Abstract
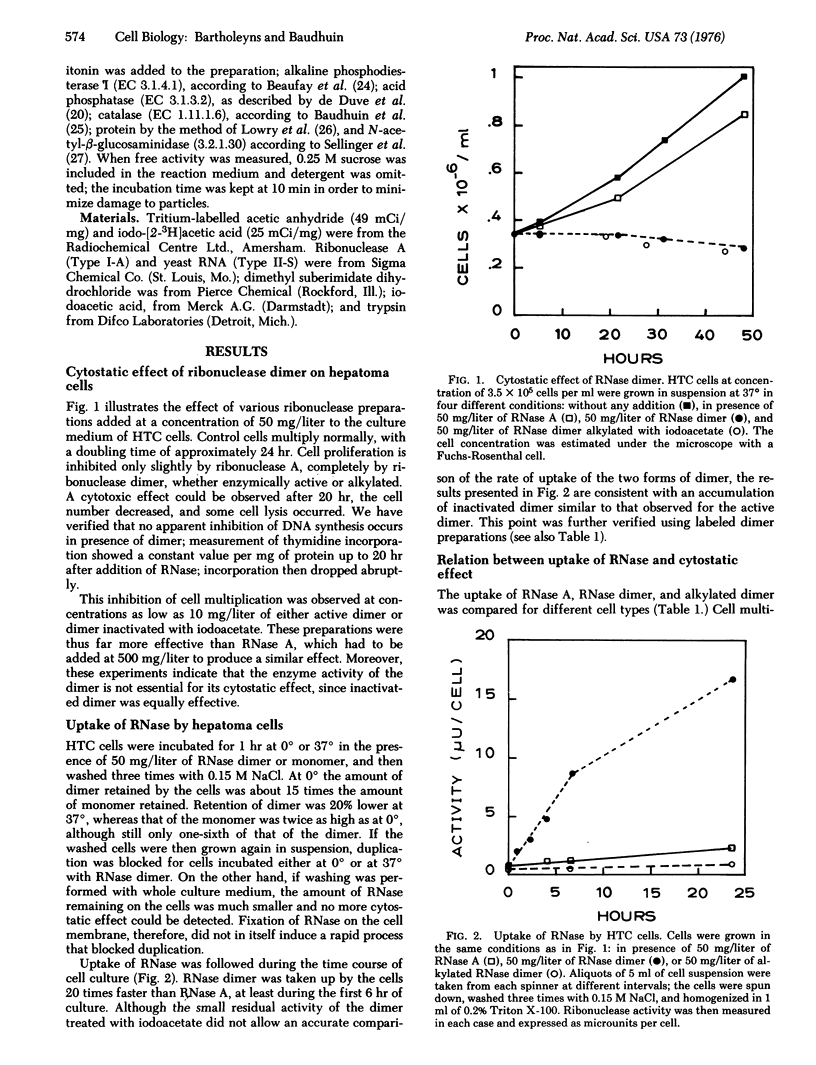
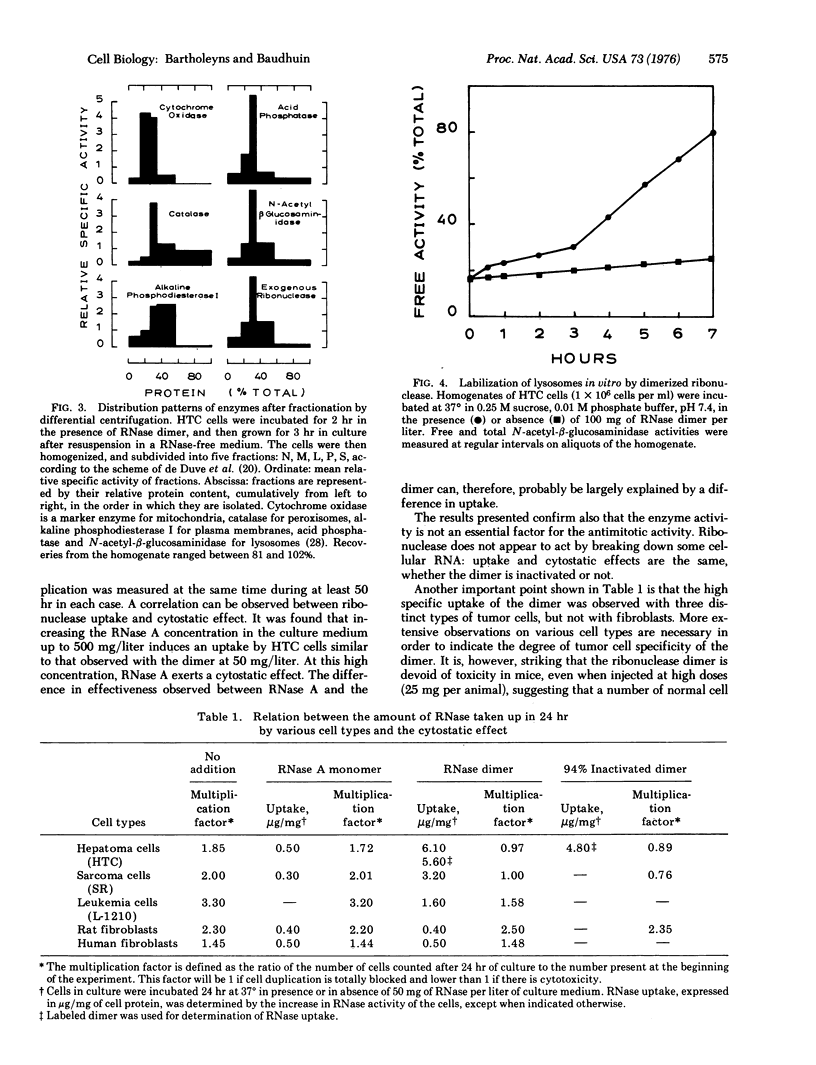
A cross-linked dimer of pancreatic ribonuclease A (ribonucleate 3'-pyrimidino-olitonucleotidohydrolase, EC 3.1.4.22), at a 10 mg/liter concentration, blocks proliferation of tumor cells. The protein retains this ability after inactivation by iodoacetate. The cytostatic effect of ribonuclease preparations on various cell lines correlates well with their rate of uptake: for example, monomeric ribonuclease A is much less effective and is taken up into the cells 10 t0 15 times more slowly. Cell fractionation studies on hepatoma cells indicate accumulation of the dimer in the lysosomal system. Ribonuclease dimer induces a labilization of the lysosomes when added to cell homogenates, raising the possibility that its antitumoral effect may be mediated by endocytosis and lysosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Isselbacher K. J. Protein synthesis by rat intestinal mucosa. The role of ribonuclease. J Biol Chem. 1967 Dec 10;242(23):5617–5622. [PubMed] [Google Scholar]
- BRACHET J. Effects of ribonuclease on the metabolism of living root-tip cells. Nature. 1954 Nov 6;174(4436):876–877. doi: 10.1038/174876a0. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J., Moore S. Pancreatic ribonuclease: enzymic and physiological properties of a cross-linked dimer. Science. 1974 Nov 1;186(4162):444–445. doi: 10.1126/science.186.4162.444. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J., Peeters-Joris C., Baudhuin P. Hepatic nucleases. Extrahepatic origin and association of neutral liver ribonuclease with lysosomes. Eur J Biochem. 1975 Dec 15;60(2):385–393. doi: 10.1111/j.1432-1033.1975.tb21014.x. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J., Peeters-Joris C., Reychler H., Baudhuin P. Hepatic nucleases. 1. Methods for the specific determination and characterization in rat liver. Eur J Biochem. 1975 Sep 1;57(1):205–211. doi: 10.1111/j.1432-1033.1975.tb02292.x. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2413–2419. [PubMed] [Google Scholar]
- D'Alessio G., Parente A., Guida C., Leone E. Dimeric structure of seminal ribonuclease. FEBS Lett. 1972 Nov 1;27(2):285–288. doi: 10.1016/0014-5793(72)80642-2. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EASTY D. M., LEDOUX L., AMBROSE E. J. The action of ribonuclease on neoplastic growth. III. Studies by interference microscopy. Biochim Biophys Acta. 1956 Jun;20(3):528–537. doi: 10.1016/0006-3002(56)90347-x. [DOI] [PubMed] [Google Scholar]
- LEDOUX L. Action of ribonuclease on two solid tumours in vivo. Nature. 1955 Jul 2;176(4470):36–37. doi: 10.1038/176036a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leroy J. G., Dumon J., Radermecker J. Deficiency of arylsulphatase A in leucocytes and skin fibroblasts in juvenile machromatic leucodystrophy. Nature. 1970 May 9;226(5245):553–554. doi: 10.1038/226553a0. [DOI] [PubMed] [Google Scholar]
- Libonati M., Floridi A. Breakdown of double-stranded RNA by bull semen ribonuclease. Eur J Biochem. 1969 Mar;8(1):81–87. doi: 10.1111/j.1432-1033.1969.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Saura P., Tulkens P., Trouet A. Characterization of subcellular constituents of hepatoma cultured cells (HTC cells). Arch Int Physiol Biochim. 1972 Dec;80(5):977–978. [PubMed] [Google Scholar]
- Matousek J., Grozdanovic J. Specific effect of bull seminal ribonuclease (AS RNase) on cell systems in mice. Comp Biochem Physiol A Comp Physiol. 1973 Oct 1;46(2):241–248. doi: 10.1016/0300-9629(73)90415-5. [DOI] [PubMed] [Google Scholar]
- Matousek J. The effect of bovine seminal ribonuclease (AS RNase) on cells of Crocker tumour in mice. Experientia. 1973;29(7):858–859. doi: 10.1007/BF01946329. [DOI] [PubMed] [Google Scholar]
- PILERI A., LEDOUX L., VANDERHAEGHE F. Etude de l'absorption de la ribonucléase par les cellules de la moelle osseuse et par les cellules des tumeurs d'ascites. Exp Cell Res. 1959 May;17(2):218–226. doi: 10.1016/0014-4827(59)90213-7. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tomkins G. M. Relation of steroid structure to enzyme induction in hepatoma tissue culture cells. J Mol Biol. 1970 Aug 28;52(1):57–74. doi: 10.1016/0022-2836(70)90177-4. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Beaufay H., Trouet A. Analytical fractionation of homogenates from cultured rat embryo fibroblasts. J Cell Biol. 1974 Nov;63(2 Pt 1):383–401. doi: 10.1083/jcb.63.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]



