Abstract
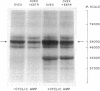
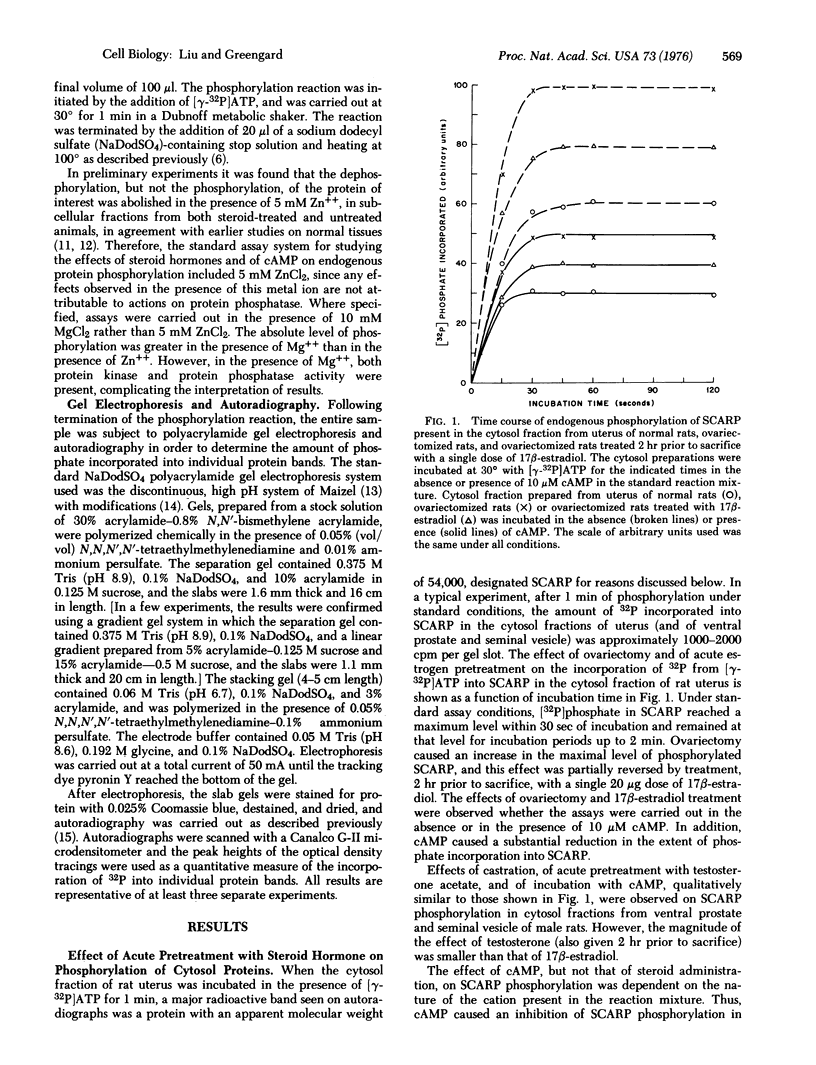
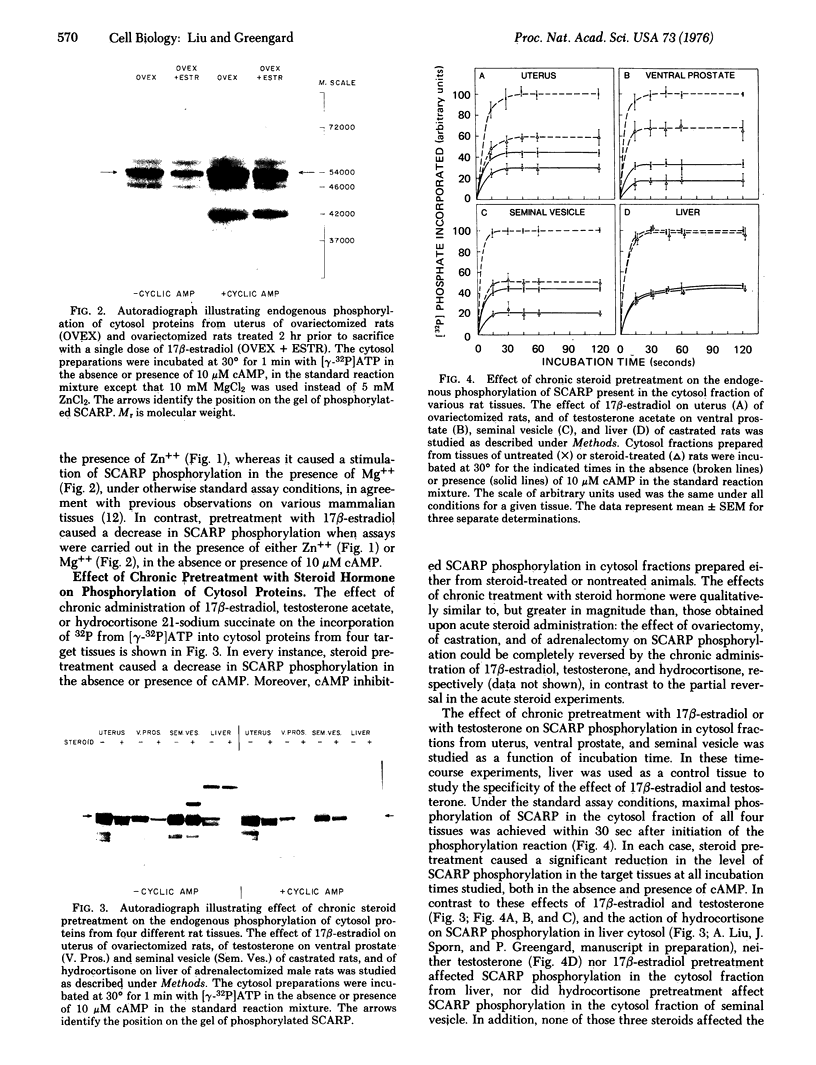
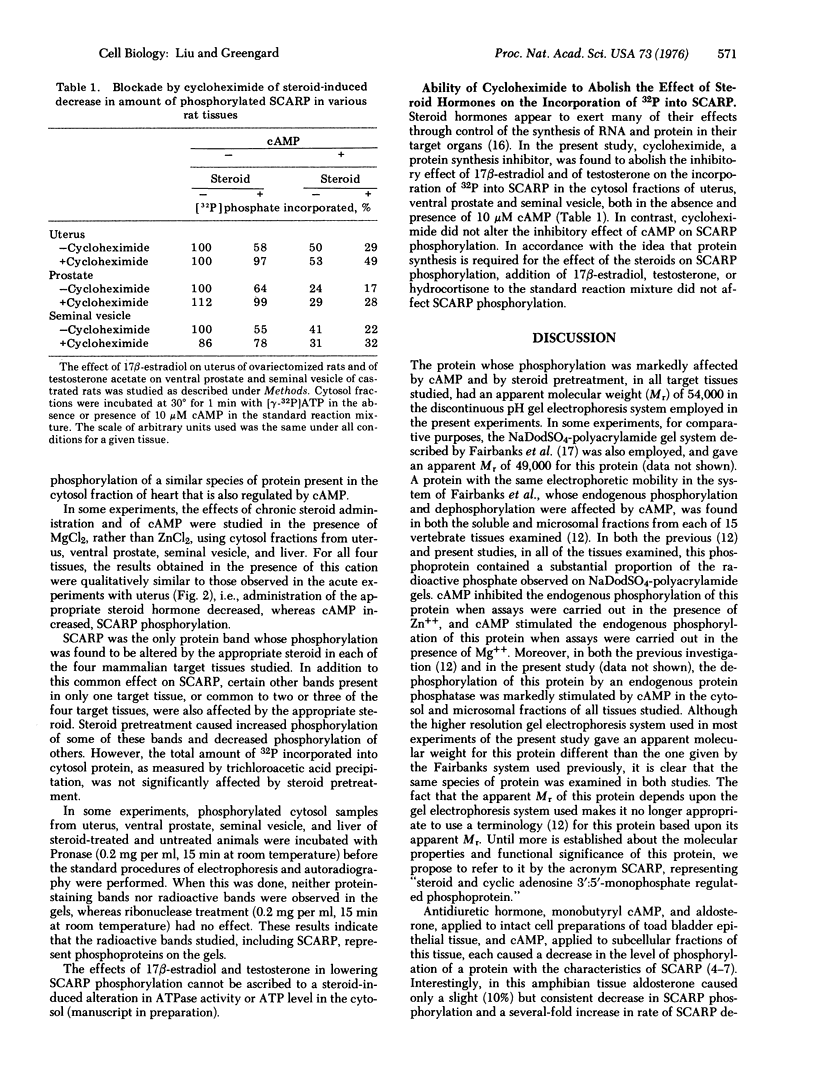
The effect of in vivo administration of steroid hormones on the endogenous phosphorylation of individual proteins in cell sap from several target tissues has been studied using the technique of discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The hormones studied (and their respective target organs) were: 17 beta-estradiol [1,3,5(10)-estratriene-3, 17beta-diol] (uterus); testosterone (17 beta-hydroxy-4-androsten-3-one) (ventral prostate and seminal vesicle)' cortisol (11beta, 17alpha, 21-trihydroxy-4-pregnene-3,20-dione) (liver); aldosterone (the 18.11-hemiacetal of 11beta,21-dihydroxy-3,20-dioxo-4-pregnen-18-al) (toad bladder). In each of the five target organs studied, pretreatment with the appropriate hormone reduced the amount of 32P incorporated from [gamma-32P]ATP into an apparently common protein band present in the cytosol fraction. The endogenous phosphorylation and dephosphorylation of this protein was also regulated by cAMP. This protein, designated SCARP (steroid and cyclic adenosine 3':5' monophosphate regulated phosphoprotein), was estimated to have an apparent molecular phoprotein), was estimated to have an apparent molecular weight of 54,000 in the gel electrophoresis system used. The effect of the steroid hormones in decreasing the phosphorylation of SCARP was specific for their respective target tissues. The effect of 17beta-estradiol and of testosterone on SCARP could be observed as early as two hours after a single dose of the steroid. A protein synthesis inhibitor, cycloheximide, abolished the effect of the steroid hormones, but not that of cAMP, on the endogenous phosphorylation of SCARP. The results suggest that steroid hormones regulate either the amount of SCARP or its ability to become phosphorylated. This regulation of a single species of protein by several types of steroid hormones in different target organs raises the possibility that this common biochemical action may be a component of the mechanism by which these steroids achieve some of their biological effects.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P. J. The physiology of the urinary bladder of amphibia. Biol Rev Camb Philos Soc. 1966 May;41(2):275–316. doi: 10.1111/j.1469-185x.1966.tb01494.x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo R. J., Greengard P. Activation by adenosine 3':5'-monophosphate of a membrane-bound phosphoprotein phosphatase from toad bladder. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1831–1835. doi: 10.1073/pnas.70.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu A. Y., Greengard P. Aldosterone-induced increase in protein phosphatase activity of toad bladder. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3869–3873. doi: 10.1073/pnas.71.10.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno H., Greengard P. Phosphoprotein phosphatases from rat cerebral cortex. Subcellular distribution and characterization. J Biol Chem. 1972 May 25;247(10):3269–3277. [PubMed] [Google Scholar]
- Maeno H., Reyes P. L., Ueda T., Rudolph S. A., Greengard P. Autophosphorylation of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. Arch Biochem Biophys. 1974 Oct;164(2):551–559. doi: 10.1016/0003-9861(74)90066-6. [DOI] [PubMed] [Google Scholar]
- Malkinson A. M., Krueger B. K., Rudolph S. A., Casnellie J. E., Haley B. E., Greengard P. Widespread occurrence of a specific protein in vertebrate tissues and regulation by cyclic AMP of its endogenous phosphorylation and dephosphorylation. Metabolism. 1975 Mar;24(3):331–341. doi: 10.1016/0026-0495(75)90114-6. [DOI] [PubMed] [Google Scholar]
- O'Malkey B. W., Woo S. L., Harris S. E., Rosen J. M., Means A. R. Steroid hormone regulation of specific messenger RNA and protein synthesis in eucaryotic cells. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):343–356. doi: 10.1002/jcp.1040850403. [DOI] [PubMed] [Google Scholar]
- Orloff J., Handler J. The role of adenosine 3',5'-phosphate in the action of antidiuretic hormone. Am J Med. 1967 May;42(5):757–768. doi: 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Lippman M. E. Mechanism of action of glucocorticoids. Metabolism. 1974 Feb;23(2):159–202. doi: 10.1016/0026-0495(74)90113-9. [DOI] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Ueda T., Rudolph S. A., Greengard P. Solubilization of a phosphoprotein and its associated cyclic AMP-dependent protein kinase and phosphoprotein phosphatase from synaptic membrane fractions, and some kinetic evidence for their existence as a complex. Arch Biochem Biophys. 1975 Oct;170(2):492–503. doi: 10.1016/0003-9861(75)90145-9. [DOI] [PubMed] [Google Scholar]
- Walton K. G., DeLorenzo R. J., Curran P. F., Greengard P. Regulation of protein phosphorylation and sodium transport in toad bladder. J Gen Physiol. 1975 Feb;65(2):153–177. doi: 10.1085/jgp.65.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]