at PF days 1 and 6 qualitatively demonstrated the formation of more complex AChR clusters (perforated, C-shaped and pretzel) in the MuSK wild-type expressing myotubes at PF day 6 (Fig. 2B). Similar morphological changes are seen in cells expressing MuSK/KA-2, although here we find complex AChR clusters already at PF day 1. To exclude the possibility that the difference in AChR cluster formation is due to different levels of AChR expression, we determined AChR α expression levels at PF day 5. We find comparable AChR α subunit expression between the used cell lines (Supplemental Fig. S2). These results demonstrate that MuSK kinase activity is required but not sufficient for substrate-induced AChR clustering.
Fig. 2.
Complex AChR cluster formation requires MuSK kinase activity. (A) MuSK−/− myotubes expressing wild-type and mutant MuSK were cultured on laminin and stained with α-BGT. Cells expressing MuSK wild-type or MuSK/KA-2 are able to form complex AChR clusters. In contrast, neither MuSK/KD nor MuSK/KA-1 and KA-3 induce substrate-dependent AChR clustering. Representative images of clusters are shown. Scale bar, 10 μm. (B) Graph showing the different cluster morphologies on PF days 1 and 6. MuSK/WT and MuSK/KA-2 are able to induce complex AChR clusters to a similar extent. n > 54 (from two independent experiments); error bars, S.E.M.
The temporal sequence of complex AChR cluster formation and the size of laminin-induced AChR clusters are altered in MuSK−/− muscle cells expressing MuSK/KA-2
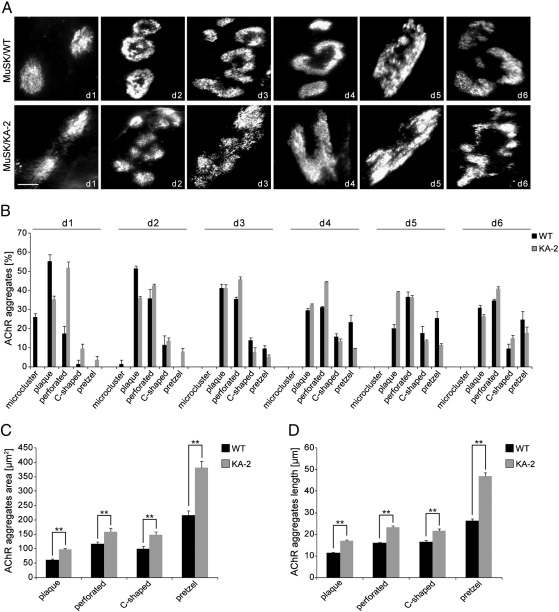
As shown in Fig. 2B, expression of constitutively active MuSK/KA-2 accelerates the formation of complex AChR clusters. To examine in more detail how an increased MuSK activity influences laminin-induced AChR clustering, we cultured muscle cells on laminin and followed the development of laminin-induced AChR clusters by staining AChRs with α-BGT from PF day 1 to day 6 (Fig. 3A). A quantitative analysis of the different cluster morphologies showed that MuSK−/− myotubes expressing MuSK/KA-2 are able to form more complex AChR cluster topologies (C-shaped and pretzel) faster than myotubes expressing MuSK wild-type (Fig. 3B). This advanced cluster maturation was evident until PF day 2, after that the distribution of morphologies was similar between MuSK wild-type and MuSK/KA-2 expressing cells. Next we quantified the length and area of the formed AChR aggregates. We found that MuSK/KA-2 induces the formation of significantly bigger AChR clusters (Figs. 3C, D). In particular, complex pretzel-shaped AChR aggregates are considerably larger in length and size compared to the clusters of the same category induced by MuSK wild-type. Taken together, constitutive activation of the MuSK kinase modulates laminin-induced AChR cluster formation by accelerating the time-course of complex cluster formation and by increasing the size of mature AChR clusters.
Fig. 3.
MuSK/KA-2 induces enhanced cluster maturation and bigger AChR clusters. (A) MuSK−/− myotubes expressing MuSK wild-type (WT) or MuSK/KA-2 were cultured on laminin and stained with α-BGT. Forced-expression of MuSK/WT and MuSK/KA-2 induces the same topological changes from plaque to pretzel. Representative images of clusters are shown. Scale bar, 10 μm. (B) Quantification of the different cluster morphologies in MuSK/WT and MuSK/KA-2 expressing myotubes. MuSK/KA-2 induced an enhanced temporal appearance of complex AChR clusters. (C and D) Graph showing the AChR cluster area and length, respectively. Cells expressing MuSK/KA-2 form significantly bigger AChR clusters. p value < 0.003; n > 40 (number of independent experiments: day1 = 2, days 2, 3, 4, 5 = 3; day 6 = 4); error bars, S.E.M.
AChR accumulation, motility and stability in MuSK−/− muscle cells expressing MuSK wild-type or MuSK/KA-2
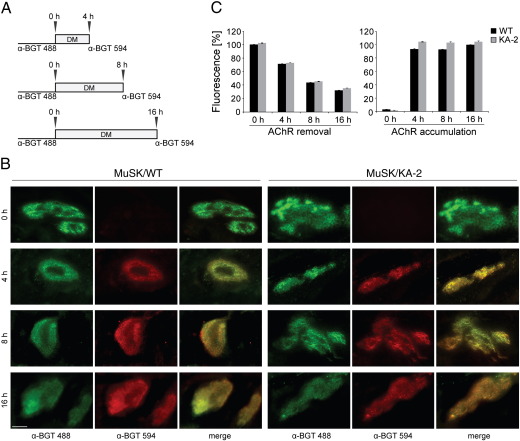
The size and number of AChR clusters can be influenced by a variety of mechanisms including accumulation rate, stability and motility of AChRs (Dai et al., 2000; Hall et al., 1981; Lee et al., 2009). To determine whether MuSK/KA-2 induced bigger AChR clusters due to an increased accumulation or removal rate we stained myotubes cultured on laminin with Alexa 488-conjugated α-BGT at PF day 5. Subsequently cells were incubated for 4, 8 or 16 h at 37 °C followed by labeling with Alexa 594-conjugated α-BGT (Fig. 4A). As shown in Fig. 4B, new AChRs are efficiently labeled with Alexa 594-conjugated α-BGT and specifically accumulated in the pre-existing laminin-induced AChR clusters. As previously reported the insertion of new AChRs occurs at the periphery of the existing cluster. This is clearly visible in MuSK/WT expressing myotubes but less pronounced in MuSK/KA-2 expressing cells. A quantitative analysis of the mean fluorescence intensity within clusters was performed using ImageJ (described in Experimental methods, Fig. 4C). We found that the loss of existing AChRs from clusters occurs gradually at a rate of 20–30% per 4 h, which corresponds to the removal rate previously reported by Bruneau and colleagues (Bruneau et al., 2005). The density of newly accumulated AChRs increases rapidly within the first 4 h. After this time period AChR density does not significantly increase presumably due to an equilibrium between removal and insertion of receptors (Bruneau et al., 2005). When we compared muscle cells expressing MuSK wild-type with cells expressing MuSK/KA-2, we found weak but non-significant differences in the removal rate of preassembled AChRs or in the densities of newly accumulated AChRs.
Fig. 4.
Accumulation of AChRs in and removal of AChRs from pre-existing laminin-induced clusters in MuSK−/− muscle cells expressing MuSK/WT or MuSK/KA-2. (A) Labeling scheme of myotubes expressing MuSK/WT or MuSK/KA-2. Cells were labeled with Alexa 488-conjugated α-BGT and relabeled with Alexa 594-conjugated α-BGT after 4, 8 and 16 h. (B) MuSK−/− myotubes expressing MuSK wild-type (WT) or MuSK/KA-2 were cultured on laminin. Representative images of clusters are shown. Scale bar, 10 μm. (C) Quantification of mean fluorescence intensities for AChR removal (Alexa 488-labeled receptors) and AChR accumulation (Alexa 594-labeled receptors) is shown. AChR densities are similar in MuSK/WT and MuSK/KA-2 expressing cells. n > 22 (from two independent experiments); error bars, S.E.M.
We also asked whether AChR stability and motility within laminin-induced AChR clusters are different in MuSK wild-type versus MuSK/KA-2 expressing myotubes after agrin stimulation, which has previously been reported to direct new receptors preferentially into agrin-induced clusters instead of pre-existing laminin clusters (Bruneau et al., 2005). Cells were stained with Alexa 488-conjugated α-BGT immediately before agrin stimulation. Labeled myotubes were kept in agrin-conditioned medium for 4, 8, 16 or 24 h, fixed and newly inserted AChRs were stained with Alexa 594-conjugated α-BGT (Supplemental Fig. S3A). A quantification of myotubes that either contain only laminin-induced clusters or laminin- and agrin-induced clusters revealed that after 4 hour agrin stimulation about 50% of myotubes contain laminin- and agrin-induced clusters (Supplemental Figs. S3B, C). By 8 h most of the myotubes (about 80%) contain both laminin- and agrin-induced clusters. This is not changed by increased incubation times with agrin. When we compare MuSK wild-type and MuSK/KA-2, we observe a slightly decreased number of myotubes with both forms of AChR clusters in cells expressing MuSK/KA-2.
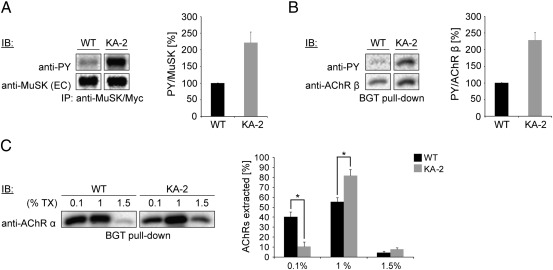
The anchorage of AChRs to the cytoskeleton is an important event during cluster formation. In particular, the extent of attachment defines the stability of AChRs within clusters (Podleski and Salpeter, 1988; Prives et al., 1982; Stya and Axelrod, 1983). Agrin stimulation and subsequent AChR phosphorylation have been shown to assist the cytoskeletal anchorage and to make AChR less detergent extractable (Borges and Ferns, 2001). Since MuSK/KA-2 displays a constitutive kinase activity, we asked whether constitutive phosphorylation of AChRs by MuSK/KA-2 increases the cytoskeletal anchorage. First, we determined the level of MuSK and AChR phosphorylation in MuSK wild-type and MuSK/KA-2 cells. For that we lysed myotubes kept on laminin until PF day 5 and assayed MuSK and AChR β phosphorylation subsequent to MuSK immunoprecipitation and AChR pull-down, respectively. As expected, MuSK/KA-2 is strongly tyrosine phosphorylated and induces an increased AChR β phosphorylation (2.5 fold increase) compared to MuSK wild-type (Figs. 5A, B). Next, on PF day 5 we lysed cells with increasing concentrations of detergent, isolated AChRs with biotin-conjugated α-BGT and determined the amount of AChR α by immunoblotting (Fig. 5C). Significantly more AChR α is extracted in MuSK wild-type expressing myotubes with 0.1% triton compared to MuSK/KA-2 expressing myotubes. In contrast, the majority of AChR α is extracted from MuSK/KA-2 expressing myotubes with 1% triton. In addition, we find that MuSK/KA-2 expressing cells contain an increased fraction of strongly attached receptors that only extracted in 1.5% triton. A similar extractability was observed for AChR β (data not shown). To further correlate phosphorylation and cytoskeletal attachment during cluster maturation we performed a comparative time course from PF days 1 to 6. We find similar phosphorylation levels for MuSK and AChR β between PF day 1 and days 5/6 (Supplemental Figs. S4A, B). In contrast, AChR extractability decreases between PF days 3 and 5 indicating an increased cytoskeletal anchorage (Supplemental Fig. S4C). This increased attachment to the cytoskeleton is more pronounced in cells expressing MuSK/KA-2. In summary, we found small but nonsignificant differences between cells expressing MuSK wild-type or MuSK/KA-2 in terms of AChR insertion, motility and stability but a significantly increased cytoskeletal anchoring of AChRs in MuSK/KA-2 expressing muscle cells, most likely caused by AChR hyperphosphorylation.
Fig. 5.
Cytoskeletal linkage of AChRs in MuSK−/− muscle cells expressing MuSK/WT or MuSK/KA-2. (A) MuSK was immunoprecipitated on PF day 5 from cell lysates of MuSK/WT and MuSK/KA-2 expressing myotubes and analyzed by immunoblotting. MuSK/KA-2 is strongly phosphorylated compared to MuSK/WT. n = 6. (B) On PF day 5, cell lysates of MuSK/WT and MuSK/KA-2 expressing myotubes were subjected to an α-BGT pull-down. Purified AChRs were analyzed by immunoblotting. AChR β phosphorylation is increased in MuSK/KA-2 expressing cells compared to cells expressing MuSK/WT. n = 4. (C) Cell lysates were extracted sub sequentially with 0.1%, 1% and 1.5% triton on PF day 5. AChRs were purified using α-BGT and assayed by immunoblotting. AChR α is weakly extracted with 0.1% triton and the majority of AChR α is extracted with 1% triton in cells expressing MuSK/KA-2. In contrast, AChR α is extracted to a similar extent in 0.1% and 1% triton in MuSK/WT expressing cells. p-value < 0.05; n = 3; error bars, S.E.M.; TX, triton X-100; PY, phosphotyrosine, IB, immunoblot.
The juxtamembrane region of MuSK is insufficient to induce the formation of complex AChR clusters on laminin
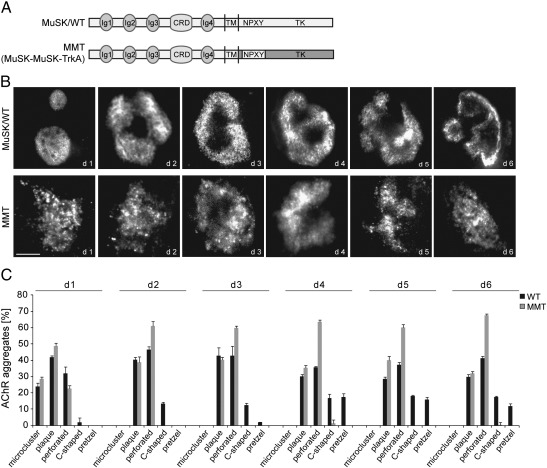
We have previously shown that the juxtamembrane region of MuSK in the context of a different kinase domain, namely TrkA, is able to rescue agrin-induced AChR clustering in response to agrin (Supplemental Fig. S5) (Herbst and Burden, 2000). Furthermore, we were able to show that MuSK mutant mice expressing the same construct (MMT) are able to survive and to form AChR clusters similar to wild-type mice. However, we also reported that NMJs are immature and less complex in muscle from adult mice (Herbst et al., 2002). To address the function of the juxtamembrane region during laminin-induced cluster formation we cultured MuSK−/− muscle cells expressing MuSK wild-type or MMT on laminin and stained AChR clusters with α-BGT. As shown in Fig. 6, MMT stimulates the formation of substrate-induced AChR clusters but these clusters display an immature morphology. A quantitative analysis of the observed morphologies revealed that muscle cells expressing MMT are able to form plaque-shaped and perforated clusters but unable to form complex pretzel-like clusters.
Fig. 6.
The MuSK/TrkA chimera, MMT, fails to induce complex AChR clusters on laminin substrate. (A) Schematic drawing of MuSK/WT and the MMT chimera. (B) MuSK−/− myotubes expressing MuSK wild-type (WT) or MMT were cultured on laminin and stained with α-BGT. Forced-expression of MMT induces the formation of immature cluster morphologies only. Representative images of clusters are shown. Scale bar, 10 μm. (C) Quantification of the different cluster morphologies in MuSK/WT and MMT expressing myotubes. MMT was unable to induce complex AChR clusters. n > 15 (number of independent experiments: days 1, 2, 4, 5 = 3; day 3 = 2; day 6 = 4); error bars; S.E.M.
The extracellular region of MuSK is necessary for substrate-dependent AChR clustering
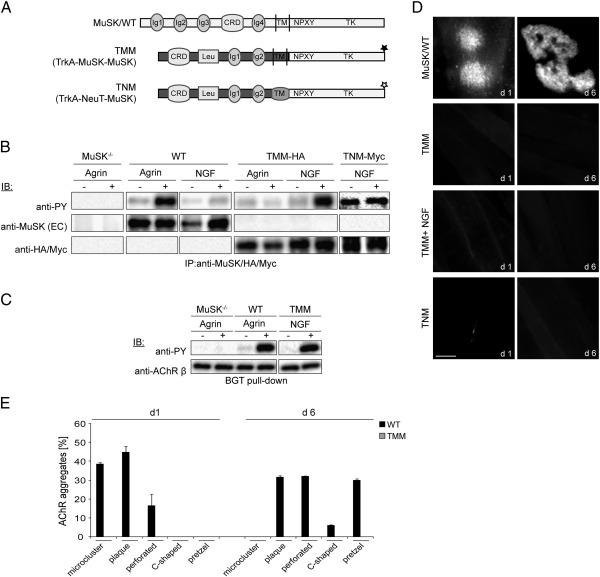
It was shown that pretzel formation does not depend on a specific substrate since laminin can be replaced by fibronectin (Kummer et al., 2004). Consequently the required activation of MuSK appears substrate-unspecific. To follow up on this observation, we asked whether the extracellular region of MuSK is required for substrate-dependent cluster formation or whether this region can be replaced by another RTK. For this we fused the extracellular domain of TrkA including the transmembrane region to the MuSK intracellular region (Fig. 7A). Analogous to previous reports on a TrkC/MuSK chimeric construct, the TrkA/MuSK (TMM) construct is activated by the TrkA-specific ligand NGF and is also able to induce AChR phosphorylation (Figs. 7B, C) (Glass et al., 1997). Next we cultured MuSK−/− myotubes expressing TMM on laminin and stained AChRs with α-BGT. We found that TMM is unable to rescue laminin-induced AChR clustering (Figs. 7D, E). To exclude the possibility that the TMM construct fails to induce AChR clusters due to an inefficient dimerization and autoactivation we designed two additional approaches: (1) TMM expressing muscle cells were cultured on laminin in continuous presence of NGF. (2) A constitutive-active chimera, designated TNM, was constructed consisting of the TrkA extracellular region, the Neu transmembrane domain and the MuSK intracellular region (Fig. 7A). TNM is phosphorylated in the absence of NGF but fails to induce AChR clusters on laminin (Figs. 7B, D). Likewise TMM in the presence of NGF is unable to cluster AChRs on laminin (Fig. 7D). These data implement an instructive role of the MuSK extracellular domain during the substrate-dependent formation of AChR clusters.
Fig. 7.
The extracellular domain of MuSK is required for substrate-induced AChR clustering. (A) Cartoon showing MuSK/WT and the chimeras TMM and TNM. Asterisks indicate the C-terminal HA/Myc tag. (B) Myotubes expressing MuSK wild-type (WT), TMM or TNM were differentiated on gelatin and stimulated with agrin or NGF on day 4 of differentiation. MuSK, TMM and TNM were immunoprecipitated from cell lysates and assayed by immunoblotting. Wild-type MuSK is phosphorylated in response to agrin but not in response to NGF. In contrast, TMM is unresponsive to agrin treatment whereas NGF stimulation induces a robust phosphorylation. TNM is constitutively phosphorylated in the absence and presence of NGF. (C) AChRs were isolated from MuSK−/− muscle cells expressing wild-type and TMM, which were differentiated on gelatin coated plates for 4 days. Phosphorylation was detected by immunoblotting. AChR β phosphorylation is induced by agrin/NGF in cells expressing MuSK wild-type or TMM. (D) MuSK−/− myotubes expressing MuSK wild-type (WT), TMM or TNM were cultured on laminin and stained with α-BGT on PF days 1 and 6. Forced expression of TMM fails to induce the formation of AChR clusters on laminin. Representative images of clusters are shown. Scale bar, 10 μm. (E) Graph showing the appearance of cluster morphologies at PF day 1 and day 6. n > 62 (from two independent experiments); error bars, S.E.M.; PY, phosphotyrosine; EC, extracellular epitope; IB, immunoblot.
Discussion
MuSK is the central master regulator of NMJ development. All known aspects of pre- and postsynaptic differentiation depend on MuSK action (DeChiara et al., 1996). To examine the role of MuSK during NMJ maturation we took advantage of a previously established assay whereby mature AChR pretzels are formed on a laminin substrate. We used MuSK−/− muscle cells to study the effect on substrate-dependent cluster formation upon expression of different MuSK mutants. Our study showed that both, the kinase domain and the extracellular region of MuSK are required for the formation of AChR clusters on laminin. Moreover, the MuSK juxtamembrane domain in the context of a different kinase domain is unable to induce cluster maturation.
Is laminin-induced AChR clustering a valid model system to study postsynaptic maturation?
Until recently it was thought that nerve-derived signals are the prime regulators of NMJ development. The discovery of aneural AChR clusters in the absence of innervation and the observation of AChR pretzels on aneurally cultured muscle cells put this neuro-centric view of NMJ development into question (Kummer et al., 2006). New models now suggest that muscle intrinsic programs exist, which influence AChR clustering. Although it is still believed that during development signals from the nerve are essential for cluster maturation, Kummer et al. uncovered a muscle-intrinsic program capable of transforming a plaque-shaped cluster into a complex array of branches (Kummer et al., 2004). In particular, this study suggests that similar mechanisms of cluster maturation exist in vitro and in vivo e.g. local AChR endocytosis and circumferential AChR addition at the periphery of clusters. Moreover, an instructive role of the muscle during cluster maturation is supported by several lines of evidence: (i) the appearance of receptor-poor perforations in the plaque often precedes local axonal removal (Balice-Gordon and Lichtman, 1993); (ii) motor axons that contact MuSK−/− or rapsyn−/− myotubes fail to form arbors (Nguyen et al., 2000); (iii) vacant regions of developing NMJs are often reoccupied by other axon branches (Walsh and Lichtman, 2003) and (iv) postsynaptic morphology determines the pattern of nerve terminal branches during reinnervation (Rich and Lichtman, 1989; Sanes et al., 1978). Considering all this, muscle-intrinsic signals appear to play a regulatory function during NMJ maturation and the substrate-induced clustering assay represents a suitable assay to study these signals.
Cluster maturation on laminin is dependent on MuSK
MuSK mutant mice lack pre- as well as postsynaptic differentiation and consequently die right after birth (DeChiara et al., 1996). Due to this early lethality the role of MuSK during later processes of NMJ development is less well understood. A previous study used cre-mediated deletion of MuSK to examine MuSK function postnatally (Hesser et al., 2006). Thereby efficient recombination was observed after postnatal day 15 and effects on NMJ morphology and function were detectable thereafter. Since NMJ maturation occurs in the first two weeks after birth no effect on the maturation was reported but a disassembly of mature NMJs was detected ultimately leading to myasthenic syndromes. Similar NMJ defects were seen in adult mice where MuSK was ablated using RNAi (Kong et al., 2004). Using the substrate-dependent clustering assay it was demonstrated that MuSK is required for the formation of aneural AChR clusters (Kummer et al., 2004). In this study we further show that forced expression of MuSK in MuSK−/− muscle cells also restores the temporal transformation of plaque-shaped clusters into pretzel-like aggregates.
MuSK function during substrate-dependent AChR clustering
MuSK kinase activity and subsequent signaling are crucial for agrin-induced AChR clustering (Wang et al., 2006). Likewise, MuSK kinase activity is required for substrate-induced AChR clustering since muscle cells expressing a kinase-deficient MuSK are unable to form clusters on laminin. In contrast, constitutive-active MuSK (MuSK/KA-2) is able to cluster AChR on laminin and like MuSK wild-type induces a topological transformation of plaques into pretzels. The formation of complex clusters occurs faster and the size of complex clusters is increased when active MuSK is expressed. Activation of MuSK induces the phosphorylation of AChRs and it has previously been shown that AChR phosphorylation regulates the cytoskeletal anchorage (Borges and Ferns, 2001; Fuhrer et al., 1997). Consistent with these results, we detected an increased AChR phosphorylation in cells expressing MuSK/KA-2. Moreover, experiments to determine the attachment of AChRs to the cytoskeleton revealed significant differences between cells expressing MuSK/KA-2 or MuSK wild-type whereas the accumulation/removal rate into clusters and/or their motility and stability within clusters is similar. Our data therefore suggest that an increased cytoskeletal attachment accounts for the differences in cluster formation between MuSK wild-type and MuSK/KA-2.
Using a chimeric protein consisting of the MuSK extracellular domain and the Neu transmembrane and intracellular regions we showed that MuSK-specific signaling is required for substrate-induced AChR clustering. Like for agrin-induced AChR clustering an active kinase is not sufficient and specific MuSK sequences are necessary for cluster formation on laminin (Herbst and Burden, 2000; Jones et al., 1999). These results suggest that agrin- and substrate-induced AChR clustering are regulated by a signaling cascade that involves the same factors. Consistent with this, we find that a previously described MuSK/TrkA chimera that has the MuSK juxtamembrane NPXY motif inserted in the TrkA intracellular domain is able to induce AChR clusters on laminin. However, the same chimeric construct is unable to stimulate the topological transformation from plaque into pretzel. Our findings in muscle cells are supported by a previous report on MuSK−/− mice expressing the MuSK/TrkA chimeric protein showing immature and fragmented NMJs (Herbst et al., 2002). This indicates that the initial localization of AChRs to synaptic sites depends on MuSK signals that are transferred via the NPXY motif and therefore consequently via Dok-7, a previously identified PTB-domain containing protein that interacts with MuSK via the NPXY motif (Okada et al., 2006). In contrast, the morphological changes that occur during cluster maturation appear to require additional MuSK specific sequences and/or signals (Supplemental Fig. S6). Alternatively, TrkA kinase that differs in kinetics and efficiency might be unable to induce the signaling cascade to the same extent as the MuSK kinase. As a consequence the maturation process is not initiated.
Interestingly, MuSK kinase activity and downstream signaling are not however sufficient for cluster formation because MuSK/KA-1, a MuSK kinase active mutant lacking Ig2–Ig4 of the extracellular domain, fails to induce clusters on laminin. It has previously been suggested that tight adhesion to the substrate independent of specific domains in MuSK is important for the cluster formation on laminin (Kummer et al., 2004). Therefore it might be envisioned that the extracellular region of MuSK/KA-1, which lacks Ig2–4 and the cysteine-rich domain is too short to ensure a strong interaction with the substrate and consequently fails to aggregate MuSK into a required primary scaffold. However, when we replaced the extracellular domain of MuSK with the equivalent domain of TrkA, no substrate-induced AChR clusters were detected. This is also the case even in the presence of a constitutive-active TrkA/MuSK chimera. These results are surprising since it was previously proposed that the substrate-bound laminin induces AChR clusters by providing a strongly adhesive interaction between muscle and substrate rather than activating a specific matrix receptor (Kummer et al., 2004). Why is the MuSK extracellular domain required to induce substrate-dependent AChR clusters? One possibility is that AChR clustering on substrate requires an interaction between Lrp4 and MuSK (Supplemental Fig. S6). Lrp4 is the recently identified agrin receptor and interacts with MuSK via its extracellular domain (Kim et al., 2008; Zhang et al., 2008, 2011). Agrin-induced Lrp4/MuSK binding leads to an activation of MuSK. In a substrate-dependent clustering assay Lrp4 would not bind to agrin but presumably bind to the substrate. This interaction might be necessary for the aggregation and activation of MuSK. Future studies will have to show whether Lrp4 is required for substrate-dependent AChR clustering. A second possibility derives from earlier studies, which proposed the existence of a transmembrane linker, called RATL, supporting the interaction between MuSK and rapsyn (Apel et al., 1997; Zhou et al., 1999). The hypothetical RATL protein has been thought to bring the AChR/rapsyn complex into close proximity to MuSK, thereby inducing MuSK-dependent AChR clustering. Considering recent data it is possible that RATL actually represents the requirement of Lrp4 binding to MuSK via the extracellular domain, which might be crucial for agrin-dependent and independent AChR clustering.
Experimental methods
Plasmid constructs
An expression plasmid encoding MuSK/KA-1 lacking Ig2–4 was generated according to Zhou et al. (1999). Briefly, SpeI cloning sites at position nucleotide (nt) 296 of the extracellular domain and at position nt 1475 of the transmembrane domain of rat MuSK cDNA were inserted by PCR. Ig1 and the transmembrane domain plus cytoplasmic domain of MuSK were ligated. A CMV driven MuSK/KA-2 expression construct was engineered from MuSK-NeuT-MuSK-Myc (a gift from Dr. Brenner, University of Basel) using EcoRI and NotI (Jones et al., 1999). For introducing a Myc tag at the C-terminal of MuSK/KA-3, a NdeI site at nt 1963 and a SpeI site was inserted at position nt 3777 into the MuSK-NeuT-Neu construct (kindly provided by Dr. Brenner, University of Basel) by PCR (Jones et al., 1999). The intracellular MuSK domain of MuSK/KA-2 was replaced with Neu to generate MuSK-NeuT-Neu-Myc. To generate the TrkA/MuSK chimera TMM, a SalI site was introduced at nt 1391 of the human TrkA cDNA and at nt 1544 of the MuSK cDNA by PCR. The TrkA fragment spanning extracellular and transmembrane domain was ligated with the MuSK cytoplasmic domain. A HA tag at the C-terminus of TMM was inserted using HindIII and BglII. The TrkA-NeuT-MuSK (TNM) chimera was constructed by introducing a NdeI site into the human TrkA cDNA (nt 1299). The extracellular TrkA was fused, via the NdeI site, to NeuT-MuSK-Myc. MuSK/KD containing the mutation K608A was a gift from Dr. Burden, NYU School of Medicine. Accuracy of the inserted fragments and tags was verified by sequencing before ligation into the retroviral vector pBabe/puro (Morgenstern and Land, 1990).
Cell culture
Phoenix cells were cultured and maintained in Dulbecco's Modified Eagle's Medium (DMEM supplemented with glutamine, 4.5 g/l glucose), 10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich) and 1% (v/v) penicillin/streptomycin (PAA) at 37 °C and 5% CO2. MuSK−/− and MuSK+/+ myoblasts isolated from embryos carrying a temperature-sensitive SV40 T antigen were propagated at 33 °C and 5% CO2 on 0.2% gelatin-coated dishes containing growth medium: DMEM containing glutamine, 4.5 g/l glucose enriched with 10% (v/v) FBS, 10% (v/v) horse serum (HS), 0.25% chick embryo extract (CEE), 20 U/ml recombinant murine interferon-γ (IFN-γ; Peprotech) and 1% (v/v) penicillin/streptomycin (Herbst and Burden, 2000; Jat et al., 1991). Stable infection of MuSK−/− muscle cells was performed according to Herbst and Burden (Herbst and Burden, 2000). Briefly, Phoenix cells were transfected with pBabe/puro plasmids and virus-containing medium was collected two days post-transfection and used immediately for the infection of MuSK−/− myoblasts in the presence of 2 μg/ml polybrene (Sigma). After 2 h the virus-containing medium was replaced with fresh growth media. 24 h post-infection, muscle cells were split and maintained in growth medium with puromycin (2 ng/ml). Clones of cells were isolated, expanded and assayed for their ability to differentiate into multinucleated myotubes by removing IFN-γ and CEE from the medium and increasing the temperature to 37 °C.
Preparation of protein extracts and immunoblotting
Differentiated myotubes were starved for 2 h, stimulated either with soluble neural A4B8 and non-neural A0B0 agrin (prepared from HEK 293T) for 30 min or with NGF (50 ng/ml) for 5 min. After stimulation proteins were extracted in NP-40 lysis buffer (1% NP-40, 5 mM EGTA, 50 mM NaCl, 30 mM triethanolamine pH 7.5, 50 mM NaF supplemented with freshly added 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 200 μM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate). Lysates were immunoprecipitated with monoclonal or polyclonal antibodies overnight and captured with Protein A or G agarose (Roche). Isolated proteins were analyzed by SDS-PAGE and immunoblotting. For AChR pull-down, the lysate was incubated with 2 ng/ml biotin-conjugated α-BGT followed by incubation with streptavidin-agarose and immunoblotting. Quantitative analysis of blots was performed by Quantity One software (Bio-Rad). Ratio of phosphorylated protein to total amount of protein was quantified. Phosphorylation of MuSK wild-type was defined as 100% and used to calculate the relative percentage phosphorylation of mutant MuSK. For MuSK activation and AChR β subunit phosphorylation, MuSK/WT and MuSK/KA-2 expressing myotubes cultured on laminin-coated substrate were lysed on PF day 5 in NP-40 lysis buffer, and proteins were isolated by immunoprecipitation or BGT-pull-down as described above.
Laminin-induced AChR clustering assay
Myoblasts were cultured and differentiated according to Kummer et al. (2004). Briefly, a 10 μg/ml solution of EHS laminin (Roche) in L-15 medium (Sigma-Aldrich) containing 0.2% NaHCO3 and 100 × glutamine was used to coat poly-l-ornithine-coated dishes overnight at 37 °C. Before plating of cells laminin was aspirated and cells were maintained at 33 °C and 5% CO2. After 24 h the growth media was replaced with differentiation medium (DM) and the temperature was increased to 37 °C to induce differentiation. After inducing fusion, cultures were maintained in DM for 1–6 days to access the prevalence of simple to complex aggregates over time as a function of PF days. 1 μg/ml TTX and 100 × FUDR was added to block the twitching of myotubes and to inhibit the growth of myoblasts. AChR clusters were stained on each consecutive PF day with 2 ng/ml of Alexa 594-coupled α-BGT, washed, fixed, mounted and imaged with 63 ×/1.4–0.60 oil objective on a Leica inverted fluorescence microscope (DMIRB) equipped with a cooled MicroMax CCD camera. Cells were photographed at equivalent exposure times using Metamorph imaging software. Multiple forms of AChR aggregates formed per PF day were classified as microclusters, plaque, perforated, C-shaped and pretzel as described previously by Kummer et al. (2004). Total number of aggregates of each individual PF day was summed up as 100% and the distribution of each form of clusters in individual PF day was plotted as a percentage of the combined total. The prevalence of morphology from 2 to 6 independent experiments of each cell line was analyzed and compared to MuSK/WT. For comparison of the size, all observed aneural AChR clusters of MuSK/WT and MuSK/KA-2 were categorized into plaque, perforated, C-shaped and pretzel. The area and length (feret diameter) of each cluster, bigger than 5 μm were measured by using free hand tool (ImageJ software).
Receptor accumulation/removal
On PF day 5, MuSK/WT and MuSK/KA-2 muscle cells on laminin-coated substrate were saturated with 2 ng/ml Alexa 488-coupled α-BGT for 30 min at 4 °C. Unbound BGT was washed away with differentiation media and cells were placed at 37 °C and 5% CO2 for 4, 8 and 16 h. After this incubation, cells were washed with PBS, fixed in 1% PFA and stained with 2 ng/ml Alexa 594-coupled α-BGT for 30 min at room temperature to identify newly accumulated receptors. Post-fixed cells were mounted in Vectashield for imaging as described above. Mean gray values of old AChRs and newly accumulated receptors for each independent time course were quantified using ImageJ and plotted as a percentage of present AChRs at time point 0 h and at time point 16 h, respectively.
Receptor stability
The extent of attachment of laminin-induced MuSK/WT and MuSK/KA-2 AChR aggregates with the cytoskeleton was determined as described previously (Sadasivam et al., 2005). On 5th day of PF, myotubes were incubated with 2 ng/μl of biotinylated α-BGT, rinsed with ice-cold PBS and surface AChRs clusters were extracted in extraction buffer (25 mM Tris, 25 mM glycine, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 200 μM phenylmethylsulfonyl fluoride) containing 0.1% triton X-100. The supernatant was collected after centrifugation and the pellet was resuspended in extraction buffer containing 1% triton, followed by a third extraction with 1.5% triton. Solubilized surface AChRs were precipitated from lysates with streptavidin-agarose and the amount of surface AChRs in each fraction was determined by immunoblotting using antibodies against the AChR-α subunit. The amount of extracted AChR-α subunits was quantified with Quantity One (Bio-Rad). The 0.1%, 1% and 1.5% fractions were calculated and summed up to 100%.
Statistical analysis
Independent samples t-test was performed using SPSS 18 (statistical software system) to compare MuSK/WT and MuSK/KA-2 muscle cell lines. A p-value < 0.05 was considered statistically significant.
Antibodies and reagents
The following antibodies were purchased from commercial sources: anti-AChR α (BD Biosciences), anti-AChR β (Sigma-Aldrich), anti-HA (Sigma-Aldrich), anti-Myc (Sigma-Aldrich), anti-phosphotyrosine PY-100 (Cell Signaling), anti-phosphotyrosine PY99 (Santa Cruz), and anti-TrkA (Santa Cruz). Polyclonal antibodies against the MuSK extracellular domain (Ig1–2) were produced in rabbits (Diederichs and Herbst, unpublished data). Antibodies to the C-terminal sequence of MuSK were provided by Dr. Fuhrer, University of Zurich (Fuhrer et al., 1997). Streptavidin- and fluorophore-conjugated α-BGT were obtained from Invitrogen. Goat anti-mouse/rat/rabbit HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch. Soluble neural A4B8 and non-neural A4B0 agrin were prepared from HEK 293T as previously described (Herbst and Burden, 2000).
Acknowledgments
We are grateful to Christian Fuhrer for providing us with anti-MuSK antibodies. We would like to thank Steve Burden and Hans-Rudolf Brenner for providing plasmids. Many thanks also go to Tamara Peneder for help with the statistical analysis. Wilfried Ellmeier provided useful comments on the manuscript. This work was supported by the Austrian Science Fund [P19223-B09] to RH and by the Higher Education Commission Pakistan (HEC) to SM.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.mcn.2011.12.007.
Appendix A. Supplementary data
Supplementary materials.
References
- Adams M.E., Kramarcy N., Fukuda T., Engel A.G., Sealock R., Froehner S.C. Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J. Neurosci. 2004;24:10302–10309. doi: 10.1523/JNEUROSCI.3408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel E.D., Glass D.J., Moscoso L.M., Yancopoulos G.D., Sanes J.R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon R.J., Lichtman J.W. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J. Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L.S., Ferns M. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 2001;153:1–12. doi: 10.1083/jcb.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau E.G., Macpherson P.C., Goldman D., Hume R.I., Akaaboune M. The effect of agrin and laminin on acetylcholine receptor dynamics in vitro. Dev. Biol. 2005;288:248–258. doi: 10.1016/j.ydbio.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Dai Z., Luo X., Xie H., Peng H.B. The actin-driven movement and formation of acetylcholine receptor clusters. J. Cell Biol. 2000;150:1321–1334. doi: 10.1083/jcb.150.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara T.M., Bowen D.C., Valenzuela D.M., Simmons M.V., Poueymirou W.T., Thomas S., Kinetz E., Compton D.L., Rojas E., Park J.S., Smith C., DiStefano P.S., Glass D.J., Burden S.J., Yancopoulos G.D. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Fuhrer C., Sugiyama J.E., Taylor R.G., Hall Z.W. Association of muscle-specific kinase MuSK with the acetylcholine receptor in mammalian muscle. EMBO J. 1997;16:4951–4960. doi: 10.1093/emboj/16.16.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfari N., Fernandez K.J., Murata Y., Morsch M., Ngo S.T., Reddel S.W., Noakes P.G., Phillips W.D. Muscle specific kinase: organiser of synaptic membrane domains. Int. J. Biochem. Cell Biol. 2011;43:295–298. doi: 10.1016/j.biocel.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Gimona M., Buccione R., Courtneidge S.A., Linder S. Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Glass D.J., Bowen D.C., Stitt T.N., Radziejewski C., Bruno J., Ryan T.E., Gies D.R., Shah S., Mattsson K., Burden S.J., DiStefano P.S., Valenzuela D.M., DeChiara T.M., Yancopoulos G.D. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Glass D.J., Apel E.D., Shah S., Bowen D.C., DeChiara T.M., Stitt T.N., Sanes J.R., Yancopoulos G.D. Kinase domain of the muscle-specific receptor tyrosine kinase (MuSK) is sufficient for phosphorylation but not clustering of acetylcholine receptors: required role for the MuSK ectodomain? Proc. Natl. Acad. Sci. U. S. A. 1997;94:8848–8853. doi: 10.1073/pnas.94.16.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady R.M., Zhou H., Cunningham J.M., Henry M.D., Campbell K.P., Sanes J.R. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- Hall Z.W., Lubit B.W., Schwartz J.H. Cytoplasmic actin in postsynaptic structures at the neuromuscular junction. J. Cell Biol. 1981;90:789–792. doi: 10.1083/jcb.90.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R., Burden S.J. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R., Avetisova E., Burden S.J. Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development. 2002;129:5449–5460. doi: 10.1242/dev.00112. [DOI] [PubMed] [Google Scholar]
- Hesser B.A., Henschel O., Witzemann V. Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol. Cell. Neurosci. 2006;31:470–480. doi: 10.1016/j.mcn.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Jacobson C., Cote P.D., Rossi S.G., Rotundo R.L., Carbonetto S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J. Cell Biol. 2001;152:435–450. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P.S., Noble M.D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Moore C., Hashemolhosseini S., Brenner H.R. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci. 1999;19:3376–3383. doi: 10.1523/JNEUROSCI.19-09-03376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Stiegler A.L., Cameron T.O., Hallock P.T., Gomez A.M., Huang J.H., Hubbard S.R., Dustin M.L., Burden S.J. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X.C., Barzaghi P., Ruegg M.A. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep. 2004;5:183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer T.T., Misgeld T., Lichtman J.W., Sanes J.R. Nerve-independent formation of a topologically complex postsynaptic apparatus. J. Cell Biol. 2004;164:1077–1087. doi: 10.1083/jcb.200401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer T.T., Misgeld T., Sanes J.R. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Han J., Bamburg J.R., Han L., Lynn R., Zheng J.Q. Regulation of acetylcholine receptor clustering by ADF/cofilin-directed vesicular trafficking. Nat. Neurosci. 2009;12:848–856. doi: 10.1038/nn.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T., Kawajiri M., Kunishige M., Endo T., Akaike M., Aki K., Matsumoto T. Functional association between nicotinic acetylcholine receptor and sarcomeric proteins via actin and desmin filaments. J. Cell. Biochem. 2000;77:584–595. doi: 10.1002/(sici)1097-4644(20000615)77:4<584::aid-jcb6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Morgenstern J.P., Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B.L., Schuetze S.M. Development of rat soleus endplate membrane following denervation at birth. J. Neurobiol. 1987;18:101–118. doi: 10.1002/neu.480180108. [DOI] [PubMed] [Google Scholar]
- Ngo S.T., Noakes P.G., Phillips W.D. Neural agrin: a synaptic stabiliser. Int. J. Biochem. Cell Biol. 2007;39:863–867. doi: 10.1016/j.biocel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.T., Son Y.J., Sanes J.R., Lichtman J.W. Nerve terminals form but fail to mature when postsynaptic differentiation is blocked: in vivo analysis using mammalian nerve-muscle chimeras. J. Neurosci. 2000;20:6077–6086. doi: 10.1523/JNEUROSCI.20-16-06077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H., Valdez G., Jarad G., Moulson C.L., Muller U., Miner J.H., Sanes J.R. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J. Cell Biol. 2008;182:1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Inoue A., Okada M., Murata Y., Kakuta S., Jigami T., Kubo S., Shiraishi H., Eguchi K., Motomura M., Akiyama T., Iwakura Y., Higuchi O., Yamanashi Y. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science (New York, N.Y.) 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- Podleski T.R., Salpeter M.M. Acetylcholine receptor clustering and triton solubility: neural effect. J. Neurobiol. 1988;19:167–185. doi: 10.1002/neu.480190206. [DOI] [PubMed] [Google Scholar]
- Prives J., Fulton A.B., Penman S., Daniels M.P., Christian C.N. Interaction of the cytoskeletal framework with acetylcholine receptor on the surface of embryonic muscle cells in culture. J. Cell Biol. 1982;92:231–236. doi: 10.1083/jcb.92.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszynski T.J., Gingras J., Valdez G., Krzewski K., Sanes J.R. Podosomes are present in a postsynaptic apparatus and participate in its maturation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18373–18378. doi: 10.1073/pnas.0910391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich M.M., Lichtman J.W. In vivo visualization of pre- and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J. Neurosci. 1989;9:1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam G., Willmann R., Lin S., Erb-Vogtli S., Kong X.C., Ruegg M.A., Fuhrer C. Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors. J. Neurosci. 2005;25:10479–10493. doi: 10.1523/JNEUROSCI.2103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J.R., Lichtman J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sanes J.R., Marshall L.M., McMahan U.J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J. Cell Biol. 1978;78:176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Butt B., Ip F.C., Dai Y., Jiang L., Yung W.H., Greenberg M.E., Fu A.K., Ip N.Y. Ephexin1 is required for structural maturation and neurotransmission at the neuromuscular junction. Neuron. 2010;65:204–216. doi: 10.1016/j.neuron.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C.R. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev. Biol. 1982;94:11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Slater C.R. Structural factors influencing the efficacy of neuromuscular transmission. Ann. N. Y. Acad. Sci. 2008;1132:1–12. doi: 10.1196/annals.1405.003. [DOI] [PubMed] [Google Scholar]
- Stya M., Axelrod D. Mobility and detergent extractability of acetylcholine receptors on cultured rat myotubes: a correlation. J. Cell Biol. 1983;97:48–51. doi: 10.1083/jcb.97.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M.K., Lichtman J.W. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang B., Xiong W.C., Mei L. MuSK signaling at the neuromuscular junction. J. Mol. Neurosci. 2006;30:223–226. doi: 10.1385/JMN:30:1:223. [DOI] [PubMed] [Google Scholar]
- Watty A., Neubauer G., Dreger M., Zimmer M., Wilm M., Burden S.J. The in vitro and in vivo phosphotyrosine map of activated MuSK. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4585–4590. doi: 10.1073/pnas.080061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Luo S., Wang Q., Suzuki T., Xiong W.C., Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Coldefy A.S., Hubbard S.R., Burden S.J. Agrin binds to the N-terminal region of Lrp4 and stimulates association between Lrp4 and the first Ig-like domain in MuSK. J. Biol. Chem. 2011;286:40624–40630. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Glass D.J., Yancopoulos G.D., Sanes J.R. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J. Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.