Abstract
Summary
Background and objectives
Recent interest has focused on wait listing patients without pretreating coronary artery disease to expedite transplantation. Our practice is to offer coronary revascularization before transplantation if indicated.
Design, setting, participants, & measurements
Between 2006 and 2009, 657 patients (427 men, 230 women; ages, 56.5 ± 9.94 years) underwent pretransplant assessment with coronary angiography. 573 of 657 (87.2%) patients were wait listed; 247 of 573 (43.1%) patients were transplanted during the follow-up period, 30.09 ± 11.67 months.
Results
Patient survival for those not wait listed was poor, 83.2% and 45.7% at 1 and 3 years, respectively. In wait-listed patients, survival was 98.9% and 95.3% at 1 and 3 years, respectively. 184 of 657 (28.0%) patients were offered revascularization. Survival in patients (n = 16) declining revascularization was poor: 75% survived 1 year and 37.1% survived 3 years. Patients undergoing revascularization followed by transplantation (n = 51) had a 98.0% and 88.4% cardiac event–free survival at 1 and 3 years, respectively. Cardiac event–free survival for patients revascularized and awaiting deceased donor transplantation was similar: 94.0% and 90.0% at 1 and 3 years, respectively.
Conclusions
Our data suggest pre-emptive coronary revascularization is not only associated with excellent survival rates in patients subsequently transplanted, but also in those patients waiting on dialysis for a deceased donor transplant.
Introduction
It is well established that chronic kidney disease (CKD) is associated with premature atherosclerosis and results in an increased risk of cardiovascular morbidity and mortality (1). Several investigators have performed coronary angiography in asymptomatic end-stage renal disease (ESRD) patients and have found the proportion with significant coronary artery stenoses (defined as stenosis >50%) to be between 37% and 53% (2–4). In a population without renal failure, there is sufficient evidence to show that coronary artery intervention in asymptomatic patients does not improve survival (5). However, this cannot be extrapolated to patients with years of renal failure and the vascular complications associated with the condition.
For patients with CKD, transplantation offers a greater survival advantage over other forms of renal replacement therapy (6). Kidneys from living or cadaver donors are a precious resource, and because cardiovascular death is a major cause of eventual graft loss (7), the decision to diagnose and treat coronary artery disease (CAD) before transplantation is an important issue, particularly because the majority of patients have no cardiac history or symptoms. A recent prospective study from a predominantly Caucasian population in Scotland challenged the practice of coronary angiographic screening before transplantation (8). The authors reported no direct evidence of patient benefit from cardiac screening and suggested that it may serve as a barrier to being placed on a waiting list.
In view of the high risk for cardiovascular disease in renal transplant candidates, our pretransplant practice involves an aggressive approach to invasive cardiac investigations and subsequent revascularization. The purpose of this study was to establish whether ESRD patients awaiting renal transplantation benefit from pre-emptive coronary angiography and intervention.
Materials and Methods
Patient Population
The Imperial College Renal and Transplant Centre in London serves an ethnically diverse population of approximately 3.5 million patients. Currently, approximately 170 to 200 kidney transplants are performed per annum at the Imperial College Renal and Transplant Centre.
Screening Criteria
Our criteria for performing screening coronary angiography on potential transplant recipients include all patients over the age of 50 years, all patients with diabetes mellitus, all patients with cardiac symptoms or disease (irrespective of age), and all patients with an electrocardiogram showing changes suggestive of ischemia or previous myocardial infarction; this included ST segment changes, left bundle branch block and other conduction abnormalities, diagnostic q wave changes in two contiguous leads, or deep T wave changes.
All patients in this study were seen by one of two consultant cardiologists in a dedicated cardiorenal clinic before angiography, and the risks of the procedure were explained. The only exclusion criterion included severe anaphylaxis with contrast administration.
Renoprotection
Patients not on renal replacement therapy had angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and diuretics stopped for the procedure unless clinically contraindicated. All of the patients had oral N-acetylcysteine (600 mg twice daily on days −1, 0, and +1), and biplane angiography with Visipaque® contrast was used. They were not fasted for fluids. Adequate hydration was achieved with the use of intravenous saline from arrival until discharge if patients had no clinical signs of fluid overload. Left ventricular assessment was performed by echocardiography rather than left ventriculography to reduce contrast dose. For those patients with CKD stage 5 and angiographically significant CAD, intervention was staged to minimize the volume of contrast administered in a single dose. Patients on dialysis who were fluid restricted did not undergo any renoprotective measures.
Optimization of Cardiac Disease and Risk Factors
Intervention was at the discretion of a single consultant cardiologist, and in the absence of symptoms the decision to intervene was guided by the angiographic severity of the lesion. If the lesion was felt to be equivocal, evidence of flow limitation of each lesion was sought with pressure wire assessment, directed dobutamine stress echo (DSE), or myocardial perfusion scanning. Hypercholesterolaemia and hypertension were aggressively managed with the use of statins (target cholesterol ≤4.0 mmol/L) and ARBs or ACEIs (target BP <140/90 mmHg) as first-line therapy.
Definitions
Significant coronary disease was defined as >75% stenosis of one or more coronary vessels (9), >50% left main stem disease (10), or an equivocal lesion with flow limitation as described above. Patients were given a diagnosis of “normal” coronary arteries only if their coronary vessels were “pristine” on angiography. Those patients with mild to moderate CAD included patients with mild atheroma or minor plaque disease, as well as those with more advanced lesions that were not severe enough to be defined as “significant.”
A cardiac event was defined as either death caused by cardiac disease, ventricular fibrillation, or pulseless electrical activity cardiac arrest with no identifiable reversible cause, non-ST elevation and ST elevation myocardial infarction (MI), or a primary cardiac arrhythmia. To further clarify “cardiac death,” patients had to have suffered either MI or a primary arrhythmia or have given a clear history of cardiac chest pain in the hours to days leading up to their subsequent death. Deaths out of hospital without postmortem evidence of MI were classified as unknown. Patients were considered “symptomatic” if they had known ischemic heart disease or if they had reported cardiac chest pain; patients with breathlessness alone, which included breathlessness on exertion, were not considered symptomatic because the cause of breathlessness in a patient with ESRD is not likely to be cardiac specific. Hypercholesterolemia was defined as present if the patients had been prescribed cholesterol-lowering agents or they had a total cholesterol of >5.0 mmol/L. Hypertension was defined as present if the patient had been prescribed BP-lowering agents or if they had a systolic BP of >140 mmHg and/or a diastolic BP of >90 mmHg. Ischemic heart disease was defined as present if the patient had evidence of previous intervention on coronary angiography, a previous episode of acute coronary syndrome, or stable angina. A history of smoking was defined as present if the patient was either an ex-smoker or a current smoker.
Statistical Analyses
Continuous variables are expressed as means ± SDs except where stated. The differences between groups were analyzed using chi-squared tests and ANOVA as appropriate. Event-free survival was estimated by the Kaplan–Meier method, with the effect of angiographic finding on outcome assessed using the Weibull proportional hazards model. Results with a P value less than 0.05 were considered significant. Statistical analyses were performed using Stata version 10 (StataCorp LP, College Station, TX).
Results
Demographics
1304 patients were assessed for transplantation between January 2006 and October 2009, with 657 of 1304 (50.3%) undergoing screening angiography. No patients were excluded from angiography unless self-directed. The clinical and demographic characteristics of the patients are outlined in Table 1. 17 patients were lost to follow-up. The population was predominantly Caucasian, although 240 of 657 (36.5%) were South Asian, reflecting the diverse ethnicity of the population in West London. 310 of 657 (47.2%) of the patients were diabetic, with diabetic nephropathy being the commonest cause of CKD in this group of patients. 116 of 657 (17.6%) patients had established coronary artery disease, and a further 42 of 657 (6.4%) of patients had cardiac symptoms as defined previously.
Table 1.
Demographics and clinical characteristics
| 2006 to 2009 (n = 657) | |
|---|---|
| Men | 427 (64.9%) |
| Women | 230 (35.1%) |
| Mean age (years) | 56.55 ± 9.94 |
| Caucasian | 273 (41.6%) |
| South Asian | 240 (36.5%) |
| Afro-Caribbean | 84 (12.8%) |
| Other ethnicity | 60 (9.1%) |
| Diabetes | 310 (47.2%) |
| Hypertension | 612 (93.2%) |
| Hypercholesterolemia | 520 (79.1%) |
| Ischemic heart disease | 116 (17.6%) |
| Smoker | 191/536 (35.6%) |
| Live donor | 122 (49.4%) |
| Deceased donor | 58 (51.6%) |
| Dialysis | 476 (72.5%) |
| Predialysis | 181 (27.5%) |
| Activated on waiting list | 573 (87.2%) |
| Not activated on waiting list | 84 (12.8%) |
| Mean follow-up (months) | 30.09 ± 11.67 |
Of the cohort studied, 573 of 657 (87.2%) patients were considered fit for transplantation after coronary angiography, and 247 of 573 (43.1%) patients were transplanted; 122 of 247 transplants (49.9%) were from a live donor. The mean time from cardiac referral to coronary angiography was 3.42 ± 3.0 months, and that from diagnostic angiography to intervention where indicated was 3.06 ± 2.82 months. The period between diagnostic and interventional angiogram resulted from a combination of planned staged procedures in patients with residual renal function and also occurred when patients with equivocal lesions underwent further noninvasive physiologic investigations.
Exclusion from Transplantation
After coronary angiography, 84 of 657 (12.8%) patients were not put forward for transplantation. The reasons for exclusion from the waiting list are outlined in Table 2. Eight patients were excluded from the waiting list because of morbid obesity, including three female patients with a body mass index of >35 and five male patients with a body mass index of >40. Seven patients were excluded from the waiting list because of infection; these infections included staphylococcal discitis (one patient), sternal wound dehiscence (one patient), osteomyelitis (three patients), and tuberculosis (two patients). With respect to the cardiac reasons for not wait listing patients, the cardiologists were unable to fully revascularize three of 184 (1.6%) patients, 16 of 184 (8.7%) declined intervention, and eight of 657 (1.2%) patients were not activated because of severe left ventricular (LV) dysfunction, defined as ejection fraction (EF) of <30%. Unfortunately, the three patients whose revascularization procedure was either abandoned or considered incomplete all died, at 0.36, 18.1, and 21.6 months after angiography.
Table 2.
Exclusion from transplantation
| 2006 to 2009 (n = 84) | |
|---|---|
| Cardiac | |
| unable to fully revascularize | 3 |
| patient declined intervention | 16 |
| severe left ventricular (LV) dysfunction EF < 30% | 8 |
| Noncardiac | |
| active lupus | 1 |
| cerebrovascular | 3 |
| encapsulating sclerosing peritonitis | 3 |
| gastrointestinal or hepatological | 3 |
| gynecological | 2 |
| hematological | 2 |
| infection | 7 |
| malignancy | 8 |
| morbid obesity | 8 |
| peripheral vascular disease | 4 |
| respiratory | 3 |
| Other | |
| noncompliance | 3 |
| patient choice | 10 |
EF, Ejection Fraction.
Waiting List Status
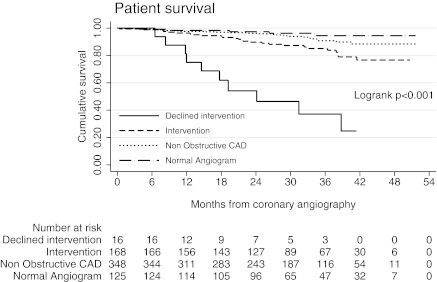
20 of 573 (3.5%) patients who were active on the transplant waiting list died during follow-up. Survival in patients wait listed was 98.9% and 95.3%, respectively, at 1 and 3 years. 40 of 84 (47.6%) patients who were excluded from the transplant waiting list died during follow-up. Survival in patients not wait listed was 83.2% and 45.7%, respectively, at 1 and 3 years. The high mortality rate in the latter group may be attributed to a number of factors; however, the most likely explanation is that the process of pretransplant assessment is rigorous enough to identify and exclude those patients unlikely to survive because of significant comorbidities, both cardiac and noncardiac. Table 3 outlines the cause of death in patients inactive on the wait list and in those patients revascularized and transplanted or revascularized and awaiting deceased donor transplants.
Table 3.
Cause of death
| Patients Inactive on Wait List (n = 84) | Patients Revascularized and Active on Wait List (n = 88) | Patients Revascularized and Transplanted (n = 51) | |
|---|---|---|---|
| Cerebrovascular | 8 (9.5%) | 2 (22.7%) | 1 (1.9%) |
| Cardiac | 12 (14.3%) | 1 (11.4%) | 1 (1.9%) |
| Gastrointestinal hemorrhage | 1 (1.2%) | 1 (11.4%) | 0 |
| Hepatic failure | 1 (1.2%) | 0 | 1 (1.9%) |
| Infection | 4 (4.8%) | 2 (22.7%) | 0 |
| Ischemic bowel | 0 | 0 | 1 (1.9%) |
| Malignancy | 5 (5.9%) | 0 | 0 |
| Vascular | 2 (2.4%) | 0 | 1 (1.9%) |
| Unknown (out of hospital) | 6 (7.1%) | 2 (22.7%) | 0 |
| Withdrawn RRT | 1 (1.2%) | 0 | 0 |
| Total | 40 (47.6%) | 8 (9.1%) | 5 (9.5%) |
RRT, renal replacement therapy.
Medical Therapy
Medical treatment for CAD was recommended where indicated by the cardiologist, and commenced by the renal physicians responsible for the patients' long-term care. Our local protocol is not to standardize the use of beta blockers, because perioperatively beta blockers, as might be used in other surgeries, limit the ability to use inotropes to increase renal perfusion pressure. In this study, 464 of 657 (70.6%) patients were on either an ACEI or ARB or both, and 520 of 637 (79.1%) patients were on statins.
Coronary Revascularization
The coronary angiography findings are outlined in Table 4. 184 of 657 (28.0%) patients were offered coronary intervention, and 168 of 184 (91.3%) of these patients underwent intervention. 93 of 168 (55.4%) patients were asymptomatic or had no previous history of CAD. 49 equivocal lesions were identified; 18 of 49 patients had negative DSE studies and underwent no further assessment, 17 of 49 had negative DSE studies but positive fractional flow reserve studies and as a result underwent intervention, and 14 of 49 patients had positive DSE studies and therefore underwent intervention. Our past experience has led us to interpret negative DSE studies with caution, because false negatives are common in patients with ESRD (11). 117 of 184 (63.5%) patients had percutaneous coronary intervention with deployment of drug-eluting stents in 97 of 117 (82.9%) patients. Bare metal stents were used in 20 of 117 (17.1%) patients, when they expressed a wish to proceed to transplantation without delay. 51 of 184 (27.7%) patients underwent coronary artery bypass graft (CABG) surgery. No serious adverse events occurred as a consequence of elective coronary angiography in this cohort of patients. Those patients who received Clopidogrel® were made active on the waiting list 4 to 6 months after their procedure, unless they received a bare metal stent, when they were able to proceed within 5 weeks of angiography. When transplantation occurred within 1 year of the patient's percutaneous coronary intervention, surgery was performed under cover of platelet transfusion.
Table 4.
Coronary angiographic findings
| Normal Coronary Arteries (n = 125/756) (19.1%) | Mild to Moderate Atheromatous Disease (n = 348/657) (52.9%) | Angiographically Significant Disease (n = 184/657) (28.0%) | |
|---|---|---|---|
| Symptomatic | 0 (0%) | 77 (22.1%) | 81 (44.0%) |
| Asymptomatic | 125 (100%) | 271 (77.9%) | 103 (54.0%) |
| Diabetic | 34 (27.2%) | 152 (43.7%) | 123 (66.8%) |
| Non-Diabetic | 91 (72.8%) | 196 (56.3%) | 61 (33.2%) |
| Intervention Advised | 0 | 0 | 184 (100%) |
| Intervention Declined | 16 (8.7%) | ||
| CABG | 51 (27.7%) | ||
| PCI | 117 (63.6%) | ||
| Deaths (all cause) | 5 | 24 | 36 |
CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
There was a 100% survival associated with the 51 patients who underwent CABG; however, the following adverse events were reported: bilateral pnuemothoraces requiring chest drains (one patient), hemofiltration for acute kidney injury in patients not established on dialysis (11 patients), sternal wound dehiscence (one patient), and tracheostomy for prolonged respiratory wean (two patients). All of the patients not on dialysis pre-CABG who required hemofiltration recovered independent renal function.
Figure 1 shows the overall survival in this group. The patients who declined intervention had 75.0% and 37.1% survival at 1 and 3 years, respectively, with 10 of 16 deaths; six of the 10 deaths were due to cardiac causes. The cardiac risk factors and comorbidities in the patients who declined intervention were similar to those who underwent intervention as advised; of note six of 16 patients were symptomatic yet still declined intervention. The association of revascularization category on cardiac outcome and survival during follow-up were determined by fitting a Weibull proportional hazards model as outlined in Table 5. Compared with those undergoing revascularization, cardiac event and death rates were significantly greater in those who were offered but declined intervention (hazard ratio [HR] 6.7, P = <0.001; and HR 6.0, P = <0.001 respectively) and significantly less frequent in those with low risk angiographic findings who were therefore not offered intervention (HR 0.4, P = 0.01; and HR 0.47, P = 0.005, respectively).
Figure 1.
Patient survival by intervention group, with risk table. CAD, coronary artery disease.
Table 5.
Patient death and cardiac events according to coronary artery disease severity
| Hazard Ratio | 95% CI | |
|---|---|---|
| Cardiac event | ||
| intervention | 1 | |
| normal | 0.1 | 0.03 to 0.56 |
| declined intervention | 6.7 | 2.91 to 15.33 |
| nonobstructive CAD | 0.4 | 0.21 to 0.81 |
| Patient survival | ||
| intervention | 1 | |
| normal | 0.25 | 0.10 to 0.65 |
| declined intervention | 6.0 | 2.91 to 12.57 |
| nonobstructive CAD | 0.47 | 0.27 to 0.81 |
CI, confidence interval; CAD, coronary artery disease.
Patient and Cardiac Event–Free Survival after Transplantation and in Patients Awaiting Transplantation
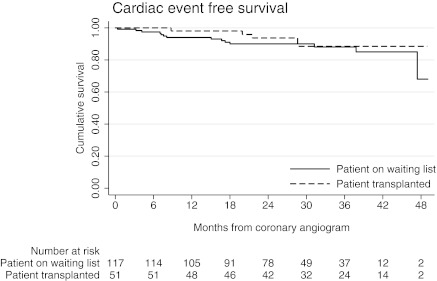
Of the 168 patients who underwent intervention, 51 (30.4%) patients have been successfully transplanted since their cardiac revascularization. Overall patient survival was 100% and 97.2%, and cardiac event–free survival was of 98.0% and 88.4% at 1 and 3 years, respectively, as shown in Figure 2. Of the remaining 117 (69.6%) patients who underwent intervention, 88 (75.2%) were activated on the waiting list for deceased donor transplantation. Overall patient survival was 94.9% and 80.7%, and cardiac event–free survival was 94.0% and 90.0% at 1 and 3 years, respectively. Table 6 summarizes overall and cardiac survival with further division according to whether patients were symptomatic or asymptomatic, intervened on or not intervened on, and transplanted or not transplanted.
Figure 2.
Cardiac event–free survival in patients who have had coronary revascularization, with risk table.
Table 6.
Patient and cardiac event–free survival according to symptoms, intervention, and transplant status
| Group | Subgroup Analysis | Overall Survival 1 Year after Angiogram | Overall Survival 3 Years after Angiogram | Cardiac Event–free Survival 1 Year after Angiogram | Cardiac Event–free Survival 3 Years after Angiogram |
|---|---|---|---|---|---|
| Entire cohort | Symptomatic | 93.8% | 77.8% | 93.8% | 84.7% |
| Asymptomatic | 97.9% | 92.0% | 99.0% | 94.4% | |
| Entire cohorta | Intervention | 96.5% | 83.5% | 95.3% | 86.8% |
| No intervention | 97.8% | 91.5% | 98.9% | 95.1% | |
| Wait-listed | Received transplant | 100% | 97.2% | 98.0% | 88.4% |
| patients | Awaiting transplant | 94.9% | 80.7% | 94.0% | 90.0% |
Excluding patients who declined intervention.
There were 30 deaths with a functioning graft recorded out of 662 patients transplanted between January 2006 and October 2009. One patient (3.3%) died from a cardiac cause. 20 deaths occurred in 324 patients active on the wait list in this study group, and one death (5%) was due to a cardiac cause.
Discussion
This is the largest reported study of cardiac screening on the basis of coronary angiography before renal transplantation. Significant CAD was identified in 184 of 657 (28.3%) angiograms, and 168 of 657 (25.6%) patients in this study underwent coronary revascularization after their cardiac assessment; no other single center observational studies have published data with an intervention rate comparable to this (12–15). This study is of relevance to all clinicians involved with pretransplant assessment when they are considering the risks and benefits of invasive cardiac investigation and treatment. UK Registry data report 16% of all deaths with a functioning graft relating to cardiac causes (16), and the US Renal Data System reports that 30% of deaths with a functioning graft are cardiac (17). Only 3.3% of our patients with functioning grafts died of cardiac causes, and it is notable that only 5% of our patients on the waiting list died of cardiac causes during this period.
Noninvasive investigations looking for reversible cardiac ischemia in asymptomatic patients with significant CAD are not considered reliable in patients with ESRD (18–21), partly because of variability in accuracy reported across centers. The sensitivity, specificity, and positive and negative predictive values for DSE in detecting angiographically significant CAD (defined as >70% visual stenosis) were 88%, 94%, 86%, and 95% in a UK-based study (22), but these are exceptional data. Despite concerns that coronary angiography in patients with stage 4 to 5 CKD may precipitate the need for chronic dialysis because of contrast nephropathy or cholesterol embolization, we have shown that coronary angiographic screening does not accelerate the decline in renal function for patients with advanced CKD (23). No major adverse events occurred as a result of the coronary angiograms carried out in this study. However, 12 adverse events occurred in 32 asymptomatic patients who underwent CABG; all adverse events were treatable, and there was no associated mortality or need for long-term renal replacement therapy after CABG surgery. It is also important to note that 10 of 16 patients with significant CAD who declined intervention died during the follow-up period, with the majority (60%) of the deaths attributed to cardiac causes as has been previously reported (24).
We detected angiographic evidence of CAD in 80.9% of the patients screened and found that 70.3% of patients with CAD had no cardiac symptoms. Cardiac symptoms are a poor indicator of ischemia in patients with diabetes; the development of an autonomic neuropathy has been proposed as the mechanism responsible for the defective anginal warning system (25). Patients with CKD may also lack symptoms, because a significant proportion will have limited mobility restricting full exercise capacity. Our screening criteria are based on a number of conventional risk factors.
Listing for transplantation was facilitated in 573 of 657 (87.2%) patients. The mean time to coronary angiography was 3.42 ± 3.0 months, and the mean time to intervention was 3.06 ± 2.82 months. These patients therefore did not experience a significant delay in being either transplanted or wait listed for a deceased donor organ, a reason used by some groups to not perform angiography in their patients (26). The primary aim of our pretransplant cardiac assessment was to revascularize coronary lesions and then have patients undergo transplantation, not to exclude them. There is an expressed understanding that transplantation provides greatest survival benefit for patients with ESRD, and this should not be denied without good reason. Table 2 shows that the reasons for exclusion from transplantation were mainly noncardiac in this study.
Our data (Figure 2) suggest that pre-emptive coronary angiography and intervention is associated with low cardiac morbidity and mortality in patients subsequently transplanted as well in those patients waiting on dialysis. It has been demonstrated that a functioning renal transplant provides significant protection from cardiovascular death (27). There is also some evidence that cardiovascular disease is slowed or halted after transplantation (6), and in cases of severe congestive heart failure, one study has shown cardiac parameters improved after transplantation (28). In this cohort, although eight patients were not waited listed because of severe LV dysfunction, this is not an absolute contraindication to transplantation. The decision to proceed to wait listing in the setting of severe LV dysfunction is dependent upon the patient accepting high perioperative mortality rates. It is encouraging to be able to demonstrate that of the patients (n = 88) who are still awaiting deceased donor transplant, cardiac event–free survival is similar to the group of patients (n = 51) who underwent intervention followed by transplantation. Cardiac disease is the major cause of death of patients on dialysis (29,30), and our study raises the question of whether or not asymptomatic patients on dialysis who are not considering transplantation should be offered screening and intervention in the same way. Evidence in the literature would support this, with a number of studies reporting the poor prognosis associated with angiographically severe CAD and death or major adverse cardiac event within 1 year of dialysis (31,32). The same group has also demonstrated improved long-term outcomes in dialysis patients with CAD after revascularization (33). The small but significant incidence of coronary events postintervention will require further study, which we are currently undertaking. Within the current renal literature, there are studies with small numbers of mainly symptomatic patients with restenosis rates of 22% to 33% up to 1 year after percutaneous intervention (34,35), and more historical reports of restenosis rates of 60% to 75% up to 1 year after balloon angioplasty (33,36).
The major limitation of this study is that it is observational, and because there is no comparison group, we cannot advocate one line of therapy over another, only comment on the low rates of cardiac events and cardiac deaths within this study group. Also noteworthy is the ethnic diversity of the patients in this study, which consisted of a significant number of South Asians (36.5%) and cannot therefore be applied to predominantly Caucasian or Afro-Caribbean populations. However, the major strength of this study lies in the large number of patients undergoing angiography and, where indicated, coronary revascularization. In addition, there is consistency of clinical practice with a clear protocol for referral, and a single cardiologist making the decision with regards to intervention. Coronary angiography and intervention before transplantation can be achieved in <6 months, and the cardiac assessment process is best done via a dedicated cardiorenal clinic.
Conclusion
Our cardiac assessment and intervention protocol during the pretransplant period may be considered aggressive, but our aim is to have fully optimized our patients' cardiac status in preparation for transplantation. CAD is an important issue for potential transplant recipients, and currently there are limited data identifying which patients will benefit from revascularization. Although coronary intervention strategies in this group remains unclear, we advocate revascularization for potential transplant recipients with flow-limiting coronary artery lesions irrespective of symptoms. This study has demonstrated that this approach is associated with low cardiac morbidity and mortality for those patients transplanted as well as for those patients who remain on dialysis awaiting a deceased donor organ.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Cardiovascular Evaluation before Renal Transplantation: To Cath or Not to Cath?,” on pages 1807–1809.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Herzog CA: How to manage the renal patient with coronary heart disease: The agony and the ecstasy of opinion-based medicine. J Am Soc Nephrol 14: 2556–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S: Cardiovascular risk evaluation before transplantation. J Am Soc Nephrol 16: 843–845, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ohtake T, Kobayashi S, Moriya H, Negishi K, Okamoto K, Maesato K, Saito S: High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: An angiographic examination. J Am Soc Nephrol 16: 1141–1148, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Hayashi T, Obi Y, Kimura T, Iio K, Sumitsuji S, Takeda Y, Nagai Y, Imai E: Cardiac troponin T predicts occult coronary artery stenosis in patients with chronic kidney disease at the start of renal replacement therapy. Nephrology Dialysis Transplantation 23: 2936–2942, 2008 [DOI] [PubMed] [Google Scholar]
- 5. McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B, Shunk K, Jaenicke C, Thottapurathu, Ellis N, Reda DJ, Henderson WG: Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 351: 2795–2804, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B: Kidney transplantation halts cardiovascular disease progression in patients with end stage renal disease. Am J Transplant 4: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 7. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Table H. 29: Mortality rates by primary cause of mortality, 2005–2007, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 8. Patel RK, Mark PB, Johnston N, McGeoch R, Lindsay M, Kingsmore DB, Dargie HJ, Jardine AG: Prognostic value of cardiovascular screening in potential renal transplant recipients: A single-centre prospective observational study. Am J Transplant 8: 1673–1683, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NHJ, Siebes M, Spaan JAE: Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 114: 1321–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Hermann HC, Hillis D, Hutter AM, Witney Lytle B, Marlow RA, Nugent WC, Orszulak TA: ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Surgery). J Am Coll Cardiol 44: e213–e311, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Preston EC, Adamson DL, Frankel A, Baker CSR, Salama AD: Coronary assessment pre-renal transplantation in asymptomatic patients. J Am Soc Nephrol 17: 171, 2006 [Google Scholar]
- 12. Lentine KL, Schnitzler MA, Brennan DC, Snyder JJ, Hauptman PJ, Abbott KC, Axelrod D, Salvalaggio PR, Kasiske B: Cardiac evaluation before kidney transplantation: A practice patterns analysis in Medicare-insured dialysis patients. Clin J Am Soc Nephrol 3: 1115–1124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kasiske BL, Malik MA, Herzog CA: Risk stratified screening for ischaemic heart disease in kidney transplant candidates. Transplantation 80: 815–820, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Lewis MS, Wilson RA, Walker K, Stegeman-Oslen J, Norman DJ, Barry JM, Bennett WM: Factors in cardiac risk stratification of candidates for renal transplantation. J Cardiovascular Risk 6: 251–255, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Patel AD, Abu-Auda WS, Davis JM, Zoghbi GJ, Deierhoi MH, Heo J, Iskandrian AE: Prognostic value of myocardial perfusion imaging in predicting outcome after renal transplantation. Am J Cardiol 92: 146–151, 2003 [DOI] [PubMed] [Google Scholar]
- 16. The Renal Association: UK Renal Registry, 10th Annual Report, 2007 [Google Scholar]
- 17. U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Figure 7.30: Causes of death with functioning graft in patients aged 18 & older, 1997–2006, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 18. Sharma R, Pellerin D, Gaze DC, Gregson H, Streather CP, Collinson PO, Brecker SJ: Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant 20: 2007–2214, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Pilmore H. Cardiac Assessment for renal transplantation. Am J Transplant 6; 659–665, 2006 [DOI] [PubMed] [Google Scholar]
- 20. De Lima JJ, Sabbaga E, Vieira ML, de Paula FJ, Ianhez LE, Krieger EM, Ramires JA: Coronary angiography is the best predictor of events in renal transplant patients when compared with non-invasive testing. Hypertension 42: 263–275, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Rosas SE, Mensah K, Weinstein RB, Bellamy SL, Rader DJ: Coronary artery calcification in renal transplant recipients. Am J Transplant 5:194207, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Sharma R, Chemla E, Tome M, Mehta RL, Gregson H, Brecker SJD, Chang R, Pellerin D: Echocardiography-based score to predict outcome after renal transplantation. Heart 93: 464–469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar N, Dahri L, Brown W, Duncan N, Singh S, Baker CSR, Mailk I, Cairns T, Palmer A, Griffith M, Taube D: The effect of coronary angiographic screening on GFR in patients with advanced CKD. Clin J Am Soc Nephrol 4: 1907–1913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Lima JJ, Gowdak LH, de Paula FJ, Arantes RL, de Oliveira ALV, Ramires JA, César LA, Krieger E: Treatment of coronary artery disease in hemodialysis patients evaluated for transplant: A registry study. Transplantation 89: 845–850, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Caracciolo EA, Chaitman BR, Forman SA, Stone PH, Bourassa MG, Sopko G, Geller NL, Conti CR: Diabetics with coronary disease have a prevalence of asymptomatic ischemia during exercise treadmill testing and ambulatory ischemia monitoring similar to that of non-diabetic patients. Circulation 93: 2097–2105, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Mark PB, Patel RK, Jardine AG: Screening for coronary artery disease before renal transplantation: Rational or rationing? Transplantation 89: 807–808, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Segoloni GP, Quaglia M, Giacosa C, Ferro M, Martina G, Piccoli GB: Renal Transplantation from cadaveric donor after myocardial revascularisation: Still a matter of concern? Transplant Proc 36: 2635, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E: Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end stage renal disease. J Am Coll Cardiol 45: 1051, 2004 [DOI] [PubMed] [Google Scholar]
- 29. The Renal Association: UK Renal Registry, 12th Annual Report, 2009 [Google Scholar]
- 30. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Figure 6.13: Adjusted cause-specific mortality in the first months of therapy: Mortality due to cardiovascular disease, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 31. Joki N, Hase H, Takahashi Y, Ishikawa H, Nakamura R, Imanura Y, Tanaka Y, Saijyo T, Fukazawa M, Inishi Y, Nakamura M, Yamaguchi T: Angiographical severity of coronary atherosclerosis predicts death in first year of hemodialysis. Int Urol Nephrol 35: 289–297, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Hase H, Tsunoda T, Tanaka Y, Takahashi Y, Imamura Y, Ishikawa H, Inishi Y, Joki N: Risk factors for de novo acute cardiac events in patients initiating hemodialysis with no previous cardiac symptom. Kidney Int 70: 1142–1148, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Hase H, Nakamura M, Joki N, Tsunoda T, Nakamura R, Saijyo T, Morishita M, Yamaguchi T: Independent predictors of restenosis after percutaneous coronary revascularization in hemodialysis patients. Nephrol Dial transplant 16: 2372–2377, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Aoyama T, Ishii H, Toriyama T, Takahashi H, Kasuga H, Murakami R, Amano T, Uetani T, Yasuda Y, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T: Sirolimus eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysis. Circ J 72: 56–60, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Sasao H, Hotta D, Maeda T, Saito N, Takagi S, Shimamoto K: Comparison of long term clinical outcome after sirolimus eluting stent implantation in patients with and without hemodialysis. Int Heart J 48: 689–700, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Schoebel FC, Gradaus F, Ivens K, Heering P, Jax TW, Grabensee B, Strauer B, Leschke M: Restenosis after elective coronary balloon angioplasty in patients with end stage renal disease: A case-control study using quantitative coronary angiography. Heart 78: 337–342, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]