With postnatal MR imaging as reference, retrospective consensus review of fetal MR images by pediatric neuroradiologists had sensitivity of 85% for polymicrogyria, 100% for schizencephaly, and 67% for heterotopia; specificity was 100% for all three cortical malformations when an abnormality was seen in two planes.
Abstract
Purpose:
To determine the diagnostic accuracy of fetal magnetic resonance (MR) imaging for malformations of cortical development by using postnatal MR imaging as reference standard.
Materials and Methods:
Eighty-one patients who had undergone fetal and postnatal MR imaging of the brain were identified in this institutional review board–approved, HIPAA-compliant study. Images were retrospectively reviewed in consensus by two pediatric neuroradiologists who were blinded to clinical information. Sensitivity and specificity were calculated according to retrospective review of the images and clinical reports for fetal MR images. The Fisher exact test was used to compare results for fetuses imaged before and after 24 gestational weeks and for image review versus clinical reports for fetal MR images.
Results:
Median gestational age at fetal MR imaging was 25.0 weeks (range, 19.71–38.14 weeks). Postnatal MR imaging depicted 13 cases of polymicrogyria, three cases of schizencephaly, and 15 cases of periventricular nodular heterotopia. Sensitivity and specificity of fetal MR imaging were 85% and 100%, respectively, for polymicrogyria; 100% each for schizencephaly; and 73% and 92%, respectively, for heterotopia. When heterotopia was seen in two planes, specificity was 100% and sensitivity was 67%. Sensitivity for heterotopia decreased to 44% for fetuses younger than 24 weeks. According to reports for fetal MR images, prospective sensitivity and specificity, respectively, were 85% and 99% for polymicrogyria, 100% and 99% for schizencephaly, and 40% and 91% for heterotopia.
Conclusion:
Fetal MR imaging had the highest sensitivity for polymicrogyria and schizencephaly. Specificity was 100% for all cortical malformations when the abnormality was seen in two planes. Sensitivity for heterotopia was lower for fetuses younger than 24 weeks. Knowledge of the gestational age is important, especially for counseling patients about heterotopia.
© RSNA, 2012
Introduction
Fetal magnetic resonance (MR) imaging can depict additional abnormalities when a finding is suspected at prenatal ultrasonography (US) (1–6). Although fetal MR imaging is increasingly used, its diagnostic accuracy for malformations of cortical development is not well studied. Our purpose was to determine the diagnostic accuracy of fetal MR imaging for malformations of cortical development by using postnatal MR imaging as the reference standard.
Materials and Methods
Our institutional review board approved this Health Insurance Portability and Accountability Act–compliant, retrospective study. For patients with both prenatal and postnatal imaging performed at our institution, consent was waived. For patients with postnatal imaging performed at outside institutions, consent and Health Insurance Portability and Accountability Act forms were obtained and were used to obtain postnatal MR images.
Clinical Data
From November 1996 to November 2009, we performed 693 fetal MR imaging examinations solely to assess the fetal brain. We identified 92 fetuses who subsequently underwent postnatal brain MR imaging with several methods. We searched our database of fetal MR imaging for women who had delivered at the University of California, San Francisco, Calif, and then reviewed imaging records to determine whether postnatal MR imaging had been performed. We also contacted women who had an abnormal finding at fetal brain MR imaging and who had live births at the University of California, San Francisco, or other hospitals. We also identified patients during routine clinical interpretation of postnatal MR images if the records stated that they had undergone fetal MR imaging. We also identified patients who were discussed in radiology multidisciplinary conferences and who had undergone fetal MR imaging.
Of the 92 fetuses who were identified as having undergone fetal MR imaging of the brain at the University of California, San Francisco, and then subsequent postnatal MR imaging of the brain, postnatal MR images could not be obtained for four cases; in addition, two fetuses were excluded from the study because of difficulty distinguishing the fetus from their co-twin on the fetal MR images. Of the remaining 86 cases, five were excluded after both reviewers determined that the fetal MR images were of poor quality (because of severe motion or technical reasons), leaving 81 patients to make up our study population. Of these 81 patients, 15 had undergone more than one fetal MR imaging examination, resulting in a total of 98 examinations. In particular, 13 patients had undergone two fetal MR imaging examinations, with mean gestational age at follow-up MR imaging of 27.9 weeks (range, 22.4–32.4 weeks). Two patients had undergone three fetal MR imaging examinations, with follow-up MR imaging performed between 29.3 and 34 gestational weeks.
Referral indications for fetal MR imaging were ventriculomegaly (n = 14); suspected callosal agenesis (n = 12); posterior fossa abnormality (n = 8); brain mass (n = 3); callosal agenesis, abnormal posterior fossa, and ventriculomegaly (n = 5); abnormal posterior fossa and ventriculomegaly (n = 4); callosal agenesis and abnormal posterior fossa (n = 1); cephalocele (n = 3); vein of Galen malformation (n = 3); large interhemispheric cyst (n = 1); suspected holoprosencephaly (n = 1); ventriculomegaly and increased brain echogenicity in known congenital cytomegalovirus infection (n = 1); increased periventricular white matter echogenicity in a case with congenital cystic adenomatoid malformation (n = 1); scalp mass (n = 2); fetal arthrogryposis and gastroschisis (n = 1); fetal congenital heart disease (n = 2); complication of monochorionic twinning (n = 14); family history of tuberous sclerosis in a fetus with cardiac rhabdomyoma (n = 1); family history of polymicrogyria (n = 1); family history of holoprosencephaly (n = 1); family history of congenital muscular dystrophy (n = 1); and maternal cardiac arrest (n = 1). Ten of these patients, including five with malformations of cortical development, have been described elsewhere (7–9).
Fetal and Postnatal MR Imaging
Fetal MR imaging was performed with single-shot fast spin-echo (SE) T2-weighted imaging in axial, sagittal, and coronal planes on a 1.5-T unit (GE Healthcare, Milwaukee, Wis), by using a torso phased-array coil. Of the 98 fetal MR examinations, 89 examinations included single-shot fast SE T2-weighted images of 3-mm thickness with no skip in sagittal, axial, or coronal planes; two examinations had only 4-mm-thick sections in sagittal, axial, and coronal planes; and seven examinations had a combination of 3-mm-thick and 4-mm-thick sections in sagittal, axial, and coronal planes.
Gestational age at fetal MR imaging was determined by the mother’s last menstrual period or by findings at first trimester US, if performed.
Postnatal MR sequences varied because they were performed at different institutions. All but one patient had T2-weighted axial images ranging from 3- to 5-mm thickness with a skip of 0–2 mm; one patient had 6-mm section thickness. All patients had sagittal T1-weighted images or three-dimensional gradient T1 images with sagittal reformations. Seventy-seven percent (62 of 81) of postnatal MR examinations included at least one three-dimensional T1 or T2 volumetric sequence.
MR Image Review
Fetal MR images were reviewed by two pediatric neuroradiologists with 10 years (O.A.G.) and 15 years (A.J.B.) of experience in fetal neuroimaging, which included prospective interpretation of the fetal MR images of the study patients. At the time of image review for this study, both reviewers were blinded to the patient’s name, indication for the fetal MR imaging, postnatal MR findings, and US findings; however, they were aware of the gestational age at the time of MR imaging. Both reviewers scored the images together for three categories of malformations of cortical development: polymicrogyria, schizencephaly, and periventricular nodular heterotopia (PVNH). Polymicrogyria was characterized by cortical infoldings located in abnormal positions, resulting in an appearance of too many infoldings for gestational age (Figs 1–3). Schizencephaly appeared as a transmantle cleft extending through the germinal matrix and cortex, was lined by T2 isointensity relative to the germinal matrix and cortex, and was associated with focal dilation of the underlying ventricle and adjacent polymicrogyria (Fig 4). PVNH was characterized by a focal round or ovoid nodule that had signal intensity similar to the intensity of the adjacent germinal matrix but that focally protruded into the ventricle, resulting in irregularity of the ventricular wall (Fig 2). In addition to these three categories, any other brain abnormalities were also recorded.
Figure 1a:
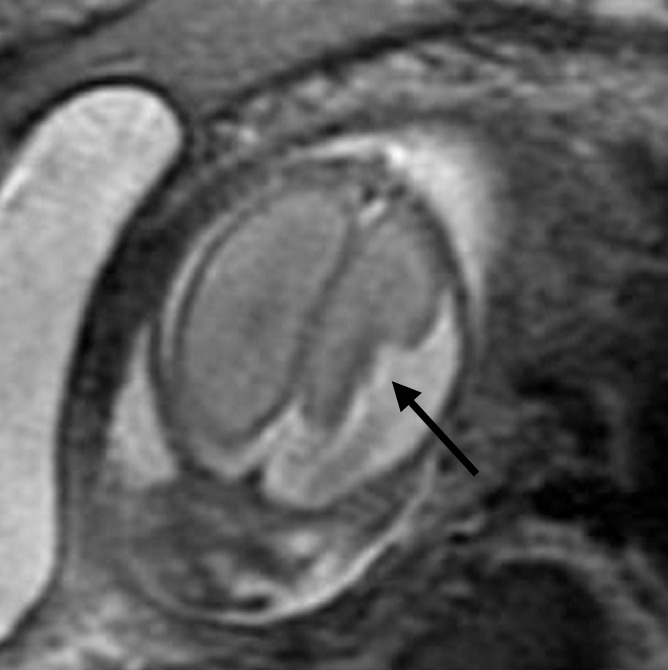
Focal polymicrogyria detected with fetal MR imaging at 23 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates a large area of encephalomalacia involving the left frontal and parietal lobes (arrow). (b) Fetal coronal single-shot fast SE T2-weighted image demonstrates abnormal cortical infoldings in the left frontal lobe in the area of encephalomalacia, consistent with polymicrogyria (arrow). (Reprinted, with permission, from reference 21.) (c) Postnatal axial T2-weighted image demonstrates volume loss and polymicrogyria involving the left frontal and parietal lobes (arrow).
Figure 3a:
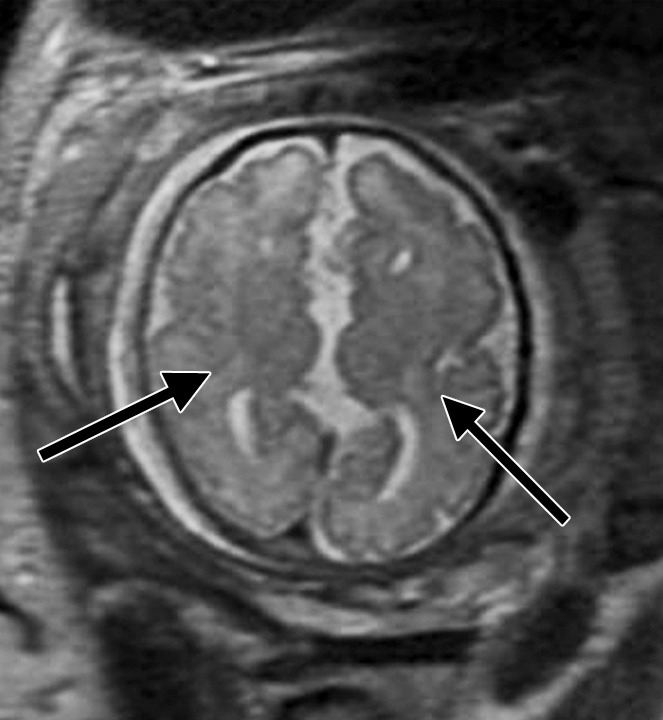
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 4a:
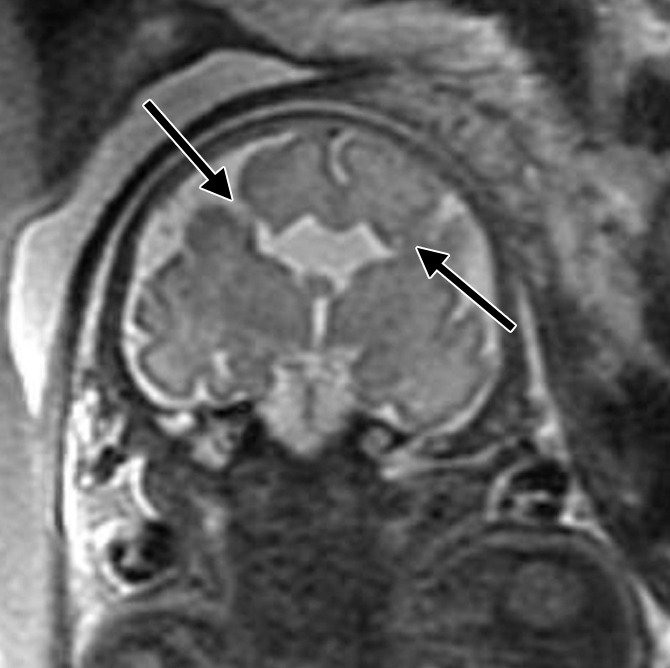
Bifrontal open-lip schizencephalies detected with fetal MR imaging at 33 gestational weeks. (a) Fetal coronal single-shot fast SE T2-weighted image demonstrates bilateral clefts (arrows) in the parenchyma that extend from the ventricular lumen to the subarachnoid space and are lined by signal intensity similar to that of the developing cortex. Findings are compatible with open-lip schizencephalic defects. Septal leaves are absent. (b) Corresponding coronal, three-dimensional, spoiled gradient-recalled acquisition in the steady state, T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows). (c) Fetal axial single-shot fast SE T2-weighted image also demonstrates the bilateral open-lip schizencephalic defects (arrows), as well as abnormal infoldings in the adjacent cortex. (d) Corresponding axial T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows), as well as adjacent polymicrogyria.
Figure 2a:
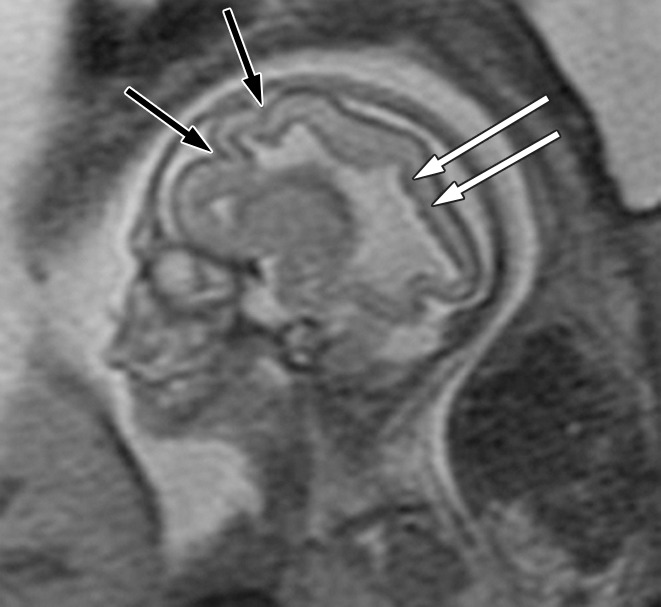
Polymicrogyria and PVNH detected with fetal MR imaging at 22 gestational weeks. (a) Sagittal single-shot fast SE T2-weighted image at 22 gestational weeks demonstrates multiple abnormal infoldings of the cortex, consistent with polymicrogyria (black arrows). PVNH appears as nodular areas along the wall of the lateral ventricular atrium, which protrude into the ventricular lumen (white arrows). (Reprinted, with permission, from reference 9.) (b) Postnatal axial fast spin-echo T2-weighted image at 2 days of age demonstrates bilateral frontal polymicrogyria, confirming fetal MR findings. (c) Fetal axial single-shot fast SE T2-weighted image demonstrates nodular area in the wall of the left lateral ventricle, which is isointense to germinal matrix and protrudes slightly into the ventricular lumen, and which was confirmed in the coronal plane, consistent with PVNH (arrows). (d) Postnatal axial spin-echo T2-weighted image at the level of the ventricular atria confirms the PVNH (arrow).
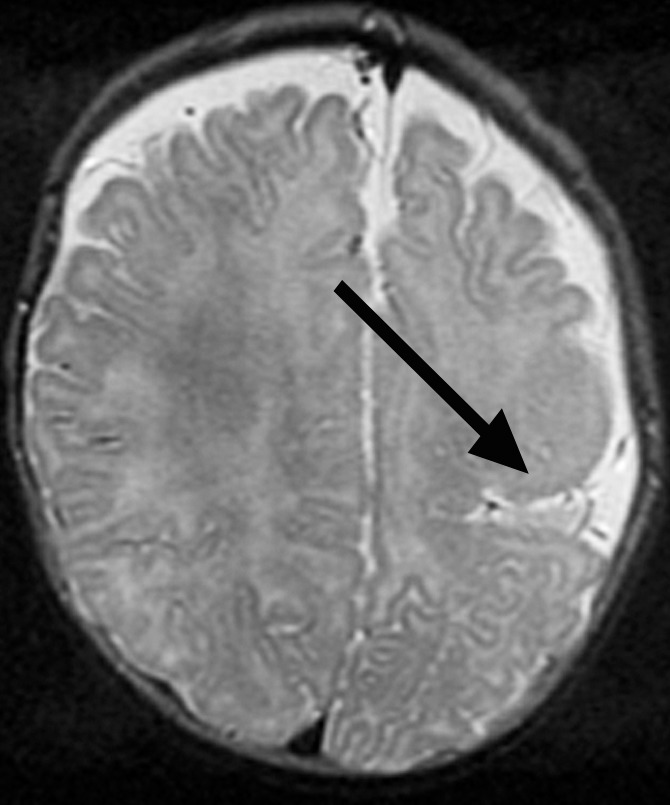
Figure 1b:
Focal polymicrogyria detected with fetal MR imaging at 23 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates a large area of encephalomalacia involving the left frontal and parietal lobes (arrow). (b) Fetal coronal single-shot fast SE T2-weighted image demonstrates abnormal cortical infoldings in the left frontal lobe in the area of encephalomalacia, consistent with polymicrogyria (arrow). (Reprinted, with permission, from reference 21.) (c) Postnatal axial T2-weighted image demonstrates volume loss and polymicrogyria involving the left frontal and parietal lobes (arrow).
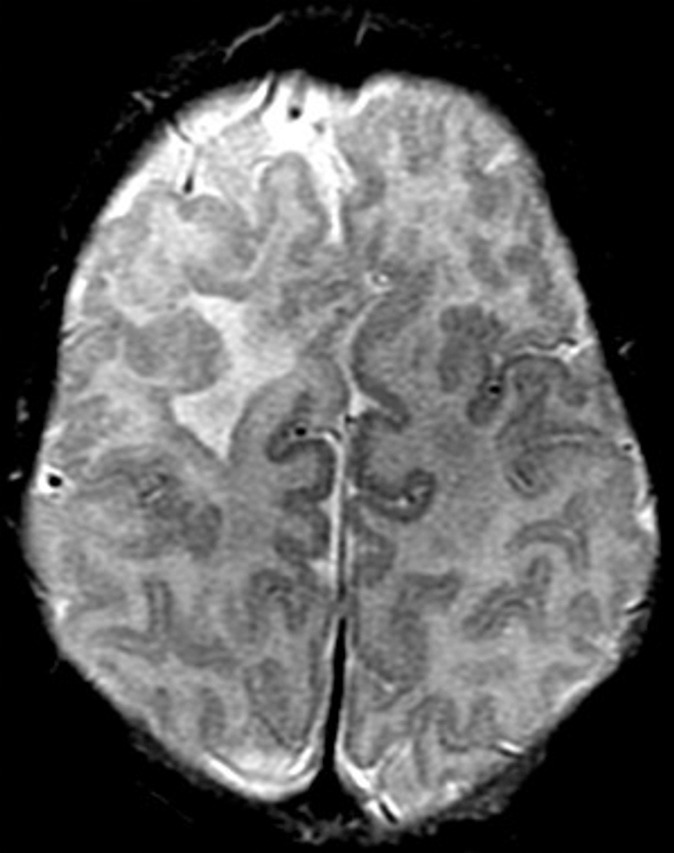
Figure 1c:
Focal polymicrogyria detected with fetal MR imaging at 23 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates a large area of encephalomalacia involving the left frontal and parietal lobes (arrow). (b) Fetal coronal single-shot fast SE T2-weighted image demonstrates abnormal cortical infoldings in the left frontal lobe in the area of encephalomalacia, consistent with polymicrogyria (arrow). (Reprinted, with permission, from reference 21.) (c) Postnatal axial T2-weighted image demonstrates volume loss and polymicrogyria involving the left frontal and parietal lobes (arrow).
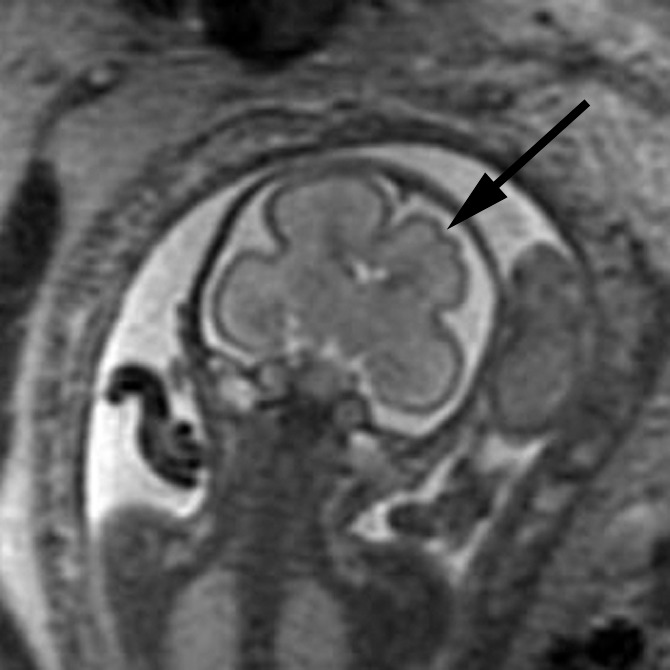
Figure 2b:
Polymicrogyria and PVNH detected with fetal MR imaging at 22 gestational weeks. (a) Sagittal single-shot fast SE T2-weighted image at 22 gestational weeks demonstrates multiple abnormal infoldings of the cortex, consistent with polymicrogyria (black arrows). PVNH appears as nodular areas along the wall of the lateral ventricular atrium, which protrude into the ventricular lumen (white arrows). (Reprinted, with permission, from reference 9.) (b) Postnatal axial fast spin-echo T2-weighted image at 2 days of age demonstrates bilateral frontal polymicrogyria, confirming fetal MR findings. (c) Fetal axial single-shot fast SE T2-weighted image demonstrates nodular area in the wall of the left lateral ventricle, which is isointense to germinal matrix and protrudes slightly into the ventricular lumen, and which was confirmed in the coronal plane, consistent with PVNH (arrows). (d) Postnatal axial spin-echo T2-weighted image at the level of the ventricular atria confirms the PVNH (arrow).
Figure 2c:
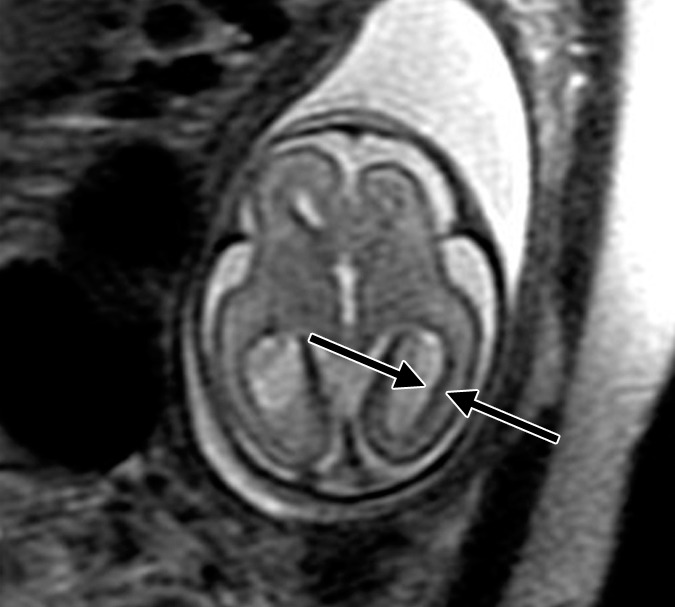
Polymicrogyria and PVNH detected with fetal MR imaging at 22 gestational weeks. (a) Sagittal single-shot fast SE T2-weighted image at 22 gestational weeks demonstrates multiple abnormal infoldings of the cortex, consistent with polymicrogyria (black arrows). PVNH appears as nodular areas along the wall of the lateral ventricular atrium, which protrude into the ventricular lumen (white arrows). (Reprinted, with permission, from reference 9.) (b) Postnatal axial fast spin-echo T2-weighted image at 2 days of age demonstrates bilateral frontal polymicrogyria, confirming fetal MR findings. (c) Fetal axial single-shot fast SE T2-weighted image demonstrates nodular area in the wall of the left lateral ventricle, which is isointense to germinal matrix and protrudes slightly into the ventricular lumen, and which was confirmed in the coronal plane, consistent with PVNH (arrows). (d) Postnatal axial spin-echo T2-weighted image at the level of the ventricular atria confirms the PVNH (arrow).
Figure 2d:
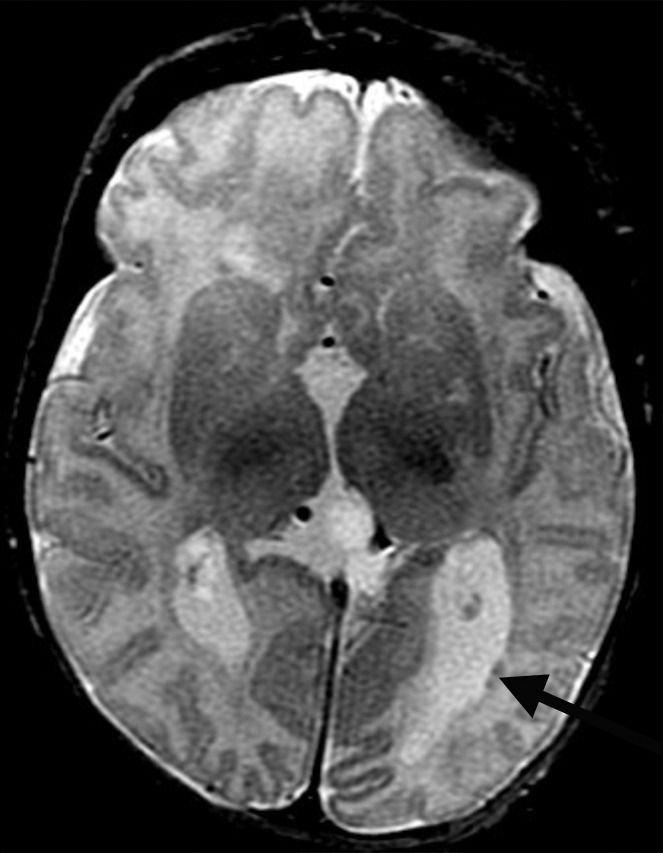
Polymicrogyria and PVNH detected with fetal MR imaging at 22 gestational weeks. (a) Sagittal single-shot fast SE T2-weighted image at 22 gestational weeks demonstrates multiple abnormal infoldings of the cortex, consistent with polymicrogyria (black arrows). PVNH appears as nodular areas along the wall of the lateral ventricular atrium, which protrude into the ventricular lumen (white arrows). (Reprinted, with permission, from reference 9.) (b) Postnatal axial fast spin-echo T2-weighted image at 2 days of age demonstrates bilateral frontal polymicrogyria, confirming fetal MR findings. (c) Fetal axial single-shot fast SE T2-weighted image demonstrates nodular area in the wall of the left lateral ventricle, which is isointense to germinal matrix and protrudes slightly into the ventricular lumen, and which was confirmed in the coronal plane, consistent with PVNH (arrows). (d) Postnatal axial spin-echo T2-weighted image at the level of the ventricular atria confirms the PVNH (arrow).
Figure 3b:
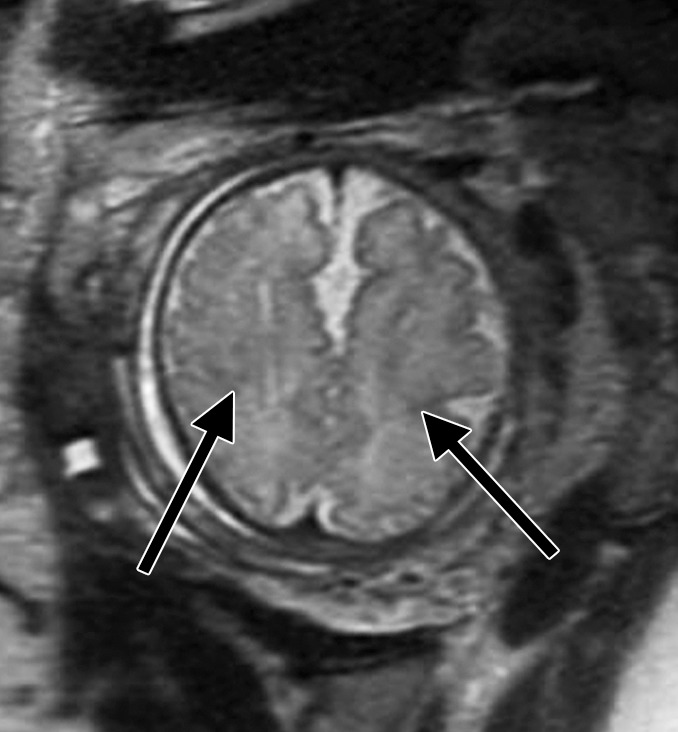
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 3c:
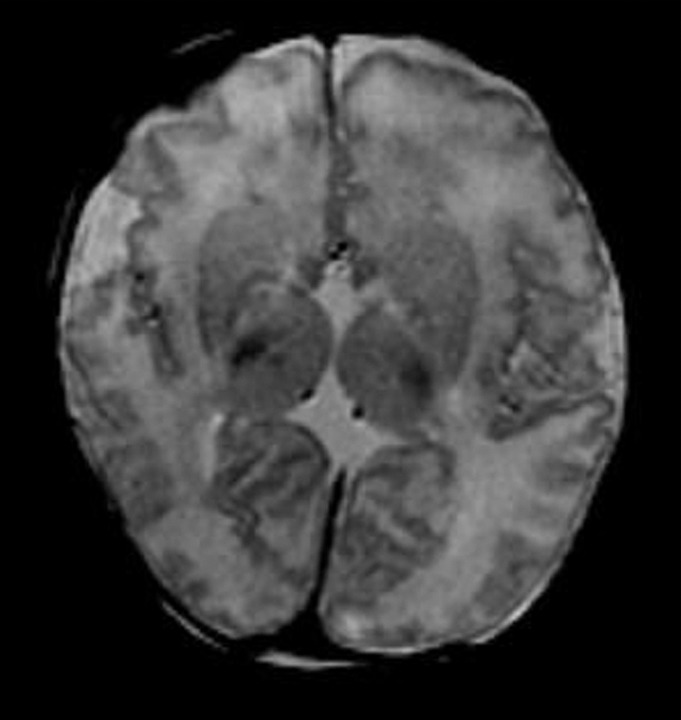
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 3d:
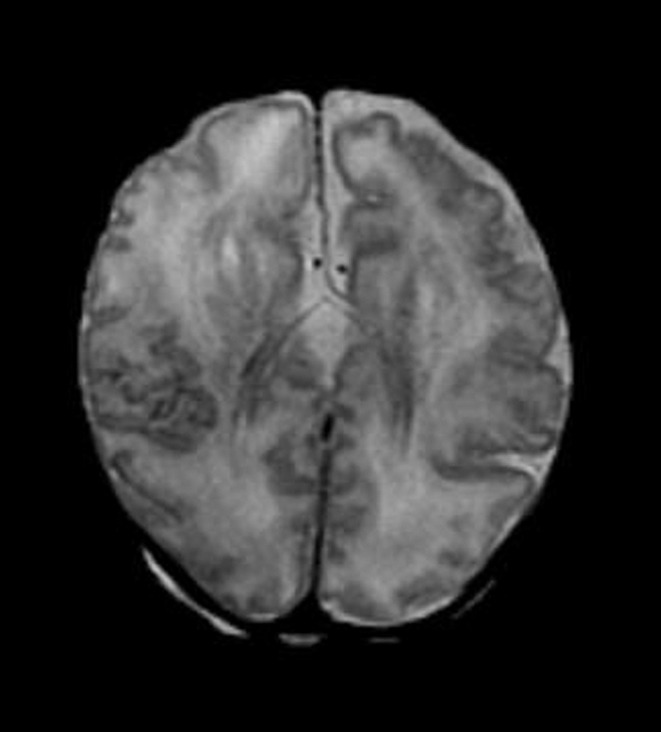
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 3e:
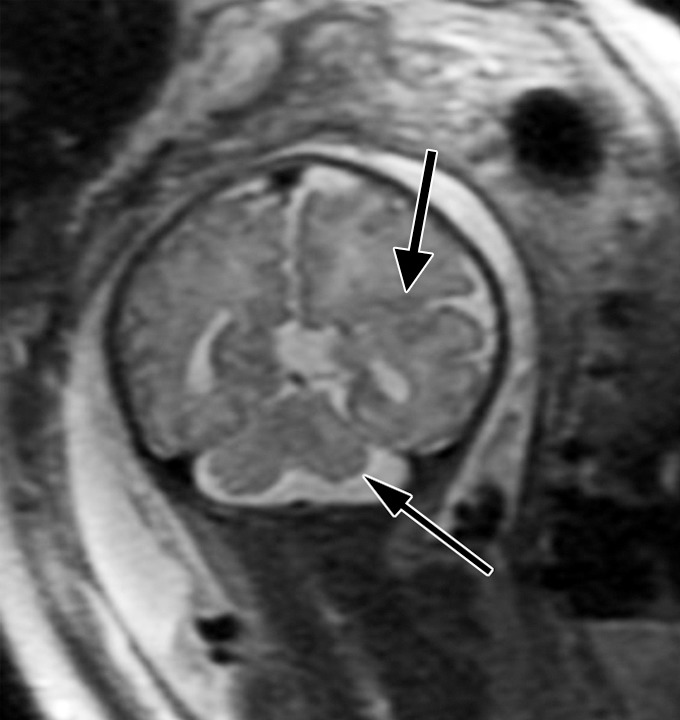
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 3f:
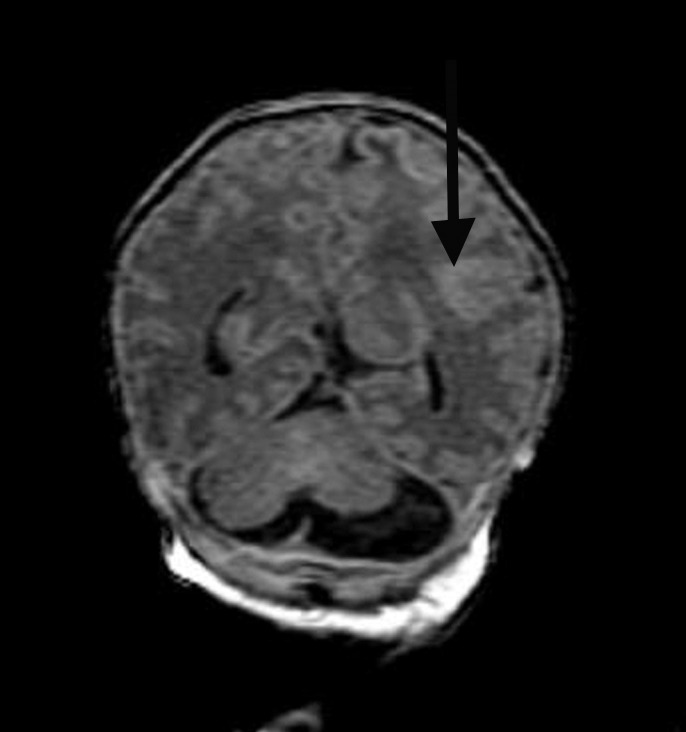
Diffuse polymicrogyria detected with fetal MR imaging at 36 gestational weeks. (a) Fetal axial single-shot fast SE T2-weighted image demonstrates bilateral abnormal cortical folding pattern characterized by too numerous sulci with abnormally small gyri, consistent with diffuse polymicrogyria. Both sylvian fissures extend too far posteriorly, which is often seen with polymicrogyria affecting the perisylvian areas (arrows). There is also callosal agenesis. (b) Axial single-shot fast SE T2-weighted image more superiorly again demonstrates diffuse polymicrogyria with abnormal posterior extent of the sylvian fissures (arrows). (c) Postnatal SE axial T2-weighted image corresponding to the same location as in a demonstrates diffuse polymicrogyria. (d) Postnatal SE axial T2-weighted image corresponding to b demonstrates abnormal posterior extent of the sylvian fissures bilaterally with diffuse polymicrogyria. (e) Fetal coronal single-shot fast SE T2-weighted image shows multiple abnormal infoldings, most prominent in the posterior left sylvian fissure (top arrow). Left cerebellar hypoplasia is also noted (bottom arrow). (Reprinted, with permission, from reference 9.) (f) Corresponding postnatal coronal three-dimensional spoiled gradient-recalled acquisition in the steady state T1-weighted image confirms numerous abnormal cortical infoldings, with apparent thickening of posterior left sylvian fissure due to multiple tiny cortical infoldings (arrow).
Figure 4b:
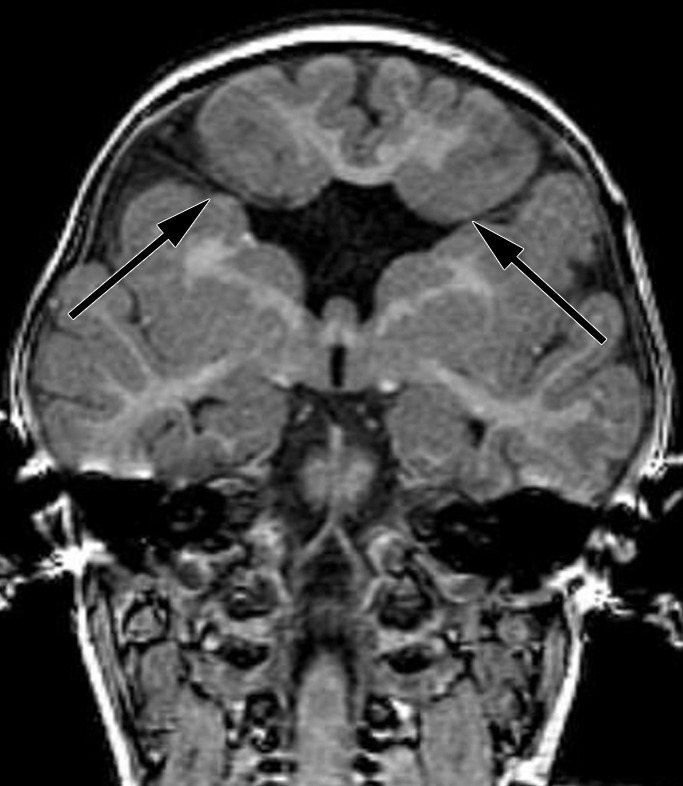
Bifrontal open-lip schizencephalies detected with fetal MR imaging at 33 gestational weeks. (a) Fetal coronal single-shot fast SE T2-weighted image demonstrates bilateral clefts (arrows) in the parenchyma that extend from the ventricular lumen to the subarachnoid space and are lined by signal intensity similar to that of the developing cortex. Findings are compatible with open-lip schizencephalic defects. Septal leaves are absent. (b) Corresponding coronal, three-dimensional, spoiled gradient-recalled acquisition in the steady state, T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows). (c) Fetal axial single-shot fast SE T2-weighted image also demonstrates the bilateral open-lip schizencephalic defects (arrows), as well as abnormal infoldings in the adjacent cortex. (d) Corresponding axial T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows), as well as adjacent polymicrogyria.
Figure 4c:
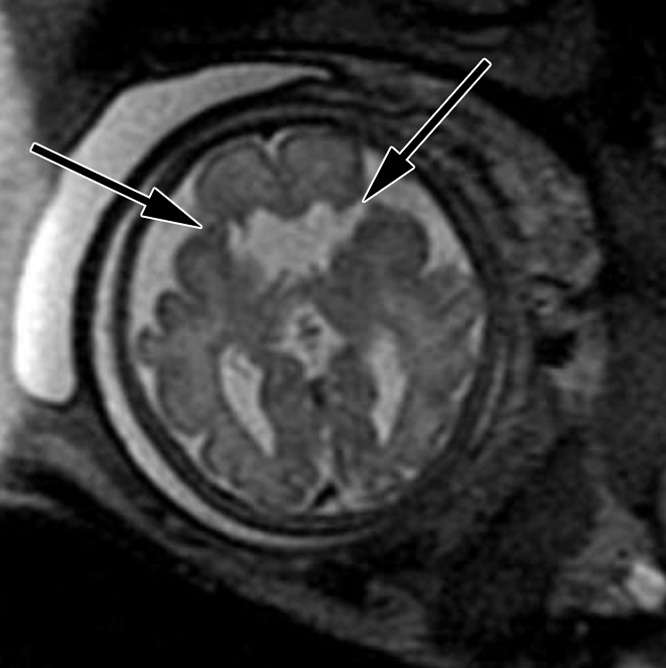
Bifrontal open-lip schizencephalies detected with fetal MR imaging at 33 gestational weeks. (a) Fetal coronal single-shot fast SE T2-weighted image demonstrates bilateral clefts (arrows) in the parenchyma that extend from the ventricular lumen to the subarachnoid space and are lined by signal intensity similar to that of the developing cortex. Findings are compatible with open-lip schizencephalic defects. Septal leaves are absent. (b) Corresponding coronal, three-dimensional, spoiled gradient-recalled acquisition in the steady state, T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows). (c) Fetal axial single-shot fast SE T2-weighted image also demonstrates the bilateral open-lip schizencephalic defects (arrows), as well as abnormal infoldings in the adjacent cortex. (d) Corresponding axial T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows), as well as adjacent polymicrogyria.
Figure 4d:
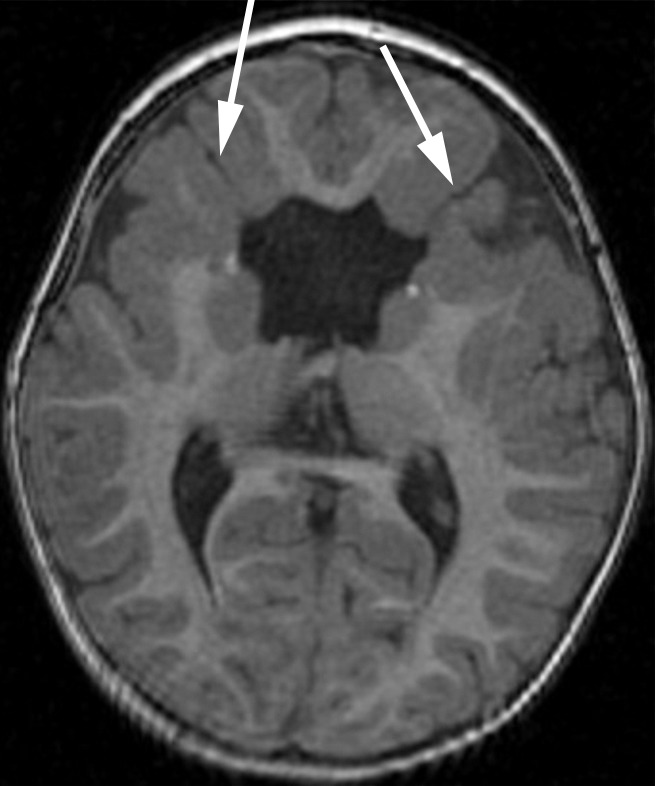
Bifrontal open-lip schizencephalies detected with fetal MR imaging at 33 gestational weeks. (a) Fetal coronal single-shot fast SE T2-weighted image demonstrates bilateral clefts (arrows) in the parenchyma that extend from the ventricular lumen to the subarachnoid space and are lined by signal intensity similar to that of the developing cortex. Findings are compatible with open-lip schizencephalic defects. Septal leaves are absent. (b) Corresponding coronal, three-dimensional, spoiled gradient-recalled acquisition in the steady state, T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows). (c) Fetal axial single-shot fast SE T2-weighted image also demonstrates the bilateral open-lip schizencephalic defects (arrows), as well as abnormal infoldings in the adjacent cortex. (d) Corresponding axial T1-weighted image obtained at 1 year of age confirms the bilateral open-lip schizencephalies (arrows), as well as adjacent polymicrogyria.
Images were scored as normal, abnormal, or unable to be assessed for each category according to consensus review. A category was scored as abnormal at fetal MR imaging only when the abnormality was seen in at least two planes; it was scored as suspicious at fetal MR imaging when the abnormality was seen only in one plane and could not be confirmed in another plane. A category was scored as unable to be assessed if it could not be well evaluated on the images. In particular, if image quality was thought to be inadequate for assessing for PVNH at fetal MR imaging, then the PVNH category was scored as unable to be assessed; these cases were excluded only from the PVNH analysis. Postnatal MR images were scored in a similar manner, with both reviewers blinded to clinical and prior imaging results. When more than one postnatal MR imaging examination was performed, the initial postnatal examination was used for this study. Postnatal MR images were always reviewed at least 2 weeks after review of the fetal MR images.
Clinical Report Review
We also reviewed the clinical reports for fetal MR images and recorded whether the diagnosis of polymicrogyria, PVNH, or schizencephaly was made prospectively at the time of the clinical interpretation of the fetal MR image. Of the 693 fetal MR imaging examinations performed during this 13-year period, 7.9% of patients (55 of 693) had polymicrogyria, PVNH, or schizencephaly mentioned in the clinical report of fetal MR imaging. Termination or spontaneous intrauterine death occurred in 45% (25 of 55) of cases, and 4% of cases were lost to follow-up (two of 55). In 51% (28 of 55) of cases, a live birth occurred. For 71% (20 of 28) of the live births, a postnatal MR image was available; all these patients were included in our study. Thus, a total of 36% (20 of 55) of cases with cortical malformation on the fetal MR imaging report were included in our study.
Statistical Analysis
With postnatal MR imaging used as the reference standard, we calculated sensitivity and specificity for each category and 95% binomial confidence intervals (CIs) on the basis of the blinded, retrospective review of the fetal MR images and the prospective clinical reports of fetal MR imaging. If a patient had undergone more than one fetal MR imaging examination, we used the earliest fetal MR image for all our analyses. We compared sensitivity of fetal MR imaging for patients in whom MR imaging was performed before 24 gestational weeks with that for patients imaged at or after 24 gestational weeks; the Fisher exact test was used for this comparison, with a significance level of P < .05.
Results
Eighty-one fetuses were included in this study. Median gestational age at fetal MR imaging was 25.0 weeks (range, 19.71–38.14 weeks). Fetal MR imaging was performed before 24 weeks in 37 patients. Age at postnatal MR imaging ranged from 0 days to 4.7 years, with a median postnatal age (corrected for delivery age < 37 weeks) of 3 days.
Thirteen postnatal examinations showed polymicrogyria, and 11 of these cases were correctly identified at fetal MR image review (Table 1). Gestational age for cases detected at fetal MR image review (29 weeks [range, 22–38.1 weeks]) did not significantly differ from that for cases missed at fetal MR image review (24.2 weeks [range, 23.4–25 weeks]) (P = .26), but this comparison is limited by small sample size. The sensitivity of fetal MR imaging for polymicrogyria was 85% (11 of 13) (95% CI: 55%, 98%). No cases were scored as suspicious for polymicrogyria at fetal MR image review. There were no false-positive cases, with a resultant specificity of 100% (68 of 68) (95% CI: 95%, 100%). Considering only cases for which fetal MR imaging was performed before 24 gestational weeks, the sensitivity of fetal MR imaging was 75% (three of four) (95% CI: 19%, 99%), with 100% (33 of 33) specificity (95% CI: 89%, 100%). Considering only cases for which fetal MR imaging was performed at or after 24 gestational weeks, fetal MR imaging had a sensitivity of 89% (eight of nine) (95% CI: 52%, 100%) and a specificity of 100% (35 of 35) (95% CI: 90%, 100%). There was no significant difference in sensitivity when fetal MR imaging was performed before 24 gestational weeks and when fetal MR imaging was performed at or after 24 gestational weeks (P = .41).
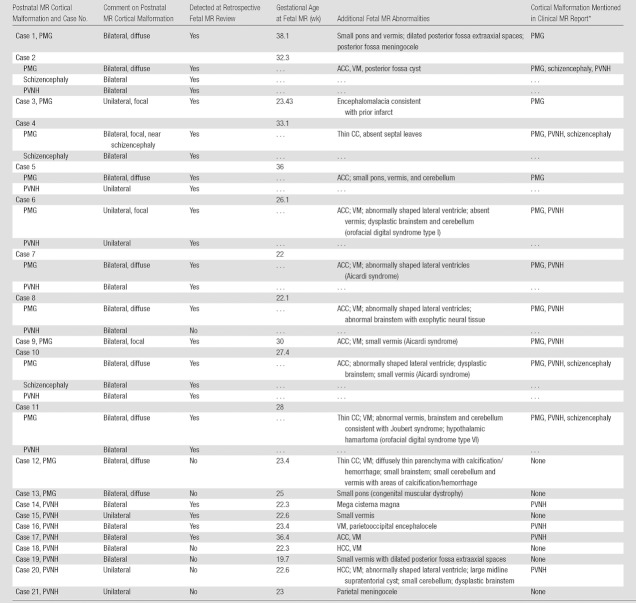
Table 1.
Cases with Malformation of Cortical Development at Postnatal MR Imaging
Note.— ACC = agenesis of the corpus callosum, CC = corpus callosum, HCC = hypogenesis of the corpus callosum, PMG = polymicrogyria, PVNH = periventricular nodular heterotopia, VM = ventriculomegaly.
Includes suspicion for malformation. Information in this column refers to detection of PMG, schizencephaly, or PVNH according to the clinical fetal MR imaging report, which was generated at the time of the initial prospective clinical interpretation of the image.
In nine of 13 cases (69%), the polymicrogyria was bilateral and diffuse (Table 1). Additional brain abnormalities were seen in all cases of polymicrogyria, most commonly involving the corpus callosum (Table 1). Specific syndromes were diagnosed prenatally or postnatally, or both, in five cases (Table 1).
Two cases of polymicrogyria were diffuse and bilateral but were not detected at fetal MR image review. In both cases, fetal MR imaging included at least two series in each plane with 3-mm section thickness and no skip. In one case of cytomegalovirus infection, fetal MR imaging at 23.4 weeks demonstrated shallow sylvian fissures and parietooccipital sulci with diffuse parenchymal destruction, ventriculomegaly, and small brainstem; follow-up fetal MR imaging at 28 weeks showed diffuse bilateral polymicrogyria. In one case with a family history of congenital muscular dystrophy, fetal MR imaging performed at 25 weeks demonstrated a small pons with normal sulcation. A second fetal MR imaging examination at 30 weeks depicted polymicrogyria in the bilateral temporal lobes, as well as the shallow appearance of the frontal sulci (Fig 5). A third image obtained at 34 gestational weeks demonstrated bilateral frontal and temporal polymicrogyria with more subtle polymicrogyria throughout the brain.
Figure 5a:
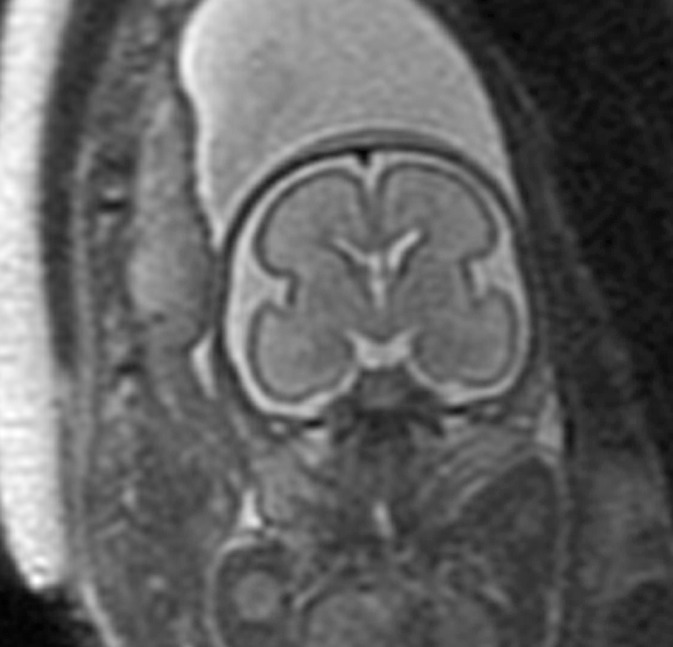
Polymicrogyria detected with fetal MR imaging at 30 gestational weeks. (a) Coronal single-shot fast SE T2-weighted image in a 25-week-old fetus demonstrates normal sulcation pattern without any evidence of developing polymicrogyria. (b) Coronal single-shot fast SE T2-weighted image in the same fetus at 30 gestational weeks demonstrates shallow appearance of the frontal sulci (arrows). (c) Axial single-shot fast SE T2-weighted image at 30 gestational weeks also demonstrates abnormal infoldings of the temporal cortex bilaterally, consistent with polymicrogyria (arrows). (d) Postnatal coronal three-dimensional fast SE T2-weighted image obtained at 3 months of age confirms the findings from 30-week fetal MR image with bifrontal polymicrogyria. Abnormal T2 hyperintensity in the white matter is also noted (and was not detected with fetal MR imaging). (e) Postnatal axial SE T2-weighted image also demonstrates bilateral temporal lobe polymicrogyria, as detected with fetal MR imaging.
Figure 5b:
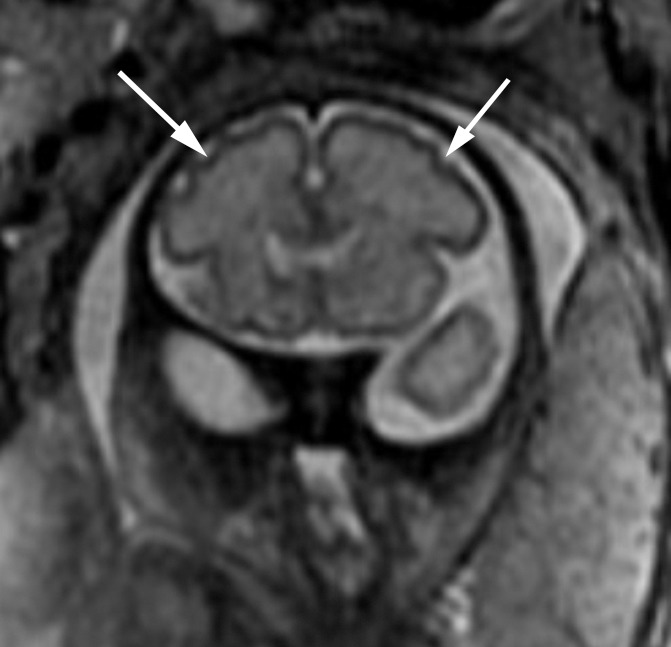
Polymicrogyria detected with fetal MR imaging at 30 gestational weeks. (a) Coronal single-shot fast SE T2-weighted image in a 25-week-old fetus demonstrates normal sulcation pattern without any evidence of developing polymicrogyria. (b) Coronal single-shot fast SE T2-weighted image in the same fetus at 30 gestational weeks demonstrates shallow appearance of the frontal sulci (arrows). (c) Axial single-shot fast SE T2-weighted image at 30 gestational weeks also demonstrates abnormal infoldings of the temporal cortex bilaterally, consistent with polymicrogyria (arrows). (d) Postnatal coronal three-dimensional fast SE T2-weighted image obtained at 3 months of age confirms the findings from 30-week fetal MR image with bifrontal polymicrogyria. Abnormal T2 hyperintensity in the white matter is also noted (and was not detected with fetal MR imaging). (e) Postnatal axial SE T2-weighted image also demonstrates bilateral temporal lobe polymicrogyria, as detected with fetal MR imaging.
Figure 5c:
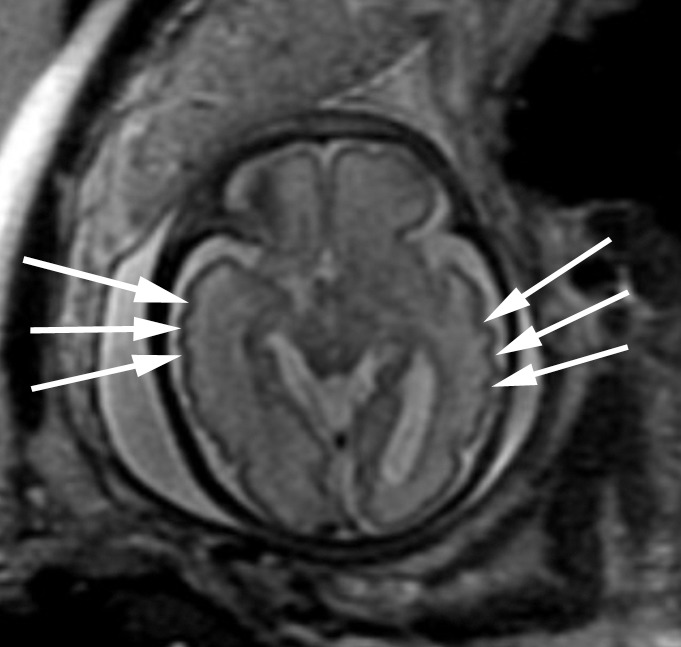
Polymicrogyria detected with fetal MR imaging at 30 gestational weeks. (a) Coronal single-shot fast SE T2-weighted image in a 25-week-old fetus demonstrates normal sulcation pattern without any evidence of developing polymicrogyria. (b) Coronal single-shot fast SE T2-weighted image in the same fetus at 30 gestational weeks demonstrates shallow appearance of the frontal sulci (arrows). (c) Axial single-shot fast SE T2-weighted image at 30 gestational weeks also demonstrates abnormal infoldings of the temporal cortex bilaterally, consistent with polymicrogyria (arrows). (d) Postnatal coronal three-dimensional fast SE T2-weighted image obtained at 3 months of age confirms the findings from 30-week fetal MR image with bifrontal polymicrogyria. Abnormal T2 hyperintensity in the white matter is also noted (and was not detected with fetal MR imaging). (e) Postnatal axial SE T2-weighted image also demonstrates bilateral temporal lobe polymicrogyria, as detected with fetal MR imaging.
Figure 5d:
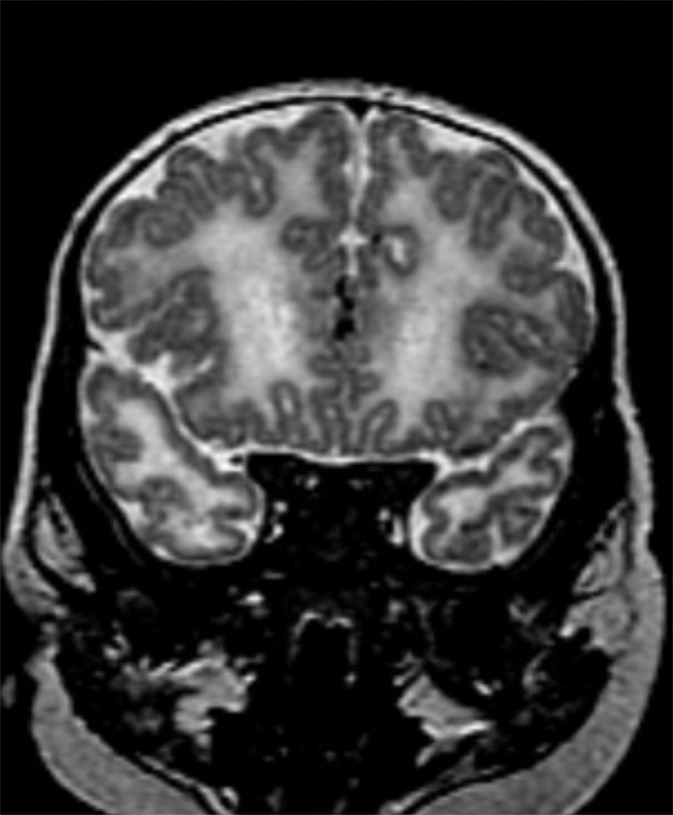
Polymicrogyria detected with fetal MR imaging at 30 gestational weeks. (a) Coronal single-shot fast SE T2-weighted image in a 25-week-old fetus demonstrates normal sulcation pattern without any evidence of developing polymicrogyria. (b) Coronal single-shot fast SE T2-weighted image in the same fetus at 30 gestational weeks demonstrates shallow appearance of the frontal sulci (arrows). (c) Axial single-shot fast SE T2-weighted image at 30 gestational weeks also demonstrates abnormal infoldings of the temporal cortex bilaterally, consistent with polymicrogyria (arrows). (d) Postnatal coronal three-dimensional fast SE T2-weighted image obtained at 3 months of age confirms the findings from 30-week fetal MR image with bifrontal polymicrogyria. Abnormal T2 hyperintensity in the white matter is also noted (and was not detected with fetal MR imaging). (e) Postnatal axial SE T2-weighted image also demonstrates bilateral temporal lobe polymicrogyria, as detected with fetal MR imaging.
Figure 5e:
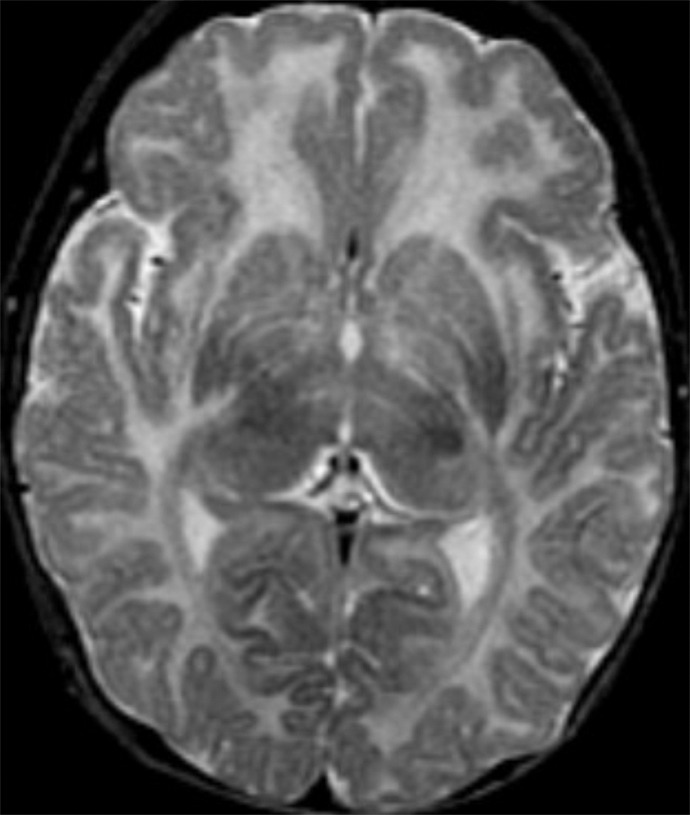
Polymicrogyria detected with fetal MR imaging at 30 gestational weeks. (a) Coronal single-shot fast SE T2-weighted image in a 25-week-old fetus demonstrates normal sulcation pattern without any evidence of developing polymicrogyria. (b) Coronal single-shot fast SE T2-weighted image in the same fetus at 30 gestational weeks demonstrates shallow appearance of the frontal sulci (arrows). (c) Axial single-shot fast SE T2-weighted image at 30 gestational weeks also demonstrates abnormal infoldings of the temporal cortex bilaterally, consistent with polymicrogyria (arrows). (d) Postnatal coronal three-dimensional fast SE T2-weighted image obtained at 3 months of age confirms the findings from 30-week fetal MR image with bifrontal polymicrogyria. Abnormal T2 hyperintensity in the white matter is also noted (and was not detected with fetal MR imaging). (e) Postnatal axial SE T2-weighted image also demonstrates bilateral temporal lobe polymicrogyria, as detected with fetal MR imaging.
Schizencephaly was detected on postnatal MR images in three cases (Table 1). It was detected at fetal MR image review at 27.4 weeks, 32.3 weeks, and 33.1 weeks, respectively, with mean gestational age of 31 weeks. Both open- and closed-lip schizencephalies were detected at fetal MR image review. No cases were scored as suspicious, and no cases were scored as false-positive. The sensitivity of fetal MR imaging was 100% (three of three) (95% CI: 29%, 100%), and specificity was 100% (78 of 78) (95% CI: 95%, 100%).
PVNH were identified on postnatal MR images in 15 cases (Table 1). At fetal MR image review, 10 of these 15 cases were abnormal, one was suspicious, and four were normal. Five false-positive cases were scored as suspicious at fetal MR image review, with no PVNH seen at postnatal MR imaging. Three of the 81 cases were scored as unable to be assessed for PVNH at fetal MR image review and were excluded from the analysis for PVNH; however, none of these cases were shown to have PVNH at postnatal MR imaging.
Mean gestational ages were 27.2 weeks (range, 22–36.4 weeks) among cases of PVNH detected at fetal MR image review and 21.9 weeks (range, 19.7–23 weeks) among cases missed at fetal MR image review (P = .09). The overall sensitivity for PVNH was 73% (11 of 15) (95% CI: 45%, 92%). Five cases were scored as suspicious at fetal MR image review but were found to be normal at postnatal MR imaging; the resulting specificity was 92% (58 of 63) (95% CI: 82%, 97%). If we included only cases for which fetal MR imaging was performed before 24 weeks (n = 35), the sensitivity was 56% (five of nine) (95% CI: 21%, 86%) and specificity was 96% (26 of 27) (95% CI: 81%, 100%). For cases for which fetal MR imaging was performed at or after 24 weeks, the sensitivity was 100% (six of six) (95% CI: 54%, 100%) and specificity was 89% (33 of 37) (95% CI: 75%, 97%). There was a trend toward lower sensitivity when fetal MR imaging was performed before 24 gestational weeks than when performed at or after 24 gestational weeks (P = .06).
When we grouped cases scored as suspicious at fetal MR image review with those scored as normal at fetal MR image review, the mean gestational ages were 27.7 weeks (range, 22–36.4 weeks) for cases with PVNH detected at fetal MR image review and 21.9 weeks (range, 19.7–23 weeks) for cases missed at fetal MR image review (P = .04). The specificity increased to 100% (63 of 63) (95% CI: 94%, 100%), and the sensitivity decreased to 67% (10 of 15) (95% CI: 38%, 88%). If we then included only cases for which fetal MR imaging was performed before 24 weeks (again grouping suspicious cases with normal), the sensitivity was 44% (four of nine) (95% CI: 14%, 79%) and specificity was 100% (26 of 26) (95% CI: 87%, 100%). For cases for which fetal MR imaging was done at or after 24 weeks (again grouping suspicious cases with normal), the sensitivity was 100% (six of six) (95% CI: 54%, 100%) and specificity was 100% (37 of 37) (95% CI: 91%, 100%). The sensitivity of fetal MR imaging was lower for examinations performed before 24 gestational weeks than for examinations performed at or after 24 gestational weeks (P = .04).
Ten of 15 cases (67%) had bilateral PVNH at postnatal MR imaging, and five of 15 (33%) had unilateral PVNH (Table 1). Of the five cases scored as normal or suspicious at fetal MR image review, PVNH was unilateral in two cases and bilateral in three cases at postnatal MR imaging. The proportion of unilateral PVNH cases (two of five) missed at fetal MR image review was not significantly different from that of bilateral PVNH cases missed at fetal MR image review (three of 10) (P = .7). All five cases missed at fetal MR image review had 3-mm single-shot fast SE T2-weighted images with no skip. Four of the 5 cases had at least two series in each plane; one case had only one series in each plane. In two of the cases detected at fetal MR image review, the axial and coronal images were 4 mm thick with no skip. All other cases detected at fetal MR image review had 3-mm section thickness.
In 14 of 15 PVNH cases, fetal MR imaging depicted other abnormalities, including polymicrogyria in seven cases (Table 1). In all five cases of PVNH at postnatal MR imaging that were scored as normal or suspicious at fetal MR image review, fetal MR imaging showed other abnormalities, including callosal agenesis, callosal hypogenesis, small vermis, dysplastic brainstem, ventriculomegaly, and meningocele.
Of the 15 fetuses who had more than one fetal MR examination, five had malformations of cortical development at postnatal MR imaging (Table 2). In two of the polymicrogyria cases, the first fetal MR examination did not depict polymicrogyria; however, subsequent fetal MR images did (discussed earlier). In the two cases of PVNH, neither the first nor the second fetal MR imaging examination (performed as late as 31.1 gestational weeks) depicted PVNH.
Table 2.
Cases with More Than One Fetal MR Imaging Examination
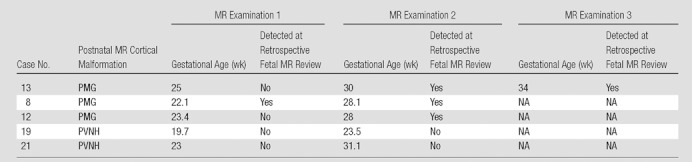
Note.— NA = not applicable.
We also calculated prospective sensitivity and specificity according to the clinical reports for fetal MR images. Review of these reports for the diagnosis of polymicrogyria revealed that 11 cases were correctly identified at the time of clinical reporting. There was one false-positive case (one case of callosal agenesis with a large interhemispheric cyst). There were also two cases for which polymicrogyria was not identified at the time of clinical interpretation; these were the same cases that were missed during retrospective review of the fetal MR images. According to the clinical reports, the sensitivity of fetal MR imaging for polymicrogyria was 85% (11 of 13) (95% CI: 55%, 98%) and the specificity was 99% (67 of 68) (95% CI: 92%, 100%). Considering only cases for which fetal MR imaging was performed before 24 gestational weeks, the sensitivity of fetal MR imaging was 75% (three of four) (95% CI: 19%, 99%), with 100% (33 of 33) specificity (95% CI: 89%, 100%). Sensitivity or specificity did not significantly differ between cases imaged before 24 gestational weeks and those imaged at or after 24 gestational weeks.
All three cases of schizencephaly were also identified in the clinical MR imaging report. In one case of orofacial digital syndrome type VI with multiple brain malformations (including polymicrogyria and PVNH), the fetal MR imaging report incorrectly diagnosed focal closed-lip schizencephaly. On the basis of clinical reports, the sensitivity and specificity of fetal MR imaging for schizencephaly were 100% (three of three) (95% CI: 29%, 100%) and 99% (77 of 78) (95% CI: 93%, 100%), respectively. No cases diagnosed with schizencephaly on the basis of the clinical fetal MR report were imaged before 24 gestational weeks.
Clinical reports for fetal MR imaging correctly listed a diagnosis of PVNH in six of the 15 cases. There were six false-positive cases. According to clinical report, the sensitivity and specificity of fetal MR imaging for PVNH were 40% (six of 15) (95% CI: 16%, 68%) and 91% (60 of 66) (95% CI: 81%, 97%), respectively. Considering only cases for which fetal MR imaging was performed before 24 gestational weeks, the sensitivity of fetal MR imaging was 22% (two of nine) (95% CI: 3%, 60%), with 96% (27 of 28) specificity (95% CI: 82%, 100%). Sensitivity and specificity did not differ between cases imaged before 24 gestational weeks and those imaged at or after 24 gestational weeks.
Discussion
In nearly all cases of malformations of cortical development, at least one additional abnormality could be identified at fetal MR imaging. This finding emphasizes the importance of searching for more than one abnormality once an abnormality is detected at fetal MR imaging.
Overall, we observed good sensitivity of fetal MR imaging for polymicrogyria, at 85%. The sensitivity decreased to 75% for fetuses younger than 24 gestational weeks; this difference was not significant, however, probably because of the small sample size. It is likely that technical difficulties in performing fetal MR imaging, including fetal motion, small size of the fetal brain, and increased distance of the coil from the fetal head, all contributed to our inability to reach 100% sensitivity for the detection of polymicrogyria. However, it is also possible that the detection of polymicrogyria at fetal MR imaging differs depending on the type or cause of polymicrogyria (10). Of note, all seven cases of polymicrogyria associated with callosal agenesis were detected at fetal MR imaging, including two cases that were imaged before 24 gestational weeks, three cases with Aicardi syndrome, one case with orofacial digital syndrome type I, and one case of orofacial digital syndrome type VI. However, in a case of postnatally diagnosed congenital muscular dystrophy, fetal MR imaging did not depict polymicrogyria at 25 gestational weeks but was able to show polymicrogyria by 30 weeks gestation. It is important to note that we observed additional brain abnormalities at fetal MR imaging in all cases of postnatally confirmed polymicrogyria, including those that were missed at fetal MR imaging. The presence of additional brain abnormalities in our cases probably alerted us to the possibility of more extensive brain abnormalities and perhaps heightened our sensitivity for the detection of polymicrogyria.
The location of the polymicrogyria and normal timing of sulcal development did not seem to influence the detection at fetal MR imaging. In particular, fetal MR imaging could depict polymicrogyria even before the expected normal appearance of primary sulci in a particular lobe. For example, we observed instances in which polymicrogyria was detected in the temporal lobe before 28 gestational weeks (which is before the expected normal development of primary sulci in the temporal lobe) and instances in which polymicrogyria was missed in the temporal lobe before 28 gestational weeks.
Although one might suspect that more extensive, diffuse polymicrogyria might be easier to detect than is focal polymicrogyria, this was not the case in our study: Both cases missed at fetal MR image review had diffuse bilateral involvement and might have been missed because we were desensitized by the symmetry of the polymicrogyria.
The sensitivity and specificity of fetal MR imaging were both 100% for schizencephaly; this includes detection of both open- and closed-lip schizencephalies. Open-lip schizencephalies, in particular, are easy to detect when images are obtained in three orthogonal planes, which allows clear depiction of the connection of the schizencephalic defect to the ventricle and subarachnoid space. However, there were only three cases of schizencephaly in our cohort, and all cases were seen in association with polymicrogyria. Additional patients are needed to confirm these results.
The sensitivity of fetal MR imaging for PVNH, 73%, was lower than that for polymicrogyria and schizencephaly. It was especially poor when we consider only cases imaged before 24 weeks’ gestation (44% [four of nine]) and was higher for fetuses imaged at or after 24 gestational weeks (100% [six of six]) when the PVNH had to be seen in at least two planes. Our results show that identifying PVNH with fetal MR imaging is particularly challenging, either when unilateral or bilateral. This is probably because of the small size of heterotopia and the similar signal intensity of heterotopia to the adjacent germinal matrix. Indeed, heterotopia are best detected when they protrude into the ventricular lumen and cause irregularity of the ventricular wall, or when they are larger than the surrounding residual germinal matrix (which decreases in thickness with increasing gestational age). Furthermore, heterotopia can be confused with germinal matrix hemorrhage, although sequences sensitive to blood products may help to differentiate these. Fetal MR imaging at younger gestational age is more affected by such limitations as increased fetal motion, smaller size of the fetal brain, and increased distance of the coil from the fetal head compared with more advanced gestational ages, probably accounting for the observed lower sensitivity when fetal MR imaging was performed before 24 gestational weeks.
It is important to note that whereas we observed 100% specificity of fetal MR imaging for both polymicrogyria and schizencephaly, we observed 92% specificity of fetal MR imaging for PVNH. This is because five cases were scored as suspicious at fetal MR image review (the finding was seen in only one plane) and were false-positive. The specificity increased to 100% if we required that the heterotopia had to be identified in at least two planes at fetal MR image review. This was associated, however, with a slight decrease in sensitivity to 67%; and with a more marked decrease in sensitivity (to 44%) for fetuses younger than 24 gestational weeks. However, the higher specificity is especially important in considering that decisions about pregnancy management can be influenced by the results of fetal MR imaging. In such cases, it is critical to minimize the number of false-positive findings at fetal MR imaging, even at the expense of a lower sensitivity.
Sensitivities for polymicrogyria and schizencephaly were identical when we used retrospective review of fetal MR images and review of prospective clinical fetal MR imaging reports. However, the latter led to identification of one false-positive case each for polymicrogyria and schizencephaly. The major difference in prospective and retrospective interpretations was seen in diagnosis of PVNH, for which sensitivities were 40% (six of 15) and 73% (11 of 15), respectively. This finding probably reflects a combination of recall bias and learning that occurred over the study period.
Although most studies of fetal MR imaging have compared this modality with prenatal US, several studies have used confirmation with autopsy (11,12), postnatal MR imaging (13,14), or autopsy or postnatal MR imaging (3,15,16). Our study contributes to the literature by systematically comparing a large number of fetal MR examinations with postnatal MR examinations to determine the sensitivity and specificity of fetal MR imaging for specific types of brain abnormalities.
In our study (and as occurs in routine clinical practice), most cases had at least two single-shot fast SE T2-weighted series performed in each of three orthogonal planes. In addition, we required that an abnormality be seen in at least two different planes before it was classified as abnormal at fetal MR imaging; this approach is also routinely used when fetal MR images are clinically interpreted. These aspects probably resulted in improved sensitivity and contributed to our high specificity. Furthermore, the images were reviewed by two pediatric neuroradiologists who had over 10 years of experience interpreting such images. Interpretation of fetal MR images has been shown to be influenced by neuroradiology experience (17).
Our study was limited to patients who were referred for clinical fetal MR imaging. In approximately two-thirds of these cases, a brain abnormality was detected at prenatal US; in one-third of cases the brain appeared normal at prenatal US but there was concern for increased risk for brain abnormality (such as in complications of monochorionic twinning). Our results therefore best apply to patients with an abnormality detected at US or to patients with increased risk for brain abnormality. Although this is a limitation of our study, our study population is similar to the population of fetuses undergoing fetal MR imaging in the clinical setting: namely, those in whom an abnormality is suspected according to US findings or those at increased risk for a brain abnormality. If fetal MR imaging is to be used as a screening modality, then additional studies on the diagnostic accuracy of fetal MR imaging in the general population will be needed.
Our cohort was also limited to patients who underwent postnatal MR imaging, resulting in a selection bias among those who had fetal MR imaging. Furthermore, it is likely that postnatal MR imaging examinations were preferentially performed in patients who had neurodevelopmental abnormalities or who had abnormalities at fetal MR imaging, rather than those with normal neurodevelopment or normal findings at fetal MR imaging, resulting in verification bias. Indeed, 25% of our patients (20 of 81) had a cortical malformation diagnosed at fetal MR imaging, as compared with 7.9% (55 of 693) of fetuses in the entire population of patients undergoing fetal MR imaging. The overrepresentation of fetuses with cortical malformations in our study may have resulted in an increased estimation of sensitivity and decreased estimation of specificity for cortical malformations (18).
In nearly all our patients with cortical malformations confirmed at postnatal MR imaging, more than one brain abnormality was detected with fetal MR imaging; thus, there was enhanced pathologic abnormality in our patient population. This bias may have also resulted in an increased estimation of sensitivity and decreased estimation of specificity (19). Of our 81 cases, however, 61 showed no cortical malformations at postnatal MR imaging. Although this limitation could be overcome in the future by performing postnatal MR imaging on all children who undergo fetal MR imaging, this seems unlikely because it is unethical to sedate a young child only for research purposes. Indeed, we have seen dozens of patients with a few heterotopia or localized polymicrogyria discovered incidentally in adulthood (20), and such patients would not have needed sedated pediatric MR imaging of the brain because they had normal development.
Our study is also limited by the lack of standardization of postnatal MR imaging because the postnatal MR imaging examinations were performed at different locations. For example, only 77% of the cases had three-dimensional volume sequences. It is possible that we missed cases of heterotopia or polymicrogyria at postnatal MR imaging in cases for which a three-dimensional sequence was not performed; that could have led to an overestimation of the sensitivity of fetal MR imaging. Of note, four of the 15 cases with heterotopia identified at postnatal MR imaging did not have volumetric sequence with thin partition size, although the heterotopia were still detectable.
Another potential limitation of our study is recall bias, because the neuroradiologists had participated in patient care at the time of initial interpretation and at subsequent imaging conferences, and some of the patients with cortical malformations had been previously described in published reports. Our study was also potentially limited by consensus review because we did not assess interobserver variability.
In summary, using postnatal MR imaging as the reference standard, retrospective consensus review of fetal MR images by pediatric neuroradiologists had 85% sensitivity for polymicrogyria, 100% sensitivity for schizencephaly, 67% sensitivity for heterotopia, and 100% specificity for all three cortical malformations when an abnormality was seen in two planes. The sensitivity for heterotopia was lower in fetuses younger than 24 gestational weeks, a finding important for the clinical counseling of women undergoing fetal MR imaging. Furthermore, to reach a specificity of 100%, heterotopia should be identified in more than one plane with fetal MR imaging before being considered abnormal. In addition, if there is any doubt, follow-up MR imaging at a later gestational age can be helpful.
Advances in Knowledge.
• Fetal MR imaging has 85% (11 of 13) sensitivity and 100% (68 of 68) specificity for the detection of polymicrogyria.
• Retrospective review of fetal MR images showed 100% (three of three) sensitivity and 100% (78 of 78) specificity for the detection of schizencephaly; prospective interpretation had a specificity of 99% (67 of 68).
•Retrospective review of fetal MR images showed 73% (11 of 15) sensitivity and 92% (58 of 63) specificity for the detection of periventricular nodular heterotopia; prospective interpretation showed sensitivity of 40% (six of 15) and specificity of 91% (60 of 66); when heterotopia was confirmed in two planes at fetal MR imaging, retrospective specificity increased to 100% (63 of 63) and sensitivity decreased to 67% (10 of 15).
• Identifying the malformation in at least two planes resulted in 100% specificity for all three cortical malformations studied.
• The sensitivity of fetal MR imaging for heterotopia in fetuses imaged before 24 gestational weeks was lower (44% [four of nine]) than that in fetuses imaged at or after 24 gestational weeks (100% [six of six]).
Implications for Patient Care.
• Data on the accuracy of fetal MR imaging for malformations of cortical development can result in improved clinical counseling of patients on the basis of their fetal MR imaging findings.
• Data comparing diagnostic accuracy when fetal MR imaging is performed before 24 gestational weeks as compared with that at or after 24 gestational weeks can result in improved clinical counseling of patients based on their gestational age.
• Identifying the abnormality in two planes results in improved specificity for heterotopia, with a resultant 100% specificity for all cortical malformations studied.
Disclosures of Potential Conflicts of Interest: O.A.G. No potential conflicts of interest to disclose. A.A.C. No potential conflicts of interest to disclose. A.J.B. No potential conflicts of interest to disclose. Z.H. No potential conflicts of interest to disclose. A.I.B. No potential conflicts of interest to disclose. D.X. No potential conflicts of interest to disclose.
Received December 21, 2010; revision requested February 11, 2011; final revision received August 8; accepted August 26; final version accepted December 13.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Funding: This research was supported by the National Institutes of Health (grant K23 NS52506).
Abbreviations:
- CI
- confidence interval
- PVNH
- periventricular nodular heterotopia
- SE
- spin echo
References
- 1.Garel C. MRI of the fetal brain: normal development and cerebral pathologies. Berlin, Germany: Springer, 2004 [Google Scholar]
- 2.Glenn OA, Barkovich AJ. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR Am J Neuroradiol 2006;27(8):1604–1611 [PMC free article] [PubMed] [Google Scholar]
- 3.Levine D, Barnes PD, Madsen JR, Abbott J, Mehta T, Edelman RR. Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstet Gynecol 1999;94(6):1011–1019 [DOI] [PubMed] [Google Scholar]
- 4.Pugash D, Brugger PC, Bettelheim D, Prayer D. Prenatal ultrasound and fetal MRI: the comparative value of each modality in prenatal diagnosis. Eur J Radiol 2008;68(2):214–226 [DOI] [PubMed] [Google Scholar]
- 5.Raybaud C, Levrier O, Brunel H, Girard N, Farnarier P. MR imaging of fetal brain malformations. Childs Nerv Syst 2003;19(7-8):455–470 [DOI] [PubMed] [Google Scholar]
- 6.Simon EM, Goldstein RB, Coakley FV, et al. Fast MR imaging of fetal CNS anomalies in utero. AJNR Am J Neuroradiol 2000;21(9):1688–1698 [PMC free article] [PubMed] [Google Scholar]
- 7.Glenn OA, Goldstein RB, Li KC, et al. Fetal magnetic resonance imaging in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med 2005;24(6):791–804 [DOI] [PubMed] [Google Scholar]
- 8.Glenn OA, Norton ME, Goldstein RB, Barkovich AJ. Prenatal diagnosis of polymicrogyria by fetal magnetic resonance imaging in monochorionic cotwin death. J Ultrasound Med 2005;24(5):711–716 [DOI] [PubMed] [Google Scholar]
- 9.Tang PH, Bartha AI, Norton ME, Barkovich AJ, Sherr EH, Glenn OA. Agenesis of the corpus callosum: an MR imaging analysis of associated abnormalities in the fetus. AJNR Am J Neuroradiol 2009;30(2):257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inder TE, Huppi PS, Zientara GP, et al. The postmigrational development of polymicrogyria documented by magnetic resonance imaging from 31 weeks’ postconceptional age. Ann Neurol 1999;45(6):798–801 [DOI] [PubMed] [Google Scholar]
- 11.Guo WY, Chang CY, Ho DMT, et al. A comparative MR and pathological study on fetal CNS disorders. Childs Nerv Syst 2001;17(9):512–518 [DOI] [PubMed] [Google Scholar]
- 12.Whitby EH, Paley MNJ, Sprigg A, et al. Comparison of ultrasound and magnetic resonance imaging in 100 singleton pregnancies with suspected brain abnormalities. BJOG 2004;111(8):784–792 [DOI] [PubMed] [Google Scholar]
- 13.Wagenvoort AM, Bekker MN, Go AT, et al. Ultrafast scan magnetic resonance in prenatal diagnosis. Fetal Diagn Ther 2000;15(6):364–372 [DOI] [PubMed] [Google Scholar]
- 14.Senapati GM, Levine D, Smith C, et al. Frequency and cause of disagreements in imaging diagnosis in children with ventriculomegaly diagnosed prenatally. Ultrasound Obstet Gynecol 2010;36(5):582–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Laveaucoupet J, Audibert F, Guis F, et al. Fetal magnetic resonance imaging (MRI) of ischemic brain injury. Prenat Diagn 2001;21(9):729–736 [DOI] [PubMed] [Google Scholar]
- 16.Sonigo PC, Rypens FF, Carteret M, Delezoide AL, Brunelle FO. MR imaging of fetal cerebral anomalies. Pediatr Radiol 1998;28(4):212–222 [DOI] [PubMed] [Google Scholar]
- 17.Levine D, Feldman HA, Tannus JF, et al. Frequency and cause of disagreements in diagnoses for fetuses referred for ventriculomegaly. Radiology 2008;247(2):516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revesz G, Kundel HL, Bonitatibus M. The effect of verification on the assessment of imaging techniques. Invest Radiol 1983;18(2):194–198 [DOI] [PubMed] [Google Scholar]
- 19.Mower WR. Evaluating bias and variability in diagnostic test reports. Ann Emerg Med 1999;33(1):85–91 [DOI] [PubMed] [Google Scholar]
- 20.Cho WH, Seidenwurm D, Barkovich AJ. Adult-onset neurologic dysfunction associated with cortical malformations. AJNR Am J Neuroradiol 1999;20(6):1037–1043 [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn OA, Barkovich J. Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis: part 2. AJNR Am J Neuroradiol 2006;27(9):1807–1814 [PMC free article] [PubMed] [Google Scholar]