Abstract
Summary
Background and objectives
Endothelial dysfunction is an early manifestation of vascular injury and contributes to the development of atherosclerotic cardiovascular disease. Recent studies have implicated hyperuricemia as a risk factor for cardiovascular disease. We hypothesized that lowering uric acid in subjects with asymptomatic hyperuricemia with allopurinol might improve endothelial dysfunction, BP, estimated GFR (eGFR), and inflammatory markers.
Design, setting, participants, & measurements
Subjects with asymptomatic hyperuricemia and no history of gout and 30 normouricemic control subjects were enrolled in this 4-month randomized prospective study. Thirty hyperuricemic patients received 300 mg/d allopurinol and were compared with 37 hyperuricemic patients and 30 normouricemic subjects in matched control groups. Flow-mediated dilation (FMD), eGFR, ambulatory BP monitoring, spot urine protein-creatine ratio, and highly sensitive C-reactive protein were measured at baseline and at 4 months.
Results
Age, gender, lipid profile, eGFR, hemoglobin, glucose, and level of proteinuria were similar in hyperuricemic subjects and controls at baseline. As expected, hyperuricemic patients had higher levels of highly sensitive C-reactive protein and lower FMD compared with normouricemic patients. Allopurinol treatment resulted in a decrease in serum uric acid, a decrease in systolic BP, an increase in FMD, and an increase in eGFR compared with baseline. No significant difference was observed in the control hyperuricemic and normouricemic groups. In a multiple regression analysis, FMD levels were independently related to uric acid both before (beta = −0.55) and after (beta = −0.40) treatment.
Conclusions
Treatment of hyperuricemia with allopurinol improves endothelial dysfunction and eGFR in subjects with asymptomatic hyperuricemia.
Introduction
Asymptomatic hyperuricemia is commonly viewed as an entity that should not be treated (1,2). However, there is increasing evidence that hyperuricemia may not be completely benign. Numerous studies and meta-analyses have found that elevated uric acid levels predict the development of hypertension, stroke, diabetes, and heart disease (3–6). The reverse seems also true: short-term trials also suggest a benefit from lowering uric acid on BP (7–9), insulin resistance (10), estimated GFR (eGFR) (9,11,12), C-reactive protein (CRP) levels (9,11), and endothelial dysfunction (13). However, most of these studies were short term or were not randomized, and only a few prospective randomized trials have been performed (8,11,14). Furthermore, many of these studies included subjects with hypertension, diabetes mellitus, chronic kidney disease, or cardiovascular disease and did not evaluate healthy individuals whose only abnormality was hyperuricemia. Thus, it is still unknown whether treatment of asymptomatic hyperuricemia in low-risk patients would provide benefit to patients in terms of renal function, endothelial dysfunction, and BP. We therefore decided to prospectively determine the effect of allopurinol treatment on renal function, proteinuria, serum CRP, BP, and endothelial dysfunction (assessed by flow-mediated dilation [FMD]) in asymptomatic hyperuricemic patients with normal renal function and no evidence of cardiovascular disease.
Materials and Methods
Study Design and Participants
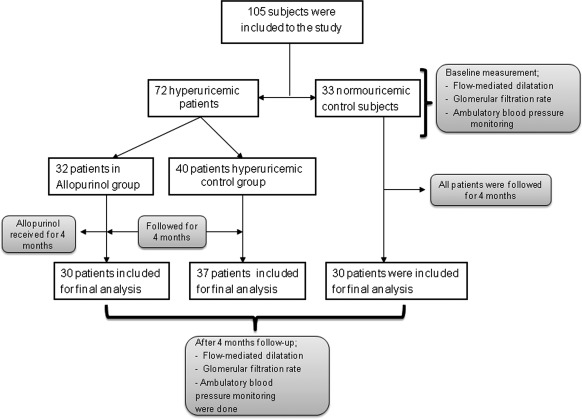
This is a prospective, randomized 7-month intervention trial conducted at Ankara Research and Training Hospital between December 2009 and June 2010. The study was approved by the Local Ethics Committee and was conducted in accordance with the ethical principles set forth by the Declaration of Helsinki. All of the participants were included after signing informed consent forms. The primary endpoint of the study was whether allopurinol treatment would affect endothelial dysfunction, BP, and eGFR in asymptomatic hyperuricemic subjects without a history of any comorbid disease compared with untreated controls. A total of 105 consecutive patients who attended the outpatient general internal medicine clinic and had normal renal function and fulfilled inclusion criteria were recruited for the study. Of these, 72 patients were hyperuricemic (defined as serum uric acid >7 mg/dl), whereas the remaining 33 patients were normouricemic. Seventy-two hyperuricemic patients were randomly assigned to receive either allopurinol 300 mg/d for 4 months or no treatment to serve as controls by means of computer-generated random numbers. A flow diagram of the study design is depicted in Figure 1. All of the groups had levels of serum uric acid, highly sensitive CRP (hsCRP), morning spot urine protein-creatine ratio, systolic and diastolic BP, eGFR, and FMD at baseline and at the end of the 4-month study period.
Figure 1.
Flow diagram of study design.
Inclusion criteria consisted of subjects with adult (>18 years) asymptomatic hyperuricemia without presence of diabetes, hypertension, heart failure, gout, or overt cardiovascular disease (n = 72). An additional control group without hyperuricemia who also had no evidence for the same comorbid conditions was also included (n = 33). To minimize any confounding effect of conditions that may influence endothelial function, patients with a history of coronary artery disease, active smokers, and patients receiving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, or supplemental vitamin pills were excluded. Subjects with a history of diabetes, those on oral hypoglycemic agents or insulin, or those with a fasting glucose level greater than 126 mg/dl were also excluded. The patients were monitored for potential adverse effects of allopurinol on a monthly basis via system review and a comprehensive physical examination.
Laboratory Testing
Blood and biochemistry panels including fasting blood glucose, serum uric acid, morning spot urine protein-creatine ratio, hsCRP, and LDL cholesterol were analyzed by a hospital autoanalyzer at onset of the study and were repeated at the end of the 4-month study period for all patients. The eGFR was calculated using the Cockcroft-Gault formula.
Flow-mediated Dilation
The determination of endothelial dysfunction was performed according to Celermajer et al. (15). Measurements were made by a single observer who was blinded to the randomization status of the patients using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA) with a 12-MHz probe. All of the vasoactive medications were withheld for 24 hours before the procedure. The subjects remained at rest in the supine position for at least 15 minutes before the examination started. The subject's arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single two-dimensional frames. All of the ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. A pneumatic tourniquet was inflated to 200 mmHg with obliteration of the radial pulse. After 5 minutes, the cuff was deflated. Flow measurements were made 60 seconds after deflation. The maximum FMD diameters were calculated as the averages of the three consecutive maximum-diameter measurements. The FMD was then calculated as the percentage of change in diameter compared with baseline resting diameters.
Ambulatory BP Monitoring
All of the subjects underwent 24 hours of ambulatory BP monitoring (ABPM) on a usual working day. They were instructed to act and work normally. ABPM was performed using a Reynolds Medical Tracker NIBP2 oscillometric monitor. Each patient used an arm cuff of similar size to the one used for routine office BP measurement in the nondominant arm. The device was programmed to measure BP every 15 minutes between 6:00 a.m. and 11:00 p.m., and every 30 minutes between 11:00 p.m. and 6:00 a.m. All of the subjects were instructed to rest or sleep between 10:00 p.m. and 6:00 a.m.
Statistical Analyses
All of the data are presented as the means ± SDs unless stated otherwise. Continuous variables were checked for the normal distribution assumption using the Kolmogorov-Smirnov statistics. ANOVA test was used for multiple group comparisons of normally distributed variables. χ2 was used to test differences in frequency distributions. All of the potential (physiologically meaningful) determinants of the FMD were investigated in a univariate screening procedure, using the Pearson coefficient of correlation test. The nonparametric Spearman rho coefficient of correlation was used to assess correlations between variables without normal distribution. One-way ANOVA, t test, and paired-sample t test were used to compare numeric data. Significant determinants identified from this analysis were studied in a multiple regression model using the F statistic. All of the variables associated with these parameters with a level of significance <0.1 were included in the tested model. The variables were forced in the model using a stepwise procedure. P < 0.05 for the final model was considered as statistically significant. The data were analyzed using the SPSS 15.0 for Windows software (SPSS Inc., Chicago, IL). The sample size was calculated by the Power and Sample Size V.3.0 program (Vanderbilt University, Department of Biostatistics, Free Software). The criteria for sample size calculation were as follows: 95% confidence intervals, 80% power, and a decrease in serum uric acid levels (2 mg/dl for allopurinol treatment, according to our previous study (9). According to these criteria, 24 patients were to be recruited in each group. Considering the possibility of laboratory and other process mishaps, we decided to include at least 30 patients in each group.
Results
Baseline Characteristics
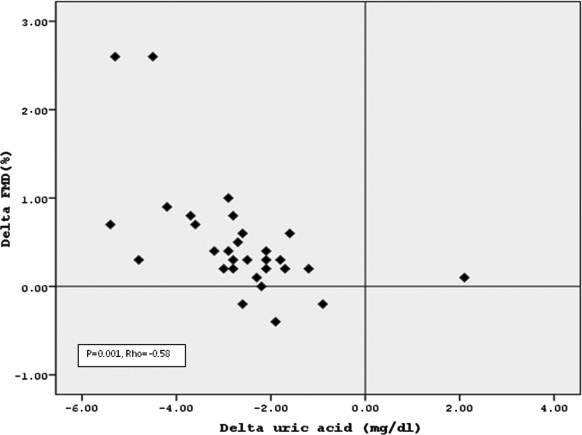
Demographic, clinical, and laboratory data at baseline in the three groups are shown in Table 1. There was no difference among the groups with respect to age, gender, blood glucose, and LDL cholesterol levels at baseline. As expected, hyperuricemic patients had higher hsCRP levels and lower FMD and eGFR values compared with normouricemic controls. FMD correlated inversely with uric acid levels when the three groups were combined (rho = −0.58, P = 0.001; Figure 2). No significant difference in baseline spot urine protein to creatinine ratio and 24-hour ABPM-derived BP levels were noted between hyperuricemic and normouricemic subjects, consistent with the inclusion criteria to study only normotensive subjects.
Table 1.
Baseline characteristics of the study population
| Normouricemic Control Group (n = 30) | Hyperuricemic Control Group (n = 37) | Allopurinol Group (n = 30) | P | |
|---|---|---|---|---|
| Age (years, mean) | 48.4 ± 9.2 | 50.4 ± 11.2 | 54.4 ± 8.0 | 0.13 |
| Male, n (%) | 14 (46) | 18 (48) | 16 (53) | 0.23 |
| Hemoglobin (g/dl) | 14.0 ± 1.4 | 13.9 ± 1.6 | 14.8 ± 1.2 | 0.07 |
| Uric acid (mg/dl) | 4.4 ± 0.9 | 7.9 ± 0.7 | 8.3 ± 1.1 | <0.01 |
| BMI (kg/m2) | 28.4 ± 3.3 | 29.7 ± 3.4 | 28.4 ± 2.9 | 0.26 |
| 24 h systolic BP (mmHg) | 119.4 ± 11.2 | 123.2 ± 13.5 | 127.6 ± 10.4 | 0.08 |
| 24 h diastolic BP (mmHg) | 77.3 ± 6.1 | 75.6 ± 8.7 | 75.1 ± 7.8 | 0.06 |
| eGFR (ml/min per 1.73 m2) | 92.8 ± 13.7 | 84.3 ± 16.7 | 86.3 ± 19.4 | 0.04 |
| Urine protein-creatinine ratio | 0.11 ± 0.04 | 0.12 ± 0.13 | 0.12 ± 0.09 | 0.52 |
| hsCRP (mg/l) | 3.3 ± 2.5 | 6.9 ± 3.4 | 7.4 ± 5.8 | 0.02 |
| LDL-cholesterol (mg/dl) | 125.7 ± 38.3 | 113.5 ± 34.4 | 123.0 ± 30.7 | 0.44 |
| Glucose (mg/dl) | 89.8 ± 7.9 | 90.3 ± 6.4 | 94.3 ± 6.9 | 0.06 |
| Flow-mediated dilation (%) | 9.16 ± 0.65 | 7.76 ± 0.86 | 7.74 ± 0.93 | <0.01 |
Mean values are reported as ± SD. The bold values indicate P < 0.05, statistically significant. BMI, body mass index; eGFR, estimated GFR; hsCRP, highly sensitive C-reactive protein.
Figure 2.
Scatter plot shows the significant negative association between the change in uric acid and flow-mediated dilation (FMD).
Effects of Allopurinol Treatment on Serum Uric Acid, hsCRP, Proteinuria, eGFR, and FMD Values
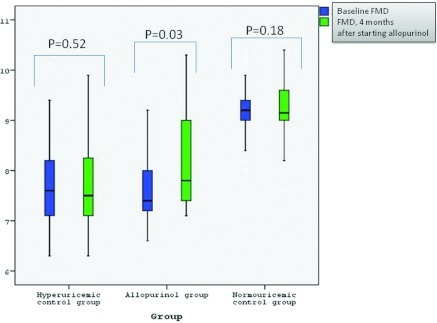
Treatment with allopurinol in the 37 hyperuricemic subjects for 4 months resulted in a significant decrease in serum uric acid, a decrease in systolic BP and hsCRP, and an increase in eGFR and FMD compared with baseline values (P < 0.05 for all [Table 2]). In contrast, control hyperuricemic and normouricemic subjects showed no change in these parameters from baseline, although a trend for improvement in uric acid levels, FMD, and systolic and diastolic BP was observed in the untreated hyperuricemic controls (Table 2). In addition, there was a significant improvement in hsCRP in the hyperuricemic control group when compared with baseline values (P < 0.05). Flow-mediated dilation at baseline and at 16 weeks in the allopurinol, hyperuricemic, and normouricemic control groups are presented in Figure 3. All of the patients in the allopurinol group tolerated the drug, and there were no adverse effects detected by physical examination or reported by patients.
Table 2.
The mean serum uric acid, flow-mediated dilation (FMD), and mean systolic (SBP) and diastolic (DBP) blood pressure at baseline and 16 weeks later
| Allopurinol Group (n = 30) |
Hyperuricemic Control Group (n = 37) |
Normouricemic Control Group (n = 30) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | P | Baseline | 16 weeks | P | Baseline | 16 weeks | P | |
| Uric acid (mg/dl) | 8.3 ± 1.1 | 5.8 ± 1.5 | <0.001 | 7.9 ± 0.7 | 7.2 ± 0.83 | 0.70 | 4.4 ± 0.9 | 4.5 ± 0.86 | 0.26 |
| FMD (%) | 7.74 ± 0.93 | 8.12 ± 1.56 | 0.003 | 7.76 ± 0.86 | 7.77 ± 0.85 | 0.52 | 9.16 ± 0.65 | 9.24 ± 0.66 | 0.18 |
| eGFR (ml/min per 1.73 m2) | 86.3 ± 19.4 | 89.6 ± 12.6 | 0.001 | 84.3 ± 16.7 | 84.4 ± 16.3 | 0.77 | 92.8 ± 13.7 | 93.3 ± 9.2 | 0.63 |
| hsCRP (mg/dl) | 7.4 ± 5.8 | 4.6 ± 3.7 | 0.003 | 6.9 ± 3.4 | 5.9 ± 3.8 | 0.04 | 3.3 ± 2.5 | 3.4 ± 2.2 | 0.79 |
| Mean SBP (mmHg) | 127.6 ± 14.4 | 116.9 ± 11.7 | 0.005 | 123.2 ± 13.5 | 116.8 ± 9.9 | 0.26 | 119.4 ± 11.2 | 116.4 ± 13.4 | 0.46 |
| Mean DBP (mmHg) | 75.1 ± 7.8 | 74.9 ± 12.4 | 0.19 | 75.6 ± 8.7 | 73.9 ± 13.9 | 0.22 | 77.3 ± 6.1 | 76.2 ± 11.8 | 0.39 |
The bold values indicate P < 0.05, statistically significant. eGFR, estimated GFR; hsCRP, highly-sensitive C-reactive protein.
Figure 3.
Flow-mediated dilation (FMD) at baseline and 16 weeks after in allopurinol, hyperuricemic, and normouricemic control group.
Univariate and Multivariate Analyses for the FMD
A multiple regression model incorporating variables expected to influence FMD (gender, age, eGFR, hsCRP, and systolic BP), as well as serum uric acid was performed both before (at baseline) and after treatment. FMD levels were independently related to uric acid levels both before (P = 0.03) and after (P = 0.024) treatment (Table 3).
Table 3.
Analysis of association between flow-mediated dilation (FMD) and some different parameters by multivariate linear regression both before and after allopurinol treatment
| Multivariate β (P) | 95% CI for β | |
|---|---|---|
| Before treatment | ||
| uric acid (mg/dl) | −0.42 (0.03) | −1.42 to −3.17 |
| age (year) | 0.57 (0.16) | −0.17 to 0.34 |
| male gender | 0.39 (0.06) | −0.83 to 1.46 |
| hsCRP (mg/L) | −0.13 (0.12) | −1.08 to 0.46 |
| eGFR (ml/min per 1.73 m2) | −0.42 (0.14) | −0.75 to 0.04 |
| systolic BP (mmHg) | 0.44 (0.17) | −0.01 to 0.03 |
| After treatment | ||
| uric acid (mg/dl) | −0.37 (0.024) | −0.52 to −0.23 |
| age (year) | 0.04 (0.87) | −0.20 to 0.34 |
| male gender | 0.32 (0.05) | −0.46 to 0.93 |
| hsCRP (mg/L) | −0.18 (0.09) | −0.70 to 0.86 |
| eGFR (ml/min per 1.73 m2) | −0.46 (0.96) | −0.10 to 0.26 |
| systolic BP (mmHg) | 0.08 (0.65) | −0.03 to 0.33 |
The bold values indicate P < 0.05, statistically significant; β, beta value; CI, confidence interval; hsCRP, highly sensitive C-reactive protein; eGFR, estimated GFR.
Discussion
The salient findings of this study were that lowering serum uric acid with allopurinol led to improvements in estimated GFR, systolic BP, and endothelial dysfunction assessed by FMD. However, we could not document any significant improvement in hsCRP, proteinuria, or diastolic BP after treatment with allopurinol, compared with untreated hyperuricemic or with normouricemic controls.
Endothelial dysfunction is considered a harbinger for hypertension and cardiovascular disease (16). FMD as a physiologic measure of endothelial function has been validated and used in many settings to investigate endothelial function (17). Several studies have reported an inverse relationship between uric acid and endothelial function/FMD (13,18–21), although this has not uniformly been noted (22). A number of studies have also reported that lowering uric acid with allopurinol may improve endothelial function in individuals with a variety of conditions, including subjects with heart failure (23–25), those with diabetes (26), heavy smokers (27), or those with obstructive sleep apnea (28). However, to our knowledge, only one study examined the effect of allopurinol on endothelial function in subjects with asymptomatic hyperuricemia (13). In this study allopurinol was administered for 4 months to 32 hyperuricemic patients who were at high risk for cardiovascular disease. Compared with baseline values and with normouricemic untreated controls, allopurinol treatment significantly increased FMD values, in parallel with a reduction in serum uric acid levels. Clearly, these subjects were not truly healthy subjects, because many of them were diabetic, hypertensive, and/or were on medications that may potentially effect endothelial dysfunction per se. In our study, we purposefully excluded potential confounding factors, which could affect endothelial function, by specifically selecting subjects who did not have diabetes, hypertension, or who were not receiving statins, angiotensin-converting enzyme inhibitors, or angiotensin-receptor blockers. Thus, the importance of our study is that it suggests that endothelial function can be improved with allopurinol therapy, even in subjects with asymptomatic hyperuricemia and no history of gout who lack all evidence of cardiovascular disease. Our results showed that with allopurinol treatment, mean FMD values were increased by 0.4%. FMD has been shown to be an independent predictor of future cardiovascular events in population-based studies (29,30). The higher the baseline FMD, the more likely the patient will experience a future cardiovascular event. Thus, reduction of FMD with administration of allopurinol might have important long-term implications. Prospective randomized trials are yet to be conducted to demonstrate whether this is the reality.
We also documented that the lowering of uric acid with allopurinol can improve eGFR. An elevated uric acid has been consistently shown to predict a fall in GFR in the adult without kidney disease (31–34). Recent small interventional trials have also shown that lowering uric acid can improve renal function in subjects with CKD (11,12). In a previous study, we showed a favorable effect of 3-month allopurinol treatment on estimated GFR, in a population of asymptomatic hyperuricemic patients (9). Mean creatinine clearance determined via collection of 24-hour urine increased from 79 ± 32 to 93 ± 37 ml/min, after allopurinol treatment. In this study, eGFR values (calculated using the Cockcroft-Gault equation) increased slightly but significantly from a baseline of 86 ± 19 to 89 ± 18 ml/min per 1.73 m2 at the end of the allopurinol treatment phase.
We documented a decrease in 24-hour systolic ambulatory BP with allopurinol treatment. The allopurinol-treated subjects had a decrease of 10 mmHg in systolic BP, whereas the hyperuricemic controls and normouricemic controls had decreases in systolic BP of 6 and 3 mmHg, respectively. Studies in experimental animal models of hyperuricemia have suggested that uric acid can raise BP, likely via the induction of oxidative stress, endothelial dysfunction, and activation of the renin-angiotensin system (35–38). These studies have suggested that an elevated level of uric acid may be more important for new-onset hypertension rather than long-standing hypertension, in which renal microvascular disease may be the key factor driving the hypertensive response (39). A recent small clinical trial reported that allopurinol can lower BP in newly diagnosed hypertension in adolescents (7,8). We also previously reported that allopurinol improved both systolic and diastolic BP in 48 adults with normal renal function, of whom most had hypertension (34 out of the 48) or high-normal BP (9). Thus, this study is the first demonstrating a BP-lowering effect of lowering serum uric acid via allopurinol, even in normotensive adults, thus extending our previous findings. Notably, allopurinol selectively reduced systolic BP but not diastolic BP. It is possible that this may relate to the normal baseline values of BP readings of the hyperuricemic patients. Our previous study (9) included patients who were hypertensive at baseline. It is recognized that the higher the baseline BP, the greater the effect of a given drug that reduces BP.
Interestingly, our study did not confirm a beneficial effect of lowering uric acid on either CRP levels or on urinary protein excretion. Other studies, however, do suggest that lowering uric acid can improve inflammatory markers in subjects with CKD (11), heart failure (10), and stroke survivors (40). We also found that allopurinol improved CRP levels in mostly hypertensive adults with asymptomatic hyperuricemia (9).
It should be mentioned that the beneficial effects of allopurinol treatment observed in this cohort may be the consequence of both lowering serum uric acid levels (thereby amelioration of uric acid mediated pathophysiologic processes) and of inhibiting the xanthine oxidase enzyme system (thereby decreasing oxidative stress generated during the production of uric acid). Some clinical and experimental studies suggest that the benefit of blocking the xanthine-oxidase system on endothelial dysfunction may relate to the inhibition of xanthine oxidase-associated oxidants as opposed to lowering uric acid. However, in a recent study (10), the uricosuric drug benzbromarone (which is not a xanthine-oxidase inhibitor) was shown to improve inflammatory markers and insulin resistance in patients with congestive heart failure, suggesting a direct effect of lowering uric acid on inflammation.
In summary, our study is the first to examine the effect of lowering uric acid in subjects with asymptomatic hyperuricemia who lack a history of gout, hypertension, or cardiovascular disease. An improvement in endothelial function, systolic BP, and eGFR was observed. The effects were mild but significant so that a larger number of patients and longer follow-up will be necessary before one can determine whether such a strategy may provide long-term survival benefits. Our study was also limited by being an open-label trial and lacked a placebo control. Allopurinol can also cause severe allergic reactions, although there are early promising studies suggesting that side effects may be reduced by screening subjects for HLA-B58 (41,42). Our study was also performed in adults, and some studies suggest that the potential benefits of allopurinol could potentially be much more significant in adolescents and younger subjects. Indeed, a multicenter trial has been proposed to the National Institutes of Health to determine whether lowering uric acid in prehypertensive adolescents and young adults may have benefit in preventing BP rise and the development of metabolic syndrome. Clearly, more studies are necessary to assess the risk/benefit of lowering uric acid in subjects with asymptomatic hyperuricemia.
Disclosures
Dr. Johnson has patent applications related to lowering uric acid as a means to treat hypertension, reduce the frequency of diabetes, and treat fatty liver. The other authors have no relationships or financial interests with companies related to the findings of this work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Duffy WB, Senekjian HO, Knight TF, Weinman EJ: Management of asymptomatic hyperuricemia. JAMA 246: 2215–2216, 1981 [PubMed] [Google Scholar]
- 2. Kanellis J, Feig DI, Johnson RJ: Does asymptomatic hyperuricaemia contribute to the development of renal and cardiovascular disease? An old controversy renewed. Nephrology 9: 394–399, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Grayson PC, Kim SY, Lavalley M, Choi HK: Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res 63: 102–110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA: Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum 61: 885–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA: Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res 62: 170–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, Saito A, Sone H: Association between serum uric acid and development of type 2 diabetes. Diabetes Care 32: 1737–1742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, Johnson RJ: Hypothesis: Uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int 66: 281–287, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Feig DI, Soletsky B, Johnson RJ: Effect of allopurinol on the blood pressure of adolescents with newly diagnosed essential hypertension. JAMA 300: 922–930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A: Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol 39: 1227–1233, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Ogino K, Kato M, Furuse Y, Kinugasa Y, Ishida K, Osaki S, Kinugawa T, Igawa O, Hisatome I, Shigemasa C, Anker SD, Doehner W: Uric acid-lowering treatment with benzbromarone in patients with heart failure: A double-blind placebo-controlled crossover preliminary study. Circ Heart Fail 3: 73–81, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, Arroyo D, Luno J: Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5: 1388–1393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, Rosano GM: Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol 94: 932–935, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Yiginer O, Ozcelik F, Inanc T, Aparci M, Ozmen N, Cingozbay BY, Kardesoglu E, Suleymanoglu S, Sener G, Cebeci BS: Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin Res Cardiol 97: 334–340, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A: Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des 15: 2988–3002, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Korkmaz H, Onalan O: Evaluation of endothelial dysfunction: Flow-mediated dilation. Endothelium 15: 157–163, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, Sezgin N, Muderrisoglu H: Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 59: 1276–1282, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Karbowska A, Boratynska M, Kusztal M, Klinger M: Hyperuricemia is a mediator of endothelial dysfunction and inflammation in renal allograft recipients. Transplant Proc 41: 3052–3055, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Kato M, Hisatome I, Tomikura Y, Kotani K, Kinugawa T, Ogino K, Ishida K, Igawa O, Shigemasa C, Somers VK: Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol 96: 1576–1578, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F: Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 17: 1466–1471, 2006 [DOI] [PubMed] [Google Scholar]
- 22. de ACT, Turner ST, Kullo IJ: Serum uric acid is associated with microvascular function in hypertensive individuals. J Hum Hypertens 21: 610–615, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R: Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation 105: 2619–2624, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD: Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 106: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 25. George J, Carr E, Davies J, Belch JJ, Struthers A: High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Butler R, Morris AD, Belch JJ, Hill A, Struthers AD: Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Guthikonda S, Sinkey C, Barenz T, Haynes WG: Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421, 2003 [DOI] [PubMed] [Google Scholar]
- 28. El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N: Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J 27: 997–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM: Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS: Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M: Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis 56: 264–272, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 44: 642–650, 2004 [PubMed] [Google Scholar]
- 33. Kuo CF, Luo SF, See LC, Ko YS, Chen YM, Hwang JS, Chou IJ, Chang HC, Chen HW, Yu KH: Hyperuricaemia and accelerated reduction in renal function. Scand J Rheumatol 40: 116–121, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS: Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19: 1204–1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodriguez-Iturbe B, Johnson RJ: Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 295: F1134–F1141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M: Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 292: F423–F429, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Sanchez-Lozada LG, Tapia E, Lopez-Molina R, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M: Effects of acute and chronic L-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol 292: F1238–F1244, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ: Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 40: 355–360, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, Walters MR: Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: A randomized, double-blind, placebo-controlled trial. Stroke 39: 3303–3307, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Chessman D, Kostenko L, Lethborg T, Purcell AW, Williamson NA, Chen Z, Kjer-Nielsen L, Mifsud NA, Tait BD, Holdsworth R, Almeida CA, Nolan D, Macdonald WA, Archbold JK, Kellerher AD, Marriott D, Mallal S, Bharadwaj M, Rossjohn J, McCluskey J: Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 28: 822–832, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Dalbeth N, Stamp L: Allopurinol dosing in renal impairment: Walking the tightrope between adequate urate lowering and adverse events. Semin Dial 20: 391–395, 2007 [DOI] [PubMed] [Google Scholar]