Abstract
Inflammation is a hallmark in many neurodegenerative diseases like Alzheimer's disease or vascular dementia. Cholesterol and homocysteine are both vascular risk factors which have been associated with dementia, inflammation and blood–brain barrier dysfunction. In previous studies we found that hypercholesterolemia but not hyperhomocysteinemia induced inflammation in rats in vivo. The aim of the present study was to investigate the effect of a combined treatment of Sprague Dawley rats with cholesterol and homocysteine for 5 months on spatial learning and memory, blood–brain barrier integrity and inflammation. Cholesterol treated rats showed severe learning deficits, while rats treated with cholesterol and homocysteine (Mix) counteracted the cholesterol-induced inflammation and partly the cortical blood–brain barrier disruptions, although cognition was still impaired. To study the potential protective effect of homocysteine, inflammation was induced in organotypic rat brain cortex slices and primary microglial cells by treatment with different inflammatory stimuli (e.g. lipopolysaccharide or tissue plasminogen activator). Tissue plasminogen activator-induced inflammation was counteracted by homocysteine. In conclusion, our data demonstrate that homocysteine significantly ameliorates cholesterol-induced inflammation and blood–brain barrier disruption but not the memory impairment, possibly involving a tissue plasminogen activator-related mechanism.
Keywords: Alzheimer's Disease, Cholesterol, Homocysteine, Blood–brain barrier, Inflammation, Spatial memory, Sprague Dawley rats, Tissue plasminogen activator
Introduction
Inflammation is a common phenomenon in many neurodegenerative diseases (Franceschi et al., 2000). In Alzheimer's disease (AD) and vascular dementia (VaD), increased levels of inflammatory proteins in the brain and plasma have been observed even before a clinical onset of dementia (Engelhart et al., 2004). Several studies suggest that inflammation may play a role in blood–brain barrier (BBB) dysfunction and cognitive decline (for review see Gorelick, 2010). The causes for AD are not known, however besides beta-Amyloid plaques and Tau inclusions, vascular pathology and inflammation is found. It became more and more clear that vascular risk factors, such as high cholesterol or homocysteine levels may play a role in the development of AD (Humpel, 2011).
Hypercholesterolemia (HChol) and Hyperhomocysteinemia (HHcy) have both been described as risk factors for VaD and AD (Garcia and Zanibbi, 2004; Humpel and Marksteiner, 2005; Kivipelto et al., 2001; Morris, 2003; Obeid et al., 2007; Puglielli et al., 2003; Raffai and Weisgraber, 2003; Seshadri et al., 2002; Simons et al., 2001). It is well established that cholesterol induces inflammation in the brain (Lominadze et al., 2006; Papatheodorou and Weiss, 2007; Poddar et al., 2001; Rahman et al., 2005; Thirumangalakudi et al., 2008; Xue et al., 2007) and oxidized metabolites of cholesterol may take part in the upregulation of inflammatory markers (Dugas et al., 2010; Joffre et al., 2007; Lemaire-Ewing et al., 2005; Morello et al., 2009; Prunet et al., 2006; Rosklint et al., 2002; Sottero et al., 2009; Trousson et al., 2009; Vejux et al., 2008). Similarly, it has also been proposed that homocysteine (Hcy) induces inflammation, possibly by enhancing oxidative stress and subsequent nuclear factor kappa B (NfκB) activation (Papatheodorou and Weiss, 2007). However, the role of Hcy on inflammation is not fully clear as some models of HHcy do not reflect the proposed pro-inflammatory properties of Hcy (Achón et al., 2009; Pirchl et al., 2010a). In previous experiments we could show that chronic treatment of rats with cholesterol (Chol) markedly increased microglial immunoreactivity and levels of several inflammatory markers in the cortex, reduced cholinergic neurons and impaired cognition (Ullrich et al., 2010a). Furthermore, we showed that HChol rats display BBB disruptions in the cortex visualized by an increased anti-rat IgG immunoreactivity (Ullrich et al., 2010a).
In the present study we hypothesized that a combined diet of Chol and Hcy potentiates inflammation and BBB disruptions in rats in vivo. However, it was unexpected to show that Hcy counteracted cholesterol-induced inflammation.
Results
Effects of cholesterol and homocysteine on spatial learning and memory
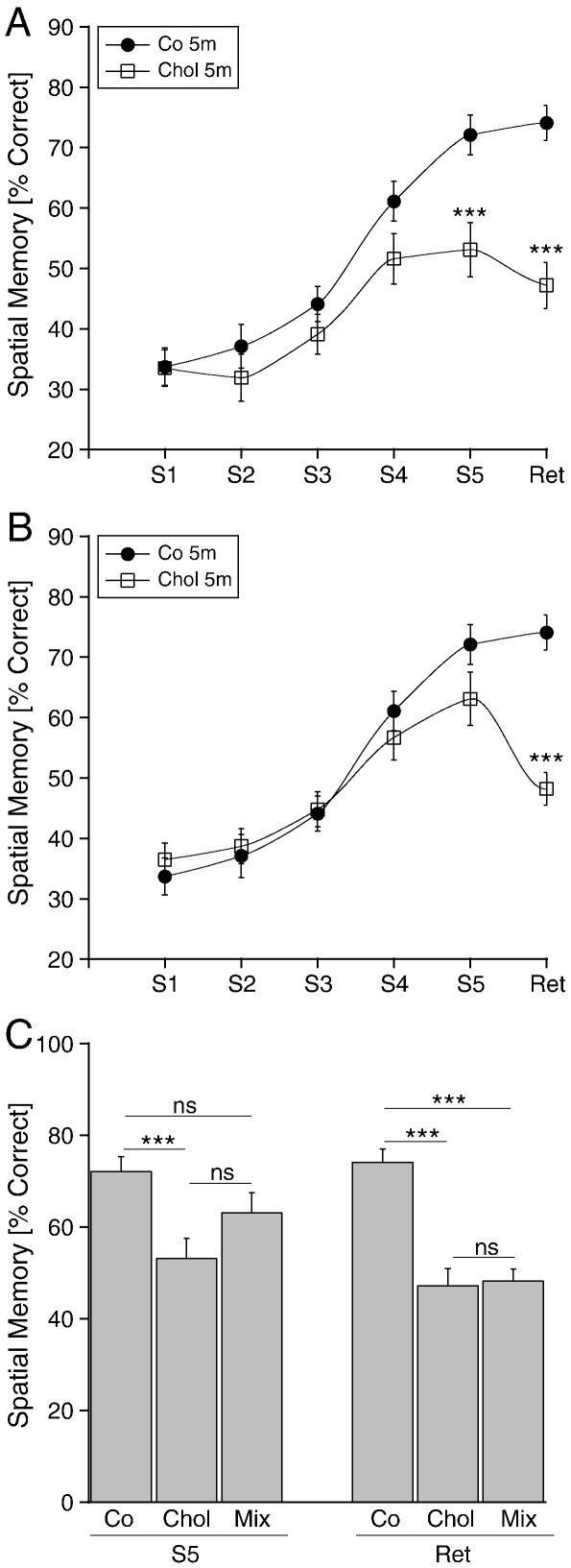
Rats treated with Chol for 5 months (Chol 5 m) showed a markedly impaired performance in the learning tasks (Fig. 1A). The percentage of correct arm choices was significantly lower at session 5 (S5) and also in the memory retention test (Ret) compared to the controls (Fig. 1A). However, the performance in the retention test (Ret) was not significantly lower compared to session 5 (S5) within the Chol group (not shown). Rats treated with Chol and Hcy for 5 months (Mix 5 m) did not show differences in the percentage of correct arm choices at sessions 1–5 (S1-S5), but spatial long term memory was significantly decreased in the memory retention test (Ret) compared to the controls (Fig. 1B), and compared to session 5 within the group (not shown). However, the spatial learning performance (S5) in the mix group (Mix 5 m) was also not significantly higher than in the cholesterol group (Chol 5 m) (Fig. 1C).
Fig. 1.
Spatial memory as tested in the 8-arm radial maze of rats treated with a diet of Chol (A) or Chol and Hcy (Mix) (B). Male Sprague Dawley rats were fed for 5 months with a special cholesterol diet (A, Chol 5 m, cholesterol, open squares, n = 10) or with cholesterol and homocysteine (B, Mix, cholesterol and homocysteine open squares, n = 10) or with normal food (A and B, Co, control; filled circles, n = 10). Spatial learning performance was assessed by testing the rats in a partially baited eight-arm radial maze on five consecutive training sessions (S1–S5), each consisting of five trials per day. Three weeks after the last training session a retention session (Ret) consisting of five trials was performed to assess long-term memory performance. To compare performance of groups at specific times a Student's t-test was performed (*** p < 0.001). C shows a comparison of all groups at session 5 (S5) and the retention test (Ret). Values are given as mean ± SEM percentage of correct arm visits (Spatial Memory [% Correct]).
Cholesterol induced anti-rat IgG and microglia in vivo
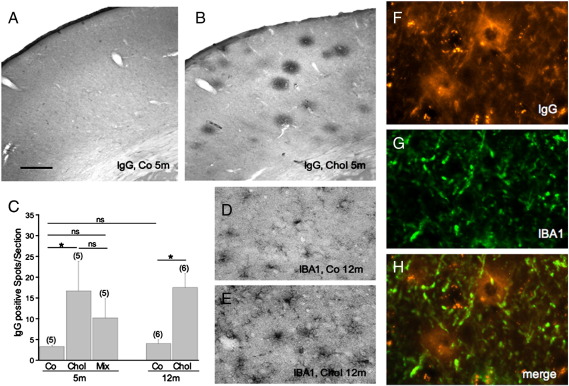
The number of anti-rat IgG-positive spots was significantly increased in hypercholesterolemic rats after 5 months (Fig. 2B) compared to the respective controls (Fig. 2A). This effect was also seen after 12 months (Fig. 2C). A slight but not significant effect was found in rats treated for 5 months with cholesterol and homocysteine (Fig. 2C). IBA1 staining revealed a weak to moderate microglial staining in control cortex (Fig. 2D), which was markedly enhanced in 12 month cholesterol-treated rats (Fig. 2E) and was also significant after 5 months (Ullrich et al., 2010a). Co-localization showed IBA1 + microglia around anti-rat IgG immunoreactive spots in 12 month old cholesterol treated rats (Fig. 2F–H).
Fig. 2.
Immunohistochemistry for anti-rat IgG (A, B, F) and microglial IBA1 (D, E, G) in the cortex of controls (A, D) and cholesterol treated rats (B, E) after 5 (A, B) or 12 (D, E) months. Fig. F–H shows co-localization of anti-rat IgG positive spots (F, Texas Red) with IBA1 + microglia (G, Alexa 488) in a merged picture (H). The number of anti-rat IgG positive spots was analyzed in the cortex of 2 sections per brain (C). The number of spots is given as mean ± SEM spots per section. The number of analyzed brains per group is given in parenthesis. Statistical analysis was performed by One Way ANOVA with a subsequent Fisher PLSD posthoc test (* p < 0.05; ns not significant). Scale bar in A = 700 μm (A, B), 90 μm (D, E) and 400 μm (F–H).
Cholesterol induced inflammation is counteracted by homocysteine in vivo
A large number of inflammatory markers were measured in the cortex by Multiplex Searchlight ELISA. Hypercholesterolemia significantly enhanced different cytokines (GM-CSF, IL-1α, IL-6, IL-10, TNFα), chemokines (MCP-1, MIP-1α, MIP-2, MIP-3α), matrix metalloproteinase-2 (MMP-2) and the growth factor PDGF-BB compared to controls (Table 1). Hyperhomocysteinemia did not enhance any of these inflammatory markers (Table 1). However Hyperhomocysteinemia counteracted the cholesterol-induced inflammation (Table 1).
Table 1.
Cholesterol-induced inflammation is counteracted by homocysteine in vivo.
| Analyte [pg/mg tissue] | Co 5 m | Chol 5 m | Hcy 5 m | Mix 5 m |
|---|---|---|---|---|
| GM-CSF | 146 ± 53 | 538 ± 16* | 312 ± 89 ns | 260 ± 32 ns |
| IL-1α | 37 ± 13 | 153 ± 61* | 85 ± 30 ns | 69 ± 8 ns |
| IL-6 | 194 ± 56 | 802 ± 205* | 538 ± 176 ns | 543 ± 49 ns |
| IL-10 | 7.6 ± 2.3 | 36 ± 6* | 21 ± 5 ns | 17 ± 3 ns |
| MCP-1 | 15 ± 4.2 | 77 ± 15*** | 38 ± 10 ns | 46 ± 4 ns |
| MIP-1α | 9.4 ± 2.3 | 18 ± 4* | 11 ± 1.7 ns | 9.4 ± 1.6 ns |
| MIP2 | 1.8 ± 0.5 | 8.3 ± 1.7** | 4.9 ± 1 ns | 3.9 ± 1.2 ns |
| MIP-3α | 51 ± 11 | 140 ± 22** | 90 ± 12 ns | 80 ± 8 ns |
| MMP2 | 264 ± 88 | 1134 ± 159*** | 660 ± 177 ns | 845 ± 95* |
| PDGF-BB | 104 ± 23 | 298 ± 64** | 198 ± 55 ns | 175 ± 15 ns |
| RANTES | 27 ± 6 | 35 ± 6 ns | 24 ± 3 ns | 43 ± 22 ns |
| TNFα | 35 ± 11 | 100 ± 18* | 60 ± 14 ns | 53 ± 7 ns |
Male Sprague Dawley Rats were fed with a special cholesterol and/or homocysteine diet for 5 months, decapitated, the brains removed and the parietal cortex dissected. Extracts were analyzed by Multiplex Searchlight ELISAs. Values are given as mean ± SEM pg/mg tissue protein (n = 5 per group). Statistical analysis was performed by One Way ANOVA with a subsequent Fisher PLSD Posthoc test (* p < 0.05; ** p < 0.01; *** p < 0.001; ns not significant). Co 5 m, control 5 months; Chol 5 m, cholesterol 5 months; Hcy 5 m, homocysteine 5 months; Mix 5 m, cholesterol and homocysteine 5 months. Proteins: GM-CSF, granulocyte macrophage colony-stimulating factor; IL-1α, interleukin-1 α; IL-6, interleukin-6; IL-10, interleukin-10; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; MIP-3α, macrophage inflammatory protein-3α; MIP2, macrophage inflammatory protein-2; MMP2, matrix metalloproteinase 2; PDGF-BB, platelet-derived growth factor-BB; RANTES, chemokine (C–C motif) ligand 5; TNFα, tumor necrosis factor-α.
Tissue plasminogen activator levels are enhanced in hypercholesterolemic rats
Total tissue plasminogen activator (tPA) levels were significantly enhanced in the plasma of rats treated for 12 months with cholesterol (Chol 12 m) compared to the controls (Table 2). Additionally, levels of active tPA in the cortex were also significantly increased in 12 months hypercholesterolemic rats (Table 2).
Table 2.
tPA is increased in plasma and cortex in Hypercholesterolemic rats.
| Co 12 m | Chol 12 m | |
|---|---|---|
| Total tPA plasma [pg/ml] | 10.71 ± 0.73 (4) | 15.54 ± 1.85 * (3) |
| Active tPA cortex [pg/mg] | 0.68 ± 0.06 (6) | 0.77 ± 0.07 * (3) |
Male Sprague Dawley Rats were fed with a special 5% cholesterol diet for 12 months, decapitated, the plasma collected, the brains removed and the parietal cortex dissected. Plasma and brain extracts were analyzed by commercial ELISAs for total and active forms of tissue plasminogen activator (tPA), respectively. The number of analyzed animals per group are given in parenthesis. Statistical analysis was performed by using student T-test (* p < 0.05). Co 12 m, control 12 months; Chol 12 m, cholesterol 12 months.
Homocysteine counteracts tPA induced inflammation in organotypic brain slices
In order to investigate the effect of homocysteine on inflammation, we used a well established organotypic brain slice model of the cortex. Lipopolysaccaride (LPS), or Polyinosinic:polycytidylic acid (Poly I:C) or tissue plasminogen activator (tPA) significantly increased the levels of MCP-1 and MIP-2 but not TNFα (Table 3). No significant changes in the levels of inflammatory markers were found when slices were treated with cholesterol (Chol), or homocysteine (Hcy), or phytohemagglutinin (PHA) (Table 3). In order to test if homocysteine can counteract inflammation, slices were co-treated with either LPS and Hcy, or tPA and Hcy (Table 3). These two agents were selected because they exhibited the most potent effect. No changes were found when slices were co-treated with LPS and Hcy compared to LPS treated slices (Table 3). However co-treatment of slices with tPA and Hcy significantly decreased the levels of all inflammatory markers compared to tPA treated slices (Table 3).
Table 3.
Inflammation in an in vitro slice model of the cortex.
| Treatment | n | MCP-1 | MIP-2 | TNFα |
|---|---|---|---|---|
| Co [pg/mg] | 33 | 1035 ± 144 | 127 ± 39 | 134 ± 22 |
| Co [%] | 33 | 100 ± 14 | 100 ± 30 | 100 ± 17 |
| Chol | 6 | 70 ± 20 ns | 63 ± 21 ns | 72 ± 19 ns |
| Poly:IC | 21 | 284 ± 23*** | 376 ± 82*** | 118 ± 27 ns |
| PHA | 11 | 61 ± 8 ns | 113 ± 22 ns | 93 ± 44 ns |
| Hcy | 24 | 140 ± 22 ns | 103 ± 26 ns | 110 ± 29 ns |
| LPS | 10 | 415 ± 36*** | 249 ± 70* | 127 ± 48 ns |
| LPS + Hcy | 16 | 551 ± 129 ns(vs LPS) | 348 ± 79 ns(vs LPS) | 58 ± 9 ns(vs LPS) |
| tPA | 14 | 410 ± 104*** | 512 ± 166*** | 147 ± 37 ns |
| tPA + Hcy | 13 | 188 ± 36***(vs tPA) | 152 ± 44***(vs tPA) | 70 ± 10*(vs tPA) |
Cortex slices were prepared from P8 rats and cultured for 2 weeks with slice medium and then stimulated for 4 days with cholesterol (Chol 2 μg/ml), polyinosinic:polycytidylic acid (Poly I:C 30 μg/ml), phytohaemagglutinin (PHA 2 μg/ml), lipopolysaccharide (LPS 1 μg/ml) or rat tissue plasminogen activator (tPA 5 μg/ml). To test the effect of homocysteine slices were incubated for 2 weeks with homocysteine (Hcy 100 μM) and then for 4 days together with LPS and or tPA (these two agents were selected because they exhibited the most potent effect). Slices were then extracted and analyzed by Multiplex Searchlight ELISAs. Values are expressed as mean ± SEM pg/mg (Co controls) or % of control. The number of slices is given as n. Statistical analysis was performed by One Way ANOVA with a subsequent Fisher PLSD Posthoc test. Comparisons were performed against controls unless other indicated (* p < 0.05; *** p < 0.001; ns not significant). MCP-1, monocyte chemotactic protein-1; MIP2, macrophage inflammatory protein-2; TNFα, tumor necrosis factor-α.
Homocysteine counteracts tPA induced inflammation in primary microglial cells
To assess if tissue plasminogen activator (tPA) stimulates microglial cells to release inflammatory markers, microglial cells were incubated with tPA and conditioned medium was collected after 4 days for Searchlight ELISA analysis. tPA significantly increased the levels of MCP-1 in the conditioned medium of primary microglial cell cultures (Table 4). However, treatment of cells with homocysteine did not induce a release of inflammatory markers (Table 4). Furthermore, co-treatment of cells with tPA and Hcy significantly counteracted the tPA induced release of MCP-1 from microglial cells (Table 4). Furthermore there was a trend that co-treatment of cells with tPA and Hcy also decreases the levels of TNFα compared to microglia treated with tPA.
Table 4.
Hcy counteracts tPA induced release of MCP1 from microglia.
| Microglia | n | MCP-1 | MIP-2 | TNFα | IL1β |
|---|---|---|---|---|---|
| Co [pg/ml] | 4 | 126 ± 13 | 1.8 ± 0.1 | 3.9 ± 1.9 | 8.6 ± 1.6 |
| Co [%] | 4 | 100 ± 11 | 100 ± 8 | 100 ± 49 | 100 ± 18 |
| Hcy | 4 | 50 ± 8 ns | 81 ± 25 ns | 58 ± 26 ns | 92 ± 33 ns |
| tPA | 4 | 340 ± 20*** | 90 ± 6 ns | 150 ± 36 ns | 90 ± 8 ns |
| tPA + Hcy | 4 | 192 ± 42***(vs tPA) | 80 ± 25 ns(vs tPA) | 45 ± 14 ns(vs tPA) | 110 ± 19 ns(vs tPA) |
Primary microglial cells were cultured for 2 weeks then stimulated for 4 days with or without rat tissue plasminogen activator (tPA 5 μg/ml) and/or homocysteine (Hcy 100 μM). Conditioned medium was then analyzed by Multiplex Searchlight ELISAs. Values are expressed as mean ± SEM pg/ml (Co controls) or % of control. The number of analyzed wells per group is given in parenthesis. Statistical analysis was performed by One Way ANOVA with a subsequent Fisher PLSD Posthoc test. Comparisons were performed against controls unless other indicated (*** p < 0.001; ns not significant). MCP-1, monocyte chemotactic protein-1; MIP2, macrophage inflammatory protein-2; TNFα, tumor necrosis factor-α, IL-1β, interleukin-1 β.
Discussion
In the present study we show that homocysteine counteracts the cholesterol-induced inflammation in vivo in rats. This effect could be mediated by a tissue plasminogen activator-linked process.
Spatial memory in vivo
Alzheimer's disease is characterized by a progressive memory loss and cognitive decline and it is suggested, that elevated cholesterol levels may increase the risk for AD (Chandra and Pandav, 1998; Kalmijn et al., 1997). It is well established that in hypercholesterolemic rats a significant decrease in spatial learning and long term memory performances is seen in the 8-arm radial maze (Granholm et al., 2008; Ullrich et al., 2010a). Similarly, hyperhomocysteinemia is also a risk factor for AD (Garcia and Zanibbi, 2004; Humpel and Marksteiner, 2005; Morris, 2003; Obeid et al., 2007; Seshadri et al., 2002) and it has been established that chronic hyperhomocysteinemia in rats induces spatial learning and long-term memory deficits (Pirchl et al., 2010a). In the present study we wanted to test if rats treated with cholesterol and homocysteine together display potential spatial memory deficits. It was unexpected to see that the combined diet did not further decline memory performance, but rather improved the spatial learning at session 5 but not in the retention test. This, points to a protective role of Hcy in hypercholesterolemic rats, which ameliorates the spatial learning performance.
Inflammation in vivo
Inflammation is a hallmark of most neurodegenerative diseases (Franceschi et al., 2000) and severe inflammatory processes have been observed in hypercholesterolemic rats (Rahman et al., 2005; Thirumangalakudi et al., 2008; Xue et al., 2007). This inflammatory process is most likely associated with activated microglia as shown after 5 months of hypercholesterolemia (Ullrich et al., 2010a) or after 12 months (this study). The mechanisms underlying the cholesterol-induced inflammation are not fully understood. Since cholesterol does not pass the BBB, it is suggested that oxygenated derivates of cholesterol (e.g. 24-OH-cholesterol) could trigger inflammation in the brain (Dugas et al., 2010; Joffre et al., 2007; Lemaire-Ewing et al., 2005; Morello et al., 2009; Prunet et al., 2006; Rosklint et al., 2002; Sottero et al., 2009; Trousson et al., 2009; Vejux et al., 2008). Hyperhomocysteinemia has often been associated with inflammation (Hofmann et al., 2001; Papatheodorou and Weiss, 2007), however, several studies, including our findings (Pirchl et al., 2010a), raise concern about the pro-inflammatory role of Hcy (Achón et al., 2009). The pro-inflammatory effects of Hcy may possibly rely on the induction of oxidative stress and subsequent activation of NFκB (Papatheodorou and Weiss, 2007). In fact, in the present study we show for the first time, that Hcy has anti-inflammatory effects and counteracts inflammation in hypercholesterolemic rats.
BBB disruptions in vivo
Considerable evidence indicates that hypercholesterolemia and derivates of cholesterol contribute to a breakdown of the BBB (Jeitner et al., 2011; Kalayci et al., 2009; Takechi et al., 2010). This is also supported by the present study, where we observed a highly increased leakage of the BBB and subsequent influx of rat-IgG into the cortex in hypercholesterolemic rats after 5 as well as 12 months. In a recent study we reported that Hcy induces similar BBB disruptions after long-term treatment (Pirchl et al., 2010a). Interestingly cholesterol and homocysteine diet for 5 months displayed slightly but not significantly lower BBB disruptions, pointing to a protective effect of homocysteine against hypercholesterolemia-induced damage.
Inflammation in an in vitro organotypic brain slice model
We have well demonstrated in our in vivo experiments that homocysteine has anti-inflammatory properties and counteracts cholesterol-induced inflammation. Thus, in order to evaluate the mechanism of this anti-inflammatory effect, we conducted further in vitro experiments. Therefore, we used a well established in vitro organotypic brain slice model (Marksteiner and Humpel, 2008; Pirchl et al., 2006). However, in the cortex slices, cholesterol did not induce inflammation, which goes in line with a previous study where we showed that cholesterol per se has a potent protective effect on cholinergic neurons in brain slices (Ullrich et al., 2010b). Similarly, we also could not induce inflammation in slices treated with Hcy. Thus, to establish a model of inflammation in vitro, we used four well established stimuli: Poly:IC, LPS, PHA or tissue plasminogen activator. In this model Poly:IC, LPS, and tPA but not PHA effectively induced an upregulation of chemokines MCP-1 and MIP-2. In order to test any protective anti-inflammatory effect of Hcy, we tested the two most potent agents (LPS and tPA) and found that Hcy only counteracted the tPA-induced inflammation in the cortex brain slices.
Role of tPA in vivo and in vitro
Tissue plasminogen activator (tPA) is a protease that cleaves zymogen plasminogen to form plasmin. tPA has numerous physiologic functions and is highly expressed in brain regions associated with learning and memory (Salles and Strickland, 2002; Seeds et al., 2003). Clinically recombinant tPA is widely used for therapy of myocardial infarction or thrombotic stroke (Collen, 1999). In an animal model of MS, tPA accumulates at sites of inflammatory damage (Teesalu et al., 2001) and tPA may contribute to blood–brain barrier breakdown (Yepes et al., 2003). In the present study, we found that hypercholesterolemia significantly increased plasma and brain tissue levels of total and active tissue plasminogen activator, respectively. Thus, our data provide evidence that vascular risk factors induce BBB disruptions in the brain, increase plasma tPA, which results in an increased influx of tPA into the brain. It is well established that tPA activates microglia (Gravanis and Tsirka, 2005; Reijerkerk et al., 2008; Rogove et al., 1999; Siao and Tsirka, 2002). Thus, in order to test if tPA can induce inflammation in microglia we cultured primary microglia and incubated the cells with recombinant tPA. In fact, we could demonstrate that tPA induced release of MCP-1, which further supports our hypothesis of a tPA-mediated inflammation via microglia.
A proposed model of Hcy-induced anti-inflammatory effects
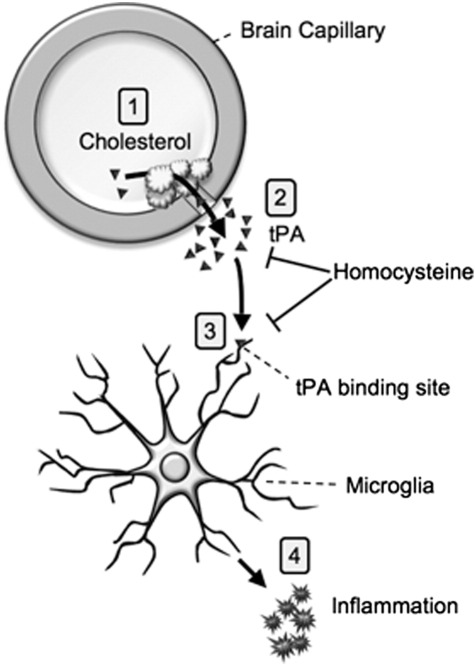
Based on our experiments, we propose that chronic mild hypercholesterolemia damages the brain capillaries and leads to BBB disruptions in the cortex. These BBB disruptions induce repair mechanisms, including activation of the blood clotting machinery. Thus, a cholesterol-induced increase of tPA in plasma may result in enhanced influx of tPA via these disrupted BBB-sites into the cortex. It is suggested that enhanced tPA in the cortex activates microglia resulting in subsequent inflammation in the brain. Indeed we have shown that hypercholesterolemia for 5 and 12 months markedly enhanced microglia in cortex (Ullrich et al., 2010a and this study). Homocysteine may exert its anti-inflammatory effect by acting on the tPA-induced process. This anti-inflammatory effect may occur at different sites. (1) Hcy may counteract cholesterol-induced damage of capillaries and reduce BBB disruptions and subsequent tPA influx. Bienvenu et al. (1993) showed that total homocysteine plasma concentrations (tHcy) in patients with thrombosis significantly correlated with tPA antigen levels and Speidl et al. (2007) found a reduced tPA activity in patients with mild HHcy. (2) Hcy may counteract the microglia-induced inflammation by blocking tPA binding sites (Siao and Tsirka, 2002). In fact, Hcy has been reported to reduce cellular binding sites for tPA on cultured endothelial cells (Hajjar, 1993; Hajjar et al., 1998). (3) Hcy may enhance plasminogen activator inhibitor-1 (PAI-1) and block the tPA effect. Indeed, Midorikawa et al. (2000) showed that homocysteine increases PAI-1 in human vascular endothelial cells (HUVEC) and smooth muscle cells (Fig. 3).
Fig. 3.
Hypothesis of Cholesterol–Homocysteine action: (1) Cholesterol increases tissue plasminogen activator (tPA) in the plasma and causes BBB disruption. (2) As a consequence tPA leaks into the brain and (3) stimulates microglial cells (4) to release inflammatory markers. Homocysteine counteracts this process by e.g. reducing tPA binding sites or enhancing plasminogen activator inhibitor-1 (PAI-1) levels.
Conclusion
Taken together our data show that Hcy has anti-inflammatory effects on cholesterol-induced inflammation in vivo. This effect may be mediated via BBB disruptions and tPA-related processes. In conclusion, the potential toxic effect of Hcy may be re-considered since high Hcy levels in the plasma may reflect anti-inflammatory responses.
Experimental methods
Controls and diets (hypercholesterolemia, hyperhomocysteinemia)
Male Sprague Dawley rats (aged 6 months) were housed at the Animal Department of the Medical University Innsbruck and had free access to food and water with a 12/12 h light–dark cycle. Animals were fed with a special diet for 5 or 12 months and randomly assigned to the following groups: group 1: controls (short-term), normal diet (n = 10) for 5 months (Co 5 m); group 2: hypercholesterolemia (short-term), diet supplemented with 5% cholesterol (n = 10) for 5 months (Chol 5 m); group 3: hyperhomocysteinemia (n = 10) for 5 months (HCy 5 m); group 4: mix, diet supplemented with 5% cholesterol and + 3 g/kg DL-homocysteine (n = 10) for 5 months (Mix 5 m), group 5: controls (long-term), normal diet (n = 12) for 12 months (Co 12 m); group 6: hypercholesterolemia (long-term), diet supplemented with 5% cholesterol (n = 9) for 12 months (Chol 12 m). The diet contains the following ingredients: 450 g/kg cornstarch, 140 g/kg casein, 155 g/kg maltodextrin, 100 g/kg sucrose, 40 g/kg soybean oil, 50 g/kg fiber, 35 g/kg mineral mix, 1.8 g/kg l-cystine, 1.4 g/kg choline chloride, 0.008 g/kg butylhydroxytoluol, 10 g/kg vitamin mix (without folic acid), 1 g/kg chocolate aroma, 0.002 g/kg folic acid (except in the Mix group) and additional 50 g/kg cholesterol (except in the Co group) and additional 3 g/kg dl-homocysteine (in the Mix group) (Ssniff special diet GmbH; Soest Germany).
Spatial memory testing in the 8-arm radial maze
Four months after start of the experiment, spatial learning and long term memory performance was assessed using a partially baited 8-arm radial maze (PanLab, Spain) as described in detail recently by us (Pirchl et al., 2010b). The maze consists of eight identical open dark Plexiglas arms with side panels and sunk-in-food cups at the end radiating from a circular platform. To facilitate spatial navigation small high contrast visual cues (triangle, vertical bars, cross and square) were placed above the doors of four arms. Before memory testing, all animals were food deprived (2 g food pellets/animal/day for 3 days) and habituated to the maze and the experimental set-up. The spatial learning performance was tested in 5 consecutive daily sessions with 5 trials per day (training). Four arms were baited with food pellets (chocolate cereals) and the trials ended when all baits were found or after 10 min exceeded. To exclude any olfactory effects additional baits were placed under the food cups of all arms (and the maze was cleaned with 70% ethanol after every trial). After 3 weeks the rats were again tested (retention) for one session (consisting of 5 trials) to assess spatial long term memory performance. The whole experiment was automatically controlled and monitored by a computer with Mazesoft Software (Version 8.1.9).
Collection of blood and brains
One day after the last retention test, animals were anesthetized by subcutaneous injection of sodium thiopental (12.5 mg/ml, Sandoz). For brain extracts, the brains were removed, the frontal cortex dissected and immediately frozen in CO2 snow (Co 5 m n = 5; Chol 5 m n = 5; HCy 5 m, n = 5; Mix 5 m n = 5; Co 12 m n = 6; Chol 12 m n = 3). From the same animals 1–2 ml blood was collected from the heart, immediately centrifuged at 2300 ×g and the plasma frozen at − 80 °C. For immunohistochemistry, rats were perfused with 4% paraformaldehyde (PFA) in PBS, the brains removed and postfixed for 90 min in 4% PFA and stored in 20% sucrose/sodium azide. Brains were then frozen in a CO2 stream and sectioned with a cryostate (Leica CM 1950) into 60 μm sections (Co 5 m n = 5; Chol 5 m n = 5; HCy 5 m, n = 5; Mix 5 m n = 5; Co 12 m n = 6; Chol 12 m n = 6).
Organotypic brain slice cultures
Organotypic rat brain slice cultures were established as described by us previously (Humpel and Weis, 2002; Weis et al., 2001). Briefly, the parietal cortex of postnatal day 8 (P8) rats was dissected under aseptic conditions. Then cortices were coronally sectioned with a tissue chopper into 400 μm slices (McIlwain, USA) which were placed on a 30 mm diameter Millicell-CM 0.4 μm membrane insert (Millipore, Austria). Slices (9 per membrane) were cultured in six-well plates (Greiner) at 37 °C and 5% CO2 with 1.2 ml/well of the following culture medium: 50% minimal essential medium (MEM)/HEPES (Gibco), 25% heat activated horse serum (Gibco/Lifetech, Austria), 6.5 mg/ml glucose (Merck), 2 mM glutamine (Merck), pH 7.2. Slices were incubated for two weeks with medium and thereafter slices were incubated for 4 days with medium with or without (Control) one of the following substances to induce inflammation: 2 μg/ml cholesterol (Chol, Sigma), or 1 μg/ml lipopolysaccharide (LPS, Sigma), or 2 μg/ml phytohaemagglutinin (PHA, Roche), or 30 μg/ml polyinosinic:polycytidylic acid (Poly I:C, Calbiochem) or 5 μg/ml rat tissue plasminogen activator (tPA, Loxo). To investigate the potential anti-inflammatory effect of homocysteine, slices were incubated with dl-homocysteine 100 μM (Sigma) for two weeks and then for 4 days with one of the before mentioned substances and additional 100 μM homocysteine.
Primary rat microglial cultures
Microglial cultures were performed as described by us in detail (Salimi and Humpel, 2002). Briefly, mixed glial cells were made from postnatal day 2–4 Sprague–Dawley rats (Himberg, Austria) by trypsinization and trituration. Cells were cultured for 7–8 days on poly-dl-ornithine-coated dishes in DMEM with 5% horse serum and 0.5% fetal calf serum (FCS). Confluent glial cultures were agitated at 180 rpm for 15 h at 37 °C and cells were seeded on poly-l-lysine-coated cell culture wells at a density of 2.5 × 104 cells per well and cultures were incubated in serum-free microglial medium (MEM-HEPES + 1 mg/ml BSA, pH 7.3) for 2 h at 37 °C to allow microglia to adhere to the wells. Plates were gently shaken for 5 min at 100 rpm, fresh medium was added and cells incubated for 2 h. Then plates were again shaken, medium discarded and the cells cultivated in fresh medium for 14 days. Thereafter, cells were treated for 4 days with or without 5 μg/ml rat tissue plasminogen activator (tPA, Loxo, Germany) and/or 100 μM DL-homocysteine (Hcy, Sigma). Finally, conditioned medium was collected for further analysis.
Evaluation of BBB disruption
Breakdown of BBB permeability was analyzed using immunohistochemistry for anti-rat IgG (Pirchl et al., 2010a; Schmidt-Kastner et al., 1993). Briefly, sections were incubated for 2 h in biotinylated rabbit anti-rat IgG (Vector, 1:400). After being washed, sections were incubated in an avidin–biotin complex solution (ABC-Elite Vectastain reagent; Vector Lab., USA) for 1 h, then washed in 50 mM Tris-buffered saline (TBS), and then the signal was detected using 0.5 mg/ml 3,3′-diaminobenzidine including 0.003% H2O2 as a substrate in TBS. The sections were mounted on glass slides, air-dried and coverslipped with Entellan (Merck, Darmstadt, Germany). Unspecific labeling was defined by omitting the primary antibody.
Measurement of inflammatory markers and tissue plasminogen activator
Inflammatory markers were analyzed using a Multiplex rat ELISA (SearchLight®, Aushon Biosystems) as described by us recently (Marksteiner et al., 2011). Standards, extracts or conditioned medium (50 μl) were added to the pre-spotted plates and incubated for 3 h at room temperature on a shaker. After washing, the plates were incubated with 50 μl of biotinylated antibody at room temperature for 30 min. Plates were again washed and 50 μl of streptavidin-HRP reagent was added to each well and incubated for 30 min. After the final washing step, 50 μl of SuperSignal® chemiluminescent substrate was added and the signal was detected with a cooled CCD camera equipped with the SearchLight® CCD imaging and analysis system. Sample values were calculated from the standard curve in a linear range. Levels of active and total rat tissue plasminogen activator (tPA) were analyzed with commercial sandwich ELISA kits (Oxford Biomedical Research, Oxford USA) as described by the manufacturer. The inflammatory markers in the 12 month old cholesterol group are not included and have been published elsewhere (Ehrlich et al., 2012). The inflammatory markers in the 15 month old homocysteine group are not included and have been published elsewhere (Pirchl et al., 2010a). Since this is a follow up experiment of our previous cholesterol study (Ullrich et al., 2010a), tPA levels could only be tested in 12 month old animals.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Salimi and Humpel, 2002). Cells were fixed for 30 min in 4% paraformaldehyde at 4 °C, washed with 0.1% Triton⁄PBS at room temperature for 30 min and pre-treated for 20 min with 5% methanol⁄1% H2O2 ⁄ PBS. Then the cells were rinsed three times for 10 min with PBS, blocked with 20% horse serum/0.2% BSA/T-PBS, and then incubated with the primary antibody against IBA1 (1:500, Wako) in 0.2% BSA/T-PBS for 2 days at 4 °C. Cells were washed and incubated with secondary biotinylated anti-goat antibody (1:200, Vector Laboratories), for 1 h at room temperature. After rinsing three times in PBS, cells were incubated in avidin–biotin complex solution (ABC; Elite Standard PK 6100, Vector Laboratories) for 1 h, then washed three times in 50 mM Tris-buffered saline (TBS), and the signal was detected using 0.5 mg/mL 3,3′diaminobenzidine (DAB) in TBS with 0.003% H2O2 as substrate. In addition, brain sections were processed for microglial IBA1 staining as described. Co-localization was performed for IBA1 using Alexa-488 (1:400, 1 h, Invitrogen) and for anti-rat IgG using Texas Red (1:500, 1 h, Amersham). Sections were mounted onto glass slides, coverslipped in Vectashield (Vector) and visualized under a fluorescence microscope.
Quantitative analysis and statistics
All quantifications were performed unbiased. Behavioral testing was statistically analyzed between groups by using student T-test and within groups using a repeated measures ANOVA. Sections were photographed under the microscope and IgG positive spots were counted in the parietal cortex of 2 sections per brain within the same area. Quantitative data are presented as mean values ± S.E.M. The significance of differences between different groups was assessed by using one way ANOVA, followed by a Fisher PLSD posthoc test or student T-test where p < 0.05 represents statistical significance. In case of multiple comparisons the α-level was adjusted by Bonferroni–Holm correction. To control for equal variances a Hartley's test (Fmax) was performed.
Acknowledgments
This study was supported by the Austrian Science Funds (P19122-B05). Michael Pirchl was partly supported by Moodinflame (Early diagnosis, treatment, and prevention of mood disorders targeting the activated inflammatory response system. FP7-Health-2007 Project no: 222963). We thank Ursula Kirzenberger-Winkler for excellent technical assistance.
References
- Achón M., Alonso-Aperte E., Varela-Moreiras G., Selhub J. Mild methionine excess does not affect thymidylate synthesis or inflammation markers expression in human aortic endothelial cells. Ann. Nutr. Metab. 2009;54:28–34. doi: 10.1159/000205317. [DOI] [PubMed] [Google Scholar]
- Bienvenu T., Ankri A., Chadefaux B., Montalescot G., Kamoun P. Elevated total plasma homocysteine, a risk factor for thrombosis. Relation to coagulation and fibrinolytic parameters. Thromb. Res. 1993;70:123–129. doi: 10.1016/0049-3848(93)90153-f. [DOI] [PubMed] [Google Scholar]
- Chandra V., Pandav R. Gene-environment interaction in Alzheimer's disease: a potential role for cholesterol. Neuroepidemiology. 1998;17:225–232. doi: 10.1159/000026175. [DOI] [PubMed] [Google Scholar]
- Collen D. The plasminogen (fibrinolytic) system. Thromb. Haemost. 1999;82:259–270. [PubMed] [Google Scholar]
- Dugas B., Charbonnier S., Baarine M., Ragot K., Delmas D., Ménétrier F., Lherminier J., Malvitte L., Khalfaoui T., Bron A., Creuzot-Garcher C., Latruffe N., Lizard G. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010;49:435–446. doi: 10.1007/s00394-010-0102-2. [DOI] [PubMed] [Google Scholar]
- Ehrlich D., Pirchl M., Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation and vascular impairment in rats. Neuroscience. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart M.J., Geerlings M.I., Meijer J., Kiliaan A., Ruitenberg A., van Swieten J.C., Stijnen T., Hofman A., Witteman J.C., Breteler M.M. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch. Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Garcia A., Zanibbi K. Homocysteine and cognitive function in elderly people. C.M.A.J. 2004;171:897–904. doi: 10.1503/cmaj.1031586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick P.B. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann. N.Y. Acad. Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- Granholm A.C., Bimonte-Nelson H.A., Moore A.B., Nelson M.E., Freeman L.R., Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008;14:133–145. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravanis I., Tsirka S.E. Tissue plasminogen activator and glial function. Glia. 2005;49:177–183. doi: 10.1002/glia.20115. [DOI] [PubMed] [Google Scholar]
- Hajjar K.A. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J. Clin. Invest. 1993;91:2873–2879. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar K.A., Mauri L., Jacovina A.T., Zhong F., Mirza U.A., Padovan J.C., Chait B.T. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J. Biol. Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- Hofmann M.A., Lalla E., Lu Y., Gleason M.R., Wolf B.M., Tanji N., Ferran L.J., Jr., Kohl B., Rao V., Kisiel W., Stern D.M., Schmidt A.M. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J. Clin. Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer's disease? Exp. Gerontol. 2011;46:225–232. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C., Marksteiner J. Cerebrovascular damage as a cause for Alzheimer's disease. Curr. Neurovasc. Res. 2005;2:341–347. doi: 10.2174/156720205774322610. [DOI] [PubMed] [Google Scholar]
- Humpel C., Weis C. Nerve growth factor and cholinergic CNS neurons studied in organotypic brain slices. Implication in Alzheimer's disease? J. Neural Transm. Suppl. 2002;62:253–263. doi: 10.1007/978-3-7091-6139-5_23. [DOI] [PubMed] [Google Scholar]
- Jeitner T.M., Voloshyna I., Reiss A.B. Oxysterol derivatives of cholesterol in neurodegenerative disorders. Curr. Med. Chem. 2011;18:1515–1525. doi: 10.2174/092986711795328445. [DOI] [PubMed] [Google Scholar]
- Joffre C., Leclère L., Buteau B., Martine L., Cabaret S., Malvitte L., Acar N., Lizard G., Bron A., Creuzot-Garcher C., Bretillon L. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 2007;32:271–280. doi: 10.1080/02713680601187951. [DOI] [PubMed] [Google Scholar]
- Kalayci R., Kaya M., Uzun H., Bilgic B., Ahishali B., Arican N., Elmas I., Küçük M. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int. J. Neurosci. 2009;119:1881–1904. doi: 10.1080/14647270802336650. [DOI] [PubMed] [Google Scholar]
- Kalmijn S., Launer L.J., Ott A., Witteman J.C., Hofman A., Breteler M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E.L., Laakso M.P., Hänninen T., Hallikainen M., Alhainen K., Soininen H., Tuomilehto J., Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. B.M.J. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessède G., Corcos L., Gambert P., Néel D., Lizard G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005;21:97–114. doi: 10.1007/s10565-005-0141-2. [DOI] [PubMed] [Google Scholar]
- Lominadze D., Roberts A.M., Tyagi N., Moshal K.S., Tyagi S.C. Homocysteine causes cerebrovascular leakage in mice. Am. J. Physiol. Heart Circ. Physiol. 2006;290:1206–1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marksteiner J., Humpel C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol. Psychiatry. 2008;13:939–952. doi: 10.1038/sj.mp.4002072. [DOI] [PubMed] [Google Scholar]
- Marksteiner J., Kemmler G., Weiss E.M., Knaus G., Ullrich C., Mechteriakov S., Oberbauer H., Auffinger S., Hinterhölzl J., Hinterhuber H., Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2011;32:539–540. doi: 10.1016/j.neurobiolaging.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa S., Sanada H., Hashimoto S., Watanabe T. Enhancement by homocysteine of plasminogen activator inhibitor-1 gene expression and secretion from vascular endothelial and smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;272:182–185. doi: 10.1006/bbrc.2000.2753. [DOI] [PubMed] [Google Scholar]
- Morello F., Saglio E., Noghero A., Schiavone D., Williams T.A., Verhovez A., Bussolino F., Veglio F., Mulatero P. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis. 2009;207:38–44. doi: 10.1016/j.atherosclerosis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Morris M.S. Homocysteine and Alzheimer's disease. Lancet Neurol. 2003;2:425–428. doi: 10.1016/s1474-4422(03)00438-1. [DOI] [PubMed] [Google Scholar]
- Obeid R., McCaddon A., Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 2007;45:1590–1606. doi: 10.1515/CCLM.2007.356. [DOI] [PubMed] [Google Scholar]
- Papatheodorou L., Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid. Redox Signal. 2007;9:1941–1958. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- Pirchl M., Marksteiner J., Humpel C. Effects of acidosis on brain capillary endothelial cells and cholinergic neurons: relevance to vascular dementia and Alzheimer's disease. Neurol. Res. 2006;28:657–664. doi: 10.1179/016164106X130371. [DOI] [PubMed] [Google Scholar]
- Pirchl M., Ullrich C., Humpel C. Differential effects of short- and long-term hyperhomocysteinaemia on cholinergic neurons, spatial memory and microbleedings in vivo in rats. Eur. J. Neurosci. 2010;32:1516–1527. doi: 10.1111/j.1460-9568.2010.07434.x. [DOI] [PubMed] [Google Scholar]
- Pirchl M., Kemmler G., Humpel C. Female Sprague Dawley rats show impaired spatial memory in the 8-arm radial maze under dim blue and red light. Int. J. Zool. 2010 http://www.hindawi.com/journals/ijz/2010/507524/ [Google Scholar]
- Poddar R., Sivasubramanian N., DiBello P.M., Robinson K., Jacobsen D.W. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- Prunet C., Montange T., Véjux A., Laubriet A., Rohmer J.F., Riedinger J.M., Athias A., Lemaire-Ewing S., Néel D., Petit J.M., Steinmetz E., Brenot R., Gambert P., Lizard G. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A. 2006;69:359–373. doi: 10.1002/cyto.a.20272. [DOI] [PubMed] [Google Scholar]
- Puglielli L., Tanzi R.E., Kovacs D.M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Raffai R.L., Weisgraber K.H. Cholesterol: from heart attacks to Alzheimer's disease. J. Lipid Res. 2003;44:1423–1430. doi: 10.1194/jlr.R300007-JLR200. [DOI] [PubMed] [Google Scholar]
- Rahman S.M., Van Dam A.M., Schultzberg M., Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. J. Neuroimmunol. 2005;169:59–67. doi: 10.1016/j.jneuroim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A., Kooij G., van der Pol S.M., Leyen T., van Het Hof B., Couraud P.O., Vivien D., Dijkstra C.D., de Vries H.E. Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J. Immunol. 2008;181:3567–3574. doi: 10.4049/jimmunol.181.5.3567. [DOI] [PubMed] [Google Scholar]
- Rogove A.D., Siao C., Keyt B., Strickland S., Tsirka S.E. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J. Cell Sci. 1999;112:4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- Rosklint T., Ohlsson B.G., Wiklund O., Norén K., Hultén L.M. Oxysterols induce interleukin-1beta production in human macrophages. Eur. J. Clin. Invest. 2002;32:35–42. doi: 10.1046/j.1365-2362.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- Salimi K., Humpel C. Down-regulation of complement receptor 3 and major histocompatibility complex I and II antigen-like immunoreactivity accompanies ramification in isolated rat microglia. Brain Res. 2002;946:283–289. doi: 10.1016/s0006-8993(02)02896-2. [DOI] [PubMed] [Google Scholar]
- Salles F.J., Strickland S. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J. Neurosci. 2002;22:2125–2134. doi: 10.1523/JNEUROSCI.22-06-02125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Meller D., Bellander B.M., Strömberg I., Olson L., Ingvar M. A one-step immunohistochemical method for detection of blood-brain barrier disturbances for immunoglobulins in lesioned rat brain with special reference to false-positive labelling in immunohistochemistry. J. Neurosci. Methods. 1993;46:121–132. doi: 10.1016/0165-0270(93)90147-j. [DOI] [PubMed] [Google Scholar]
- Seeds N.W., Basham M.E., Ferguson J.E. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J. Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D'Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Siao C.J., Tsirka S.E. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Keller P., Dichgans J., Schulz J.B. Cholesterol and Alzheimer's disease: is there a link? Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- Sottero B., Gamba P., Gargiulo S., Leonarduzzi G., Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr. Med. Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- Speidl W.S., Nikfardjam M., Niessner A., Zeiner A., Jordanova N., Zorn G., Maurer G., Schreiber W., Wojta J., Huber K. Mild hyperhomocysteinemia is associated with a decreased fibrinolytic activity in patients after ST-elevation myocardial infarction. Thromb. Res. 2007;119:331–336. doi: 10.1016/j.thromres.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Takechi R., Galloway S., Pallebage-Gamarallage M.M., Lam V., Mamo J.C. Dietary fats, cerebrovasculature integrity and Alzheimer's disease risk. Prog. Lipid Res. 2010;49:159–170. doi: 10.1016/j.plipres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Teesalu T., Hinkkanen A.E., Vaheri A. Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice. Am. J. Pathol. 2001;159:2227–2237. doi: 10.1016/S0002-9440(10)63073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L., Prakasam A., Zhang R., Bimonte-Nelson H., Sambamurti K., Kindy M.S., Bhat N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousson A., Bernard S., Petit P.X., Liere P., Pianos A., El Hadri K., Lobaccaro J.M., Ghandour M.S., Raymondjean M., Schumacher M., Massaad C. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J. Neurochem. 2009;109:945–958. doi: 10.1111/j.1471-4159.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- Ullrich C., Pirchl M., Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol. Cell. Neurosci. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich C., Pirchl M., Humpel C. Effects of cholesterol and its 24S-OH and 25-OH oxysterols on choline acetyltransferase-positive neurons in brain slices. Pharmacology. 2010;86:15–21. doi: 10.1159/000314333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejux A., Malvitte L., Lizard G. Side effects of oxysterols: cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. 2008;41:545–556. doi: 10.1590/s0100-879x2008000700001. [DOI] [PubMed] [Google Scholar]
- Weis C., Marksteiner J., Humpel C. Nerve growth factor and glial cell line-derived neurotrophic factor restore the cholinergic neuronal phenotype in organotypic brain slices of the basal nucleus of Meynert. Neuroscience. 2001;102:129–138. doi: 10.1016/s0306-4522(00)00452-8. [DOI] [PubMed] [Google Scholar]
- Xue Q.S., Sparks D.L., Streit W.J. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J. Neuroinflammation. 2007;4:20. doi: 10.1186/1742-2094-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M., Sandkvist M., Moore E.G., Bugge T.H., Strickland D.K., Lawrence D.A. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]