Abstract
Summary
Background and objectives
Interventional trials and some observational studies show target hemoglobin >13 g/dl to be associated with higher mortality in erythropoiesis-stimulating agent–treated (ESA-treated) hemodialysis patients; data for peritoneal dialysis (PD) patients are limited.
Design, setting, participants, & measurements
We tested our hypothesis that higher and lower achieved hemoglobin levels are associated with increased mortality in 9269 ESA-treated PD patients from all DaVita dialysis clinics during the time period July 2001 through June 2006 followed through June 2007 using a time-dependent analysis.
Results
Lower hemoglobin was associated with significantly higher all-cause mortality in ESA-treated PD patients: with hemoglobin of 11.0 to <12.0 g/dl as reference, the time-dependent adjusted death hazard ratios for hemoglobin levels of 10.0 to <11.0, 9.0 to <10.0, and ≤9.0 g/dl were 1.12 (1.00 to 1.24), 1.30 (1.12 to 1.50), and 1.38 (1.14 to 1.67), respectively. The time-dependent adjusted hazard ratios for cardiovascular death with hemoglobin levels of 10.0 to <11.0, 9.0 to <10.0, and ≤9.0 g/dl were 1.11 (0.93 to 1.32), 1.37 (1.09 to 1.72), and 1.12 (0.79 to 1.57), respectively. The same trend for association of lower hemoglobin level with higher mortality was seen in African-American and non–African American men and women. In contrast, there was no association between higher achieved hemoglobin and all-cause or cardiovascular mortality in ESA-treated PD patients.
Conclusions
Lower, but not higher, achieved hemoglobin is associated with higher mortality in ESA-treated PD patients. Randomized controlled trials are needed to examine the target hemoglobin level with lowest mortality in PD patients.
Introduction
Observational studies in end-stage renal disease (ESRD) patients treated with hemodialysis and those with earlier stages of chronic kidney disease (CKD) have demonstrated a consistent association between anemia and subsequent morbidity and mortality (1–3). Furthermore, interventional trials in CKD patients have shown a higher risk for adverse cardiovascular events in patients targeted to achieve a higher hemoglobin level using erythropoiesis-stimulating agents (ESAs) (4–6). Consequently, the U.S. Food and Drug Administration has added a “black-box” warning to the product label for erythropoietin and recommends physicians to “individualize dosing to achieve and maintain hemoglobin levels within the range of 10 and 12 g/dl.” However, there is a relative paucity of data for ESRD patients treated with peritoneal dialysis (PD) (7–9). To our knowledge, only three studies have previously examined the association between achieved hemoglobin and outcomes in PD-treated patients; the most recent of these studies evaluated outcomes of incident patients between 1991 and 1998. Although one of these three studies identified a significantly higher risk for adverse outcomes in PD patients with anemia, there was no demonstrably higher risk with higher achieved hemoglobin levels (7–9).
There are several compelling reasons to re-examine the association of achieved hemoglobin levels with outcomes in a contemporary cohort of PD patients. First, only 25% of patients who started PD treatment between 1995 and 2000 were treated with ESAs in the first 12 months (10). In addition, the achieved hemoglobin in recent years is significantly higher than that in the 1990s (11). Third, ESAs are administered subcutaneously in PD patients in contrast to intravenous administration in hemodialysis patients, and hence, doses are lower (10). Fourth, ESA administration and achieved hemoglobin are substantially more dependent upon patient adherence for PD patients than among those treated with hemodialysis. Finally, reimbursement for dialysis services in the United States has changed in a manner that is anticipated to result in a greater use of PD. Starting January 2011, dialysis units will receive a fixed payment to cover the costs of provision of dialysis as well as injectable medications (12). The utilization of injectable drugs is lower for PD patients, and this is anticipated to result in the therapy to be used for a larger number of ESRD patients than is currently the case.
To better inform future decision-making, we undertook this study to test the hypothesis that lower as well as higher achieved hemoglobin is associated with a higher risk for death in a contemporary cohort of PD patients. Furthermore, because a recent study of hemodialysis patients has shown differences in associations of hemoglobin with outcome by race, we sought to examine whether the same racial differences are evident in PD patients (13).
Materials and Methods
Patients
We extracted, refined, and examined data from all individuals with ESRD who underwent PD treatment from July 2001 through June 2006 in any one of the 580 outpatient dialysis facilities of DaVita, a large dialysis organization in the United States (before its acquisition of units owned by Gambro). Of the 164,789 cumulative patients treated in all DaVita units over the 5-year period, 10,528 were undergoing PD treatment at the time of entry into the cohort; of these, 9269 (88%) were receiving ESA therapy at baseline and constituted the study cohort (Supplemental Figure S1). Additional inclusion criteria included dialysis vintage ≥90 days, and availability of at least one hemoglobin measurement in the first quarter of entry into the cohort. The study was approved by relevant Institutional Review Committees.
Clinical and Demographic Measures
The creation of the cohort has been described previously (14,15). To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, (i.e., over a 13-week interval) were averaged and the summary estimate was used in all models. Average hemoglobin values were obtained from up to 20 calendar quarters (q1 through q20). The first (baseline) studied quarter for each patient was the calendar quarter in which the patient's dialysis vintage was >90 days. The presence or absence of diabetes at baseline and the history of tobacco smoking was obtained by linking the DaVita database to the data from Medical Evidence Form 2728 from the U.S. Renal Data System (USRDS). The presence of pre-existing comorbid conditions was similarly ascertained and grouped into 10 categories: ischemic heart disease, congestive heart failure, history of cardiac arrest, history of myocardial infarction, pericarditis, cardiac dysrhythmia, cerebrovascular events, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer (16).
Patients were followed for outcomes through June 30, 2007. The recorded causes of death were obtained from the USRDS, and cardiovascular death was defined as death due to myocardial infarction, cardiac arrest, heart failure, cerebrovascular accident, and other cardiac causes.
Laboratory Measures
Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, within 24 hours. All laboratory values, including hemoglobin, were measured by automated and standardized methods. Hemoglobin was measured at least monthly in virtually all patients. The 3-month-averaged hemoglobin for each quarter was used in our analyses. We divided hemoglobin a priori into seven categories (<9.0, 9.0 to <10.0, 10.0 to <11.0, 11.0 to <12.0, 12.0 to <13.0, 13.0 to <14.0, and ≥14.0 g/dl).
Epidemiologic and Statistical Methods
Data were summarized using proportions and means (±SD). The significance of difference between categorical variables was determined using the chi-squared test and continuous variables using t test or ANOVA as appropriate. Survival analysis was performed using a time-dependent analysis to examine whether hemoglobin predicted survival in ESA-treated PD patients. The primary analysis examined the association between time-dependent hemoglobin with all-cause mortality and cardiovascular mortality. The presence of nonlinearity in the association was tested by adding the quadratic term of hemoglobin to the models already containing the linear term. Additional subgroups analyses were performed in African-American women, African-American men, non–African American women, and non–African American men. For each analysis, including subgroup analyses, three models were examined:
Unadjusted or minimally adjusted models included hemoglobin categories and entry calendar quarter (q1 through q20);
Case-mix adjusted models that included hemoglobin categories and entry calendar quarter plus age, gender, race/ethnicity (African Americans and other self-categorized blacks, non-Hispanic Caucasians, Asians, Hispanics, and others), 10 pre-existing comorbid conditions, history of tobacco smoking, categories of dialysis vintage (<6 months, 6 months to 2 years, 2 to 5 years, and ≥5 years), primary insurance (Medicare, Medicaid, private, and others), and marital status (married, single, divorced, widowed, and other or unknown), presence of diabetes, serum ferritin, iron saturation, and weekly ESA dose; and
Case-mix plus malnutrition-inflammation complex syndrome (MICS) adjusted models included all of the covariates in the case-mix model as well as body mass index, and eight laboratory surrogates with known association with clinical outcomes in hemodialysis patients including serum levels of albumin, total iron-binding capacity, creatinine, phosphorus, calcium, bicarbonate, white blood cell count, and lymphocyte percentage.
Restricted cubic spline graphs were also used to examine nonlinear associations of hemoglobin and all-cause and cardiovascular mortality across four subgroups based on gender and race (African-American men and women and non–African American men and women) using case-mix and MICS adjusted models (17).
Missing covariate data were imputed by the mean or median of the existing values as appropriate. For all analyses, two-sided P values are reported and results considered statistically significant if P < 0.05. All statistical analyses were carried out with SAS, version 9.1 (SAS Institute, Inc., Cary, NC) and STATA version 11.1 (STATA Corporation, College Station, TX).
Results
The baseline demographic, clinical, and laboratory characteristics of the 9269 ESA-treated PD patients, divided into four subgroups based on gender and race, are summarized in Table 1. There was a modest positive correlation between achieved hemoglobin and serum albumin (r = 0.20), and total iron-binding capacity (r = 0.16).
Table 1.
Baseline (first calendar quarter) data of 9269 erythropoietin-stimulating agent–treated peritoneal dialysis patients subdivided based on race and gender
| Entire Cohort | African-American Men | Non-African American Men | African-American Women | Non-African American Women | |
|---|---|---|---|---|---|
| (n = 9269) | (n = 912) | (n = 3935) | (n = 1097) | (n = 3264) | |
| Age (years) | 55 ± 16 | 49 ± 15 | 57 ± 16 | 51 ± 15 | 54 ± 16 |
| Diabetes (%) | 46 | 43 | 49 | 42 | 44 |
| Vintage category (%) | |||||
| <6 months | 65 | 58 | 68 | 57 | 66 |
| 6 to 24 months | 16 | 15 | 17 | 17 | 15 |
| 2 to 5 years | 13 | 17 | 10 | 17 | 13 |
| >5 years | 6 | 10 | 5 | 9 | 6 |
| Primary insurance: Medicare (%) | 58 | 54 | 58 | 59 | 59 |
| Marital status: married (%) | 60 | 58 | 71 | 35 | 56 |
| Presence of ischemic heart disease (%) | 14 | 8 | 20 | 8 | 11 |
| Presence of congestive heart failure (%) | 17 | 14 | 20 | 15 | 15 |
| Presence of history of cardiac arrest (%) | 0.5 | 0.4 | 0.5 | 0.2 | 0.5 |
| Presence of history of myocardial infarction (%) | 6 | 3 | 8 | 3 | 4 |
| Presence of pericarditis (%) | 0.6 | 0.4 | 0.4 | 0.9 | 0.7 |
| Presence of cardiac dysrhythmia (%) | 3.2 | 1.8 | 4.5 | 1.3 | 2.6 |
| Presence of cerebrovascular events (%) | 4.6 | 4.1 | 5.5 | 2.4 | 4.6 |
| Presence of peripheral vascular disease (%) | 8.3 | 5.2 | 10.7 | 4.8 | 7.4 |
| Presence of chronic obstructive pulmonary disease (%) | 3.5 | 1.7 | 4.3 | 2.0 | 3.6 |
| Presence of cancer (%) | 3.5 | 1.9 | 4.1 | 2.3 | 3.5 |
| Body mass index (kg/m2) | 27.0 ± 7.2 | 25.4 ± 4.9 | 25.7 ± 7.7 | 28.3 ± 6.0 | 28.5 ± 7.1 |
| Serum albumin (g/dl) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 |
| Serum creatinine (mg/dl) | 8.4 ± 3.9 | 11.4 ± 4.8 | 8.4 ± 3.7 | 9.5 ± 4.0 | 7.3 ± 3.1 |
| Serum ferritin (ng/ml) | 364 ± 422 | 403 ± 422 | 347 ± 345 | 424 ± 656 | 352 ± 403 |
| Total iron-binding capacity (mg/dl) | 240 ± 50 | 227 ± 48 | 244 ± 48 | 226 ± 48 | 244 ± 53 |
| Serum bicarbonate (mEq/L) | 25 ± 3 | 25 ± 3 | 25 ± 3 | 25 ± 3 | 25 ± 3 |
| Serum phosphorus (mg/dl) | 5.3 ± 1.4 | 5.4 ± 1.5 | 5.3 ± 1.5 | 5.3 ± 1.4 | 5.3 ± 1.4 |
| Serum calcium (mg/dl) | 9.2 ± 0.8 | 9.1 ± 0.8 | 9.1 ± 0.8 | 9.2 ± 0.8 | 9.3 ± 0.8 |
| Hemoglobin (g/dl) | 12.1 ± 1.5 | 12.0 ± 1.6 | 12.3 ± 1.5 | 11.7 ± 1.6 | 12.1 ± 1.5 |
| White blood cell count (×103/μl) | 7.6 ± 2.6 | 6.7 ± 2.4 | 7.7 ± 2.7 | 7.1 ± 2.4 | 7.9 ± 2.5 |
| Lymphocyte (% of total white blood cell count) | 19 ± 8 | 23 ± 8 | 18 ± 7 | 23 ± 8 | 19 ± 7 |
| Erythropoietin stimulating agent dose (units per week) | 7998 ± 8410 | 8218 ± 7549 | 7685 ± 8592 | 8695 ± 7938 | 8024 ± 7313 |
9208 patients have information for race and gender. Mean values are presented as the means ± SDs.
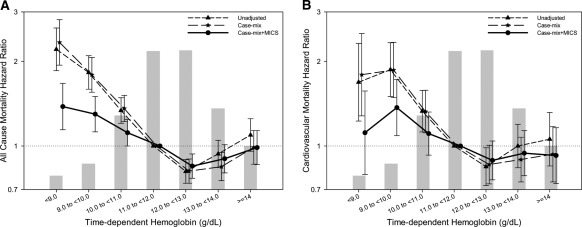
The median follow-up time for the cohort was 755 days (interquartile range: 453 to 1230 days). Figure 1, A and B, shows unadjusted, case-mix, and MICS-adjusted all-cause and cardiovascular death hazard ratios (HR), respectively for time-dependent hemoglobin levels (P < 0.001 for the quadratic hemoglobin term in all models). The HR for the unadjusted and adjusted models for all-cause and cardiovascular mortality are summarized in Table 2. Although individuals with hemoglobin <11.0 g/dl had a higher risk for all-cause and cardiovascular mortality, the risk for all-cause mortality was lowest among those with achieved hemoglobin 12.0 to <13.0 g/dl (reference: 11.0 to <12.0 g/dl). In sensitivity analyses, the cohort was divided into two groups based upon three different cutoff values of hemoglobin (10.0, 11.0, or 12.0 g/dl). In each of the three analyses, individuals with a lower achieved hemoglobin level had a higher unadjusted and adjusted risk for death (Table 3).
Figure 1.
Hazard ratios of mortality for the entire range of hemoglobin in 9269 peritoneal dialysis patients using a time-dependent analysis for all-cause (A) and cardiovascular (B) mortality. Case-mix model is adjusted for age, gender, race/ethnicity, pre-existing comorbid states, tobacco smoking, dialysis vintage, and primary insurance, marital status, presence of diabetes, serum ferritin, iron saturation, and weekly erythropoiesis-stimulating agent dose. MICS-adjusted model includes all of the case-mix covariates as well as body mass index and eight laboratory surrogate variables for nutrition and inflammation. MICS, malnutrition-inflammation complex syndrome.
Table 2.
Hazard ratio of death for time-dependent hemoglobin categories for the entire peritoneal dialysis patient population
| Hemoglobin (g/dl) | Unadjusted |
Case-Mix Adjusted |
Case-Mix and MICS Adjusted |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| All-cause mortality | ||||||
| <9.0 | 2.21 (1.85 to 2.63) | <0.001 | 2.34 (1.94 to 2.82) | <0.001 | 1.38 (1.14 to 1.67) | <0.01 |
| 9.0 to <10.0 | 1.83 (1.59 to 2.10) | <0.001 | 1.79 (1.55 to 2.07) | <0.001 | 1.30 (1.12 to 1.50) | <0.01 |
| 10.0 to <11.0 | 1.34 (1.20 to 1.49) | <0.001 | 1.36 (1.22 to 1.51) | <0.001 | 1.12 (1.00 to 1.24) | 0.05 |
| 11.0 to <12.0 | Reference | Reference | Reference | |||
| 12.0 to <13.0 | 0.81 (0.74 to 0.90) | <0.001 | 0.81 (0.74 to 0.90) | <0.001 | 0.85 (0.77 to 0.94) | <0.01 |
| 13.0 to <14.0 | 0.94 (0.84 to 1.05) | 0.25 | 0.84 (0.75 to 0.95) | <0.001 | 0.90 (0.80 to 1.01) | 0.08 |
| ≥14 | 1.09 (0.96 to 1.25) | 0.18 | 0.99 (0.86 to 1.14) | 0.88 | 0.99 (0.86 to 1.14) | 0.86 |
| Cardiovascular mortality | ||||||
| <9.0 | 1.69 (1.23 to 2.32) | <0.001 | 1.79 (1.28 to 2.51) | <0.001 | 1.12 (0.79 to 1.57) | 0.53 |
| 9.0 to <10.0 | 1.87 (1.50 to 2.33) | <0.001 | 1.86 (1.49 to 2.34) | <0.001 | 1.37 (1.09 to 1.72) | <0.01 |
| 10.0 to <11.0 | 1.33 (1.12 to 1.58) | <0.001 | 1.33 (1.11 to 1.58) | <0.001 | 1.11 (0.93 to 1.32) | 0.26 |
| 11.0 to <12.0 | Reference | Reference | Reference | |||
| 12.0 to <13.0 | 0.84 (0.72 to 0.99) | 0.03 | 0.86 (0.73 to 1.01) | 0.06 | 0.89 (0.76 to 1.04) | 0.15 |
| 13.0 to <14.0 | 1.00 (0.84 to 1.19) | 0.99 | 0.90 (0.75 to 1.08) | 0.24 | 0.94 (0.78 to 1.13) | 0.53 |
| ≥14 | 1.06 (0.85 to 1.32) | 0.62 | 0.94 (0.75 to 1.17) | 0.56 | 0.93 (0.74 to 1.16) | 0.50 |
CI, confidence interval; HR, hazard ratio; MICS, malnutrition-inflammation complex syndrome.
Table 3.
Hazard ratios of all-cause death for different time-dependent hemoglobin categories for the entire peritoneal dialysis patient population
| Hemoglobin (g/dl) | Unadjusted |
Case-Mix Adjusted |
Case-Mix and MICS Adjusted |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| <10 (reference: ≥10) | 1.97 (1.78 to 2.18) | <0.001 | 1.98 (1.77 to 2.21) | <0.001 | 1.36 (1.21 to 1.52) | <0.001 |
| <11 (reference: ≥11) | 1.65 (1.54 to 1.78) | <0.001 | 1.71 (1.58 to 1.85) | <0.001 | 1.28 (1.18 to 1.38) | <0.001 |
| <12 (≥12 as reference) | 1.37 (1.28 to 1.47) | <0.001 | 1.43 (1.33 to 1.53) | <0.001 | 1.22 (1.13 to 1.31) | <0.001 |
CI, confidence interval; HR, hazard ratio; MICS, malnutrition-inflammation complex syndrome.
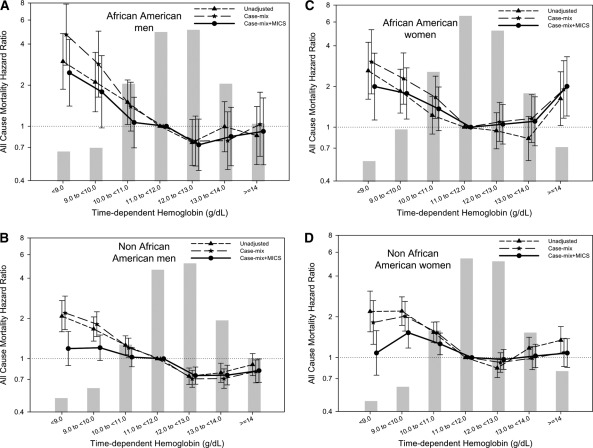
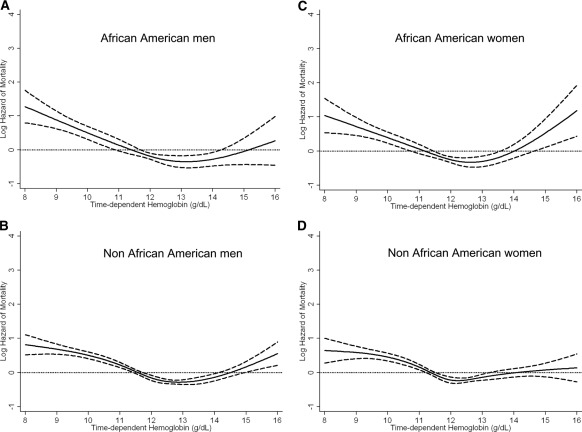
Similar qualitative results with a significant association of lower time-dependent hemoglobin levels with higher all-cause mortality was evident in each of the four subgroups based upon gender and race (African-American men and women and non–African American men and women) using a time-dependent analysis (Figure 2, A through D). Figure 3 shows similar cubic spline trends in all four subgroups. There was no significant effect modification by either gender (P value for the interaction term = 0.08) or race (P = 0.18 for the interaction term) for the association of hemoglobin with all-cause mortality.
Figure 2.
Hazard ratios of all-cause mortality for the entire range of hemoglobin in African-American men (A), non–African American men (B), African-American women (C), and non–African American women (D) treated with peritoneal dialysis using a time-dependent analysis. Case-mix model is adjusted for age, gender, race/ethnicity, pre-existing comorbid states, tobacco smoking, dialysis vintage, primary insurance, marital status, presence of diabetes, serum ferritin, iron saturation, and weekly erythropoiesis-stimulating agent dose. MICS-adjusted model includes all of the case-mix covariates as well as body mass index and eight laboratory surrogate variables for nutrition and inflammation. MICS, malnutrition-inflammation complex syndrome.
Figure 3.
Cubic spline and 95% confidence level of all-cause mortality for the entire range of hemoglobin across four subgroups based on gender and race (African-American men [A], non–African American men [B], African-American women [C], and non–African American women [D]) treated with peritoneal dialysis using a time-dependent analysis. Model adjusted for age, gender, race/ethnicity, pre-existing comorbid states, tobacco smoking, dialysis vintage, primary insurance, marital status, presence of diabetes, serum ferritin, iron saturation, weekly erythropoiesis-stimulating agent dose, body mass index, and eight laboratory surrogate variables for nutrition and inflammation.
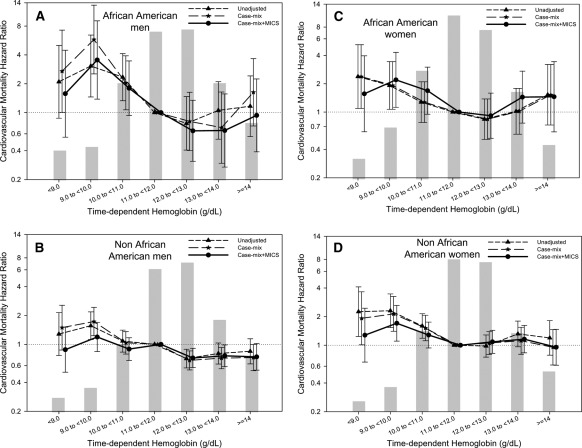
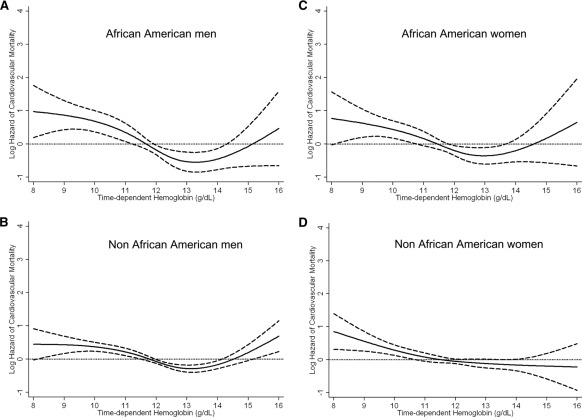
Figure 4 shows the association between time-dependent hemoglobin and cardiovascular death in four different subgroups. Figure 5 shows similar cubic spline trends in all four subgroups. There was no significant effect modification by either gender (P value for the interaction term = 0.98) or race (P value = 0.11 for the interaction term) for the association of hemoglobin with cardiovascular mortality.
Figure 4.
Hazard ratios for cardiovascular mortality for the entire range of hemoglobin in African-American men (A), non–African American men (B), African-American women (C), and non–African American women (D) treated with peritoneal dialysis using a time-dependent analysis. Case-mix model is adjusted for age, gender, race/ethnicity, pre-existing comorbid states, tobacco smoking, dialysis vintage, primary insurance, marital status, presence of diabetes, serum ferritin, iron saturation, and weekly erythropoiesis-stimulating agent dose. MICS-adjusted model includes all of the case-mix covariates as well as body mass index and eight laboratory surrogate variables for nutrition and inflammation. MICS, malnutrition-inflammation complex syndrome.
Figure 5.
Cubic spline and 95% confidence level of cardiovascular mortality of the entire range of hemoglobin across four subgroups based on gender and race (African-American men [A], non–African American men [B], African-American women [C], and non–African American women [D]) using a time-dependent analysis. Model adjusted for age, gender, race/ethnicity, pre-existing comorbid states, tobacco smoking, dialysis vintage, primary insurance, marital status, presence of diabetes, serum ferritin, iron saturation, weekly erythropoiesis-stimulating agent dose, body mass index, and eight laboratory surrogate variables for nutrition and inflammation.
In a sensitivity analysis, qualitatively similar results were obtained for all-cause mortality in both incident and prevalent patients (Figures S2 through S4). In incident PD patients (dialysis time <6 months), time-dependent hemoglobin <9.0 g/dl was associated with higher risk of mortality (Figure S2A). In the prevalent patients, time-dependent hemoglobin <10.0 g/dl was associated with elevated mortality risk (Figure S2B). This trend, in the two groups based on vintage, was also detected in gender and race subgroups (Figures S3 and S4). Similarly, qualitatively similar trends were obtained for cardiovascular mortality in both incident and prevalent patients (Figures S5 through S7).
Discussion
This analysis of data from a large and nationally representative contemporary cohort of 9269 ESA-treated PD patients allows us to make several important observations. First, we confirm the previous findings of an association between lower achieved hemoglobin and higher risk for death in PD patients using a time-dependent analysis. Second, there were no meaningful differences in the relationship between achieved hemoglobin and outcome in subgroups based on gender, race, and dialysis vintage. Third, the range of hemoglobin associated with the lowest risk for death (12.0 to <13.0 g/dl) in this observational study of PD patients has been associated with a higher risk for adverse cardiovascular events in randomized controlled clinical trials of CKD patients, raising the question whether target hemoglobin level should vary for different groups of patients with kidney disease. Furthermore, there was no demonstrable association between higher achieved hemoglobin levels and mortality in ESA-treated PD patients.
In our study, we found an association between lower hemoglobin level and higher risk of all-cause and cardiovascular mortality. Specifically, patients with a time-dependent hemoglobin <9.0 and between 9.0 and <10.0 had a 38% and 30% higher risk of death, respectively. These findings are consistent with previous studies of hemodialysis and CKD patients (1–3). At least three previous studies have evaluated the relationship of achieved baseline hemoglobin levels and outcomes in PD patients; our findings using a time-dependent analysis limited to ESA-treated patients extend the findings from the previous studies (7–9). Two of these three studies enrolled patients from a single center and were unable to demonstrate an association of hemoglobin with any of the clinical outcomes examined in the subgroup of PD-treated patients (sample sizes of 171 and 326 PD patients in studies by Foley et al. and Avram et al., respectively) (7,8). The sample size—and hence the event rate—of these two negative studies in PD patients was rather modest and the studies may have been underpowered to detect this association (7,8). The largest study that has examined this question in the past enrolled 13,974 ESA-treated patients who started PD between 1991 and 1998. In this study, similar to ours, there was no significant difference in the risk for either death or hospitalization in patients with hemoglobin ≥12.0 g/dl, or 11.0 to 11.9 g/dl (9).
Beginning January 2012, the Centers for Medicare and Medicaid Services will implement its first pay-for-performance program—the proposed rules for the Quality Improvement Program place greater emphasis on minimizing the number of patients with hemoglobin levels <10 g/dl compared with those with hemoglobin >12 g/dl. Our findings provide the first such support for that approach for patients treated with PD. Our study does not allow us to definitively determine the reasons for the higher risk with lower hemoglobin levels in PD patients. Hence, the risk for death with lower hemoglobin levels may be attributable to the consequences of anemia (i.e., left ventricular hypertrophy and congestive heart failure), or the underlying conditions that lead to ESA-hyporesponsiveness (i.e., systemic inflammation), or direct consequence of high ESA doses administered in anemic individuals (6,18,19). The higher risk in patients with anemia persisted despite adjusting the data for ESA dose, comorbid conditions, and laboratory surrogates for inflammation. This may suggest that the higher risk for death may, at least in part, be secondary to direct effects of anemia. However, residual confounding is possible, and underlying conditions leading to ESA hyporesponsiveness not accounted for in our multivariate analysis may have contributed to the higher death risk at lower hemoglobin levels.
This study was unable to confirm the findings from a recent study in which the hemoglobin level below which death risk increased was higher in African Americans (<11 g/dl) than in Caucasians (<10 g/dl) (13). Furthermore, there was no difference in this relationship by gender, race, or dialysis vintage. Hence, our findings support the same target hemoglobin level for all patients, irrespective of race, gender, or dialysis vintage.
Finally, in this observational study of PD patients, the lowest risk for death was observed for patients with hemoglobin levels between 12.0 and 13.0 g/dl. This is the same range of hemoglobin previously reported to be associated with the lowest risk for death for patients treated with hemodialysis (3). Furthermore, there was no demonstrable increase in risk with hemoglobin ≥13.0 g/dl. These findings, however, stand in contrast to the observations made in two recent interventional trials in CKD patients (4,5). In the CHOIR study, patients randomized to a target hemoglobin level of 13.5 g/dl had a significantly higher risk for the primary outcome of death, myocardial infarction, hospitalization for congestive heart failure, and stroke; the achieved hemoglobin level in this group was 12.6 g/dl (4). Similarly, CKD patients with diabetes randomized to target hemoglobin of 13.0 g/dl had a twofold higher risk for stroke; the achieved hemoglobin level in these patients was 12.5 g/dl (5). The reasons for these apparently discrepant findings are not clear. It is likely that the relationship between achieved hemoglobin levels and outcomes in ESA-treated patients are influenced by the competing effects of hemoglobin level, ESA responsiveness, and ESA toxicity. In observational studies, patients most likely to achieve hemoglobin between 12.0 and 13.0 g/dl are possibly individuals with the greatest ESA responsiveness and, hence, best overall health. This better overall health may be a more important determinant of outcome than the possible adverse effects of achieved hemoglobin level or ESA toxicity. In contrast, patients with different levels of ESA responsiveness are equally represented in both the intervention and control group in randomized controlled trials, and hence, the differences in outcomes are a direct result of either the achieved hemoglobin level or ESA toxicity. The best way to define the optimal target level for ESA-treated PD patients is a randomized controlled trial. It is unclear if such a clinical trial will be undertaken, and until such a trial is available, it appears prudent to use the targets defined by the Food and Drug Administration for ESA-treated CKD patients for those treated with PD as well.
Our study is notable for its large sample size. The detailed information on baseline covariates allowed us to control for a range of factors known to be related to the mortality of PD patients. The use of a time-dependent analysis further bolsters our findings. However, it is not without limitations. Observational studies such as ours cannot prove causal associations between predictors and outcomes. The information on comorbidities in our study was limited to that obtained from Medical Evidence Form 2728, a form in which comorbid conditions are underreported (16). Another potential limitation is the lack of information of iron therapy and explicit laboratory markers of inflammation such as C-reactive protein. However, we used data on serum albumin, total iron-binding capacity, white blood cell count, and lymphocyte percentage, which have significant associations with inflammation in dialysis patients (20). We also did not have data on PD modality (continuous ambulatory or automated PD), dialysis prescription, peritoneal transport rate, or adequacy of dialysis.
Conclusions
We report that there is a progressively higher risk of death with lower achieved time-dependent hemoglobin in ESA-treated PD patients. This observation is consistent across race, gender, and dialysis vintage. Randomized controlled trials are needed to best define the target hemoglobin levels for PD patients.
Disclosures
Rajnish Mehrotra has received grant support and/or honoraria from Amgen, Baxter Health Care, and Takeda Pharmaceuticals.
The study was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R21 DK 077341 (KKZ and RM), a philanthropist grant from Mr. Harold Simmons (KKZ), and research grants from DaVita Clinical Research (KKZ and RM). MZM received grants from the National Research Fund (NKTH-OTKA-EU 7KP-HUMAN-MB08-A-81231), was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (2008–2011), and is recipient of the Hungarian Eötvös Scholarship (MÖB/66-2/2010).
Supplementary Material
Acknowledgments
We express our sincere appreciation to the teammates in nearly 1600 DaVita clinics who work every day, not only to take care of patients but also to ensure the extensive data collection on which our work is based. We thank DaVita Clinical Research (DCR) for providing the clinical data, analysis, and review for this research project and for advancing the knowledge and practice of kidney care.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W: Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12: 2465–2473, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE: Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int 69: 560–564, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K: Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF: Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 153: 23–33, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 28: 53–61, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Avram MM, Blaustein D, Fein PA, Goel N, Chattopadhyay J, Mittman N: Hemoglobin predicts long-term survival in dialysis patients: A 15-year single-center longitudinal study and a correlation trend between prealbumin and hemoglobin. Kidney Int Suppl (87): S6–S11, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Li S, Foley RN, Collins AJ: Anemia, hospitalization, and mortality in patients receiving peritoneal dialysis in the United States. Kidney Int 65: 1864–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ: Hemoglobin levels and erythropoietin doses in hemodialysis and peritoneal dialysis patients in the United States. J Am Soc Nephrol 15: 174–179, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Medicare and Medicaid Services: 2008 Annual Report, End Stage Renal Disease Clinical Performance Measures Project, in Baltimore, Maryland, Department of Health and Human Services, Center for Medicare and Medicaid Services, Office of Clinical Standards and Quality, December 2008 [Google Scholar]
- 12. Sedor JR, Watnick S, Patel UD, Cheung A, Harmon W, Himmelfarb J, Hostetter TH, Inrig JK, Mehrotra R, Robinson E, Smedberg PC, Shaffer RN: ASN End-Stage Renal Disease Task Force: Perspective on prospective payments for renal dialysis facilities. J Am Soc Nephrol 21: 1235–1237, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Servilla KS, Singh AK, Hunt WC, Harford AM, Miskulin D, Meyer KB, Bedrick EJ, Rohrscheib MR, Tzamaloukas AH, Johnson HK, Zager PG: Anemia management and association of race with mortality and hospitalization in a large not-for-profit dialysis organization. Am J Kidney Dis 54: 498–510, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, Kalantar-Zadeh K: Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 6: 1041–1048, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, Kalantar-Zadeh K: Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. Am J Kidney Dis 2011, in press [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR: Validation of comorbid conditions on the end-stage renal disease medical evidence report: The CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol 11: 520–529, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Durrleman S, Simon R: Flexible regression models with cubic splines. Stat Med 8: 551–561, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Duke M, Abelmann WH: The hemodynamic response to chronic anemia. Circulation 39: 503–515, 1969 [DOI] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Lee GH, Miller JE, Streja E, Jing J, Robertson JA, Kovesdy CP: Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis 53: 823–834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilpatrick RD, Block G, Horwich T, Derose SF: Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 18: 293–303, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.