Abstract
Summary
Background and objectives
Retinal abnormalities are common in inherited and acquired renal disease. This study determined the prevalence of retinal abnormalities in chronic kidney disease (CKD) stages 3 to 5.
Design, setting, participants, & measurements
One hundred fifty patients with CKD stages 3 to 5 and 150 age- and gender-matched hospital patients with CKD stages 1 to 2 underwent bilateral retinal photography. These images were reviewed for incidental abnormalities, microvascular (Wong and Mitchell classification) and diabetic retinopathy (Airlie House criteria), and macular degeneration (Seddon classification).
Results
Three (2%) patients with CKD stages 3 to 5 had retinal features characteristic of inherited renal disease (atrophy in Myopathy, Encephalopathy, Lactic Acidosis, Stroke-like episodes [MELAS] syndrome; and 2 with drusen in dense deposit disease). Fifty-nine (39%) patients had moderate-severe microvascular retinopathy (hemorrhages, exudates, etc.) compared with 19 (13%) with CKD stages 1 to 2. Forty-one (28%) had moderate-severe diabetic retinopathy (microaneurysms, exudates, etc.) compared with 16 (11%) with CKD stages 1 to 2. Ten (7%) had severe macular degeneration (geographic atrophy, hemorrhage, exudates, membranes) compared with one (1%) with CKD stages 1 to 2. Renal failure was an independent risk factor for microvascular retinopathy, diabetic retinopathy, and macular degeneration. Eleven (7.3%) patients with renal failure and one (0.7%) with CKD stages 1 to 2 had previously unrecognized vision-threatening retinal abnormalities that required immediate ophthalmologic attention.
Conclusions
Retinal abnormalities are common in CKD stages 3 to 5, and are more severe and more likely to threaten vision than in hospital patients with CKD stages 1 to 2.
Introduction
Ocular disease, particularly cataracts and subconjunctival calcification, is common in chronic kidney disease (CKD) stages 3 to 5 (1,2), but retinal abnormalities also occur. These include microvascular and diabetic retinopathy (2), macular degeneration (3), hemorrhage, and calcification (4–6).
Retinal hemorrhage occurs in renal failure as a feature of the moderate-severe forms of microvascular and diabetic retinopathy and of macular degeneration (7–9), and is exaggerated by the bleeding tendency in uremia. Retinal microvascular abnormalities are common because hypertension, renovascular disease, and diabetes account for more than half of all patients with renal failure and also represent “traditional” risk factors for macro- and microvascular disease. “Nontraditional” risk factors such as inflammation, calcification, and endothelial dysfunction may contribute to the increased vascular risk, too (10–12). Diabetes is the single most common cause of CKD worldwide, and many patients with diabetes-associated renal failure also have retinopathy. In addition, recent population-based studies suggest that macular degeneration is increased in renal impairment (3). Risk factors common to renal failure and macular degeneration include increasing age, smoking, and possibly hypertension (13,14).
Many renal diseases also have characteristic retinal features. This is particularly true of inherited renal disease because the inner retina and glomerular filtration barrier share developmental pathways (15) and structural features (16), including ciliated epithelial cells (17), basement membranes comprising α3α4α5 collagen IV, and the extensive capillary beds seen in the choriocapillaris and glomerulus (18). Retinal abnormalities in inherited renal disease include drusen (Alport syndrome, dense deposit disease), coloboma (reflux nephropathy), retinitis pigmentosa (nephronophthisis; Myopathy, Encephalopathy, Lactic Acidosis, Stroke-like episodes [MELAS] syndrome), crystal deposits (oxalosis, cystinosis), and vascular anomalies (Hereditary Angiopathy, Nephropathy, and muscle Cramps syndrome; Fabry disease) (19,20). Retinal effects in acquired renal disease include vasculitis and infarcts in systemic lupus erythematosus, Wegener granulomatosis and microscopic polyangitis (21,22), and possibly central serous retinopathy in Goodpasture syndrome, in which antibodies bind to the internal limiting and Bruch membranes (23). These features are all helpful diagnostically, but it is unclear how often they occur in the typical hospital patient.
We describe here a cross-sectional observational study of patients with CKD stages 3 to 5 from a hospital ward or ambulatory clinic that demonstrated the prevalence of retinal abnormalities, their severity, and their relationship with renal failure.
Study Population and Methods
Study Population
This was a cross-sectional observational study of 150 hospital patients with CKD stages 3 to 5 (estimated GFR [eGFR] <60 ml/min per 1.73 m2 for at least 3 months) and 150 age- and gender-matched patients with CKD stages 1 to 2 (eGFR ≥60 ml/min per 1.73 m2) who were recruited from a renal ambulatory clinic at one teaching hospital, and a renal clinic and general medical ward at another. The participation rate was >80% of individuals approached. The clinic patients were recruited over an 18-month period, and the ward patients over 3 months. eGFR was calculated using serum creatinine estimated with a Beckman Coulter instrument and the Modification of Diet in Renal Disease 175 equation. The laboratory did not stipulate values for eGFR >60 or >90 ml/min per 1.73 m2 for the first year and the final 6 months, respectively, of the study, so all patients with these levels were classified as CKD stages 1 to 2. Proteinuria was not measured. Patients on dialysis were included in the study because they represented a large proportion of those with CKD stage 5. Patients with a renal transplant were excluded because of a possible effect on vascular disease.
Controls were age- and gender- matched patients with CKD stages 1 to 2 from the clinics and ward who were recruited in parallel with those with CKD stages 3 to 5. Hospital patients were chosen as controls because they were well characterized and had similar comorbidities (hypertension, diabetes) as the patients with CKD stages 3 to 5.
The study was approved by the Human Research Ethics Committee of Northern Health and Austin Health in accordance with the principles of the Declaration of Helsinki, and all participants provided signed, informed consent.
Study Assessments
Participants underwent a structured medical interview to identify the underlying renal disease and previously diagnosed hypertension (≥140/90 mmHg), diabetes (random serum glucose ≥11.0 mmol/L), dyslipidemia (serum cholesterol >5.0 mmol/L, HDL <2.0 mmol/L, or statin use), smoking history, and ophthalmologic disease. Laboratory test results (hemoglobin, lipids, hemoglobin A1c) were obtained from the patients' medical records.
Retinal Imaging and Grading
Participants underwent bilateral retinal photography after dark adaptation or dilation with 0.5% tropicamide. At least one image was centered on the macula and another on the optic disc using a KOWA 7 (Kowa Optimed, Inc., Torrance, CA) or Canon CR5-45 (Canon, Tokyo, Japan) nonmydriatic retinal camera.
Photographs were coded, reviewed for incidental abnormalities and hemorrhage, and graded for microvascular retinopathy (7), diabetic retinopathy (8), and macular degeneration by an ophthalmologist and trained observer independently (9). “Red-free” images were examined in all patients to identify hemorrhage and other features. The grade of the more severely affected eye was used in any individual.
Statistical Analyses
Fisher two-tailed test or chi-squared test with Yates correction was used to compare dichotomous variables, and a t test was used for continuous variables. Multivariate logistic regression was performed to determine whether variables had an independent effect on outcome. A result was significant if the odds ratio (OR) was greater or less than 1, the 95% confidence interval (CI) did not include 1.00, and the P value was less than 0.05. Statistical analyses were performed using STATA version 10 software (Stata Corporation, College Station, TX).
Results
Clinical Features of Patients with CKD Stages 3 to 5 or CKD Stages 1 to 2
Both groups comprised 96 men (64%) and 54 women (36%) with a median age of 62 years (range 20 to 85 years). In the patients with CKD stages 3 to 5, renal failure was due to diabetes (54, 36%); glomerulonephritis (38, 25%); hypertension/renovascular disease (26, 17%); reflux or other structural malformations (8, 5%); polycystic kidney disease (7, 5%); cancer, trauma, and nephrotoxic agents (9, 6%); or unknown causes (8, 5%). These patients had CKD stage 3 (n = 48, 32%), stage 4 (n = 40, 27%) or stage 5 (n = 62, 42%), and 49 patients (32%) were dialysis-dependent. Their overall median eGFR was 19 ml/min per 1.73 m2 (range 3 to 59 ml/min per 1.73 m2) compared with 90 ml/min per 1.73 m2 (range 60 to 90 ml/min per 1.73 m2) in patients with CKD stages 1 to 2 (P < 0.001).
Patients with CKD stages 3 to 5 had more hypertension than those with CKD stages 1 to 2 (123, 82% and 68, 45%, respectively; OR 5.49, CI 3.25 to 9.30, P < 0.001), more diabetes (64, 43% and 35, 23%, respectively; OR 2.45, CI 1.49 to 4.02, P < 0.001) and more dyslipidemia (106 of 143, 74% and 66 of 143, 46%, respectively; OR 3.34, CI 2.03 to 5.50, P < 0.001). They were less likely to have smoked than patients with CKD 1 and 2 (78, 52% and 100, 67%, respectively; OR 0.54, CI 0.34 to 0.86, P = 0.01), and they had a lower mean hemoglobin level (119.9 ± 20 g/L compared with 135.3 ± 18 g/L, P < 0.0001).
Incidental Retinal Abnormalities
Three (16.7%) of the 18 patients with CKD stages 3 to 5 and known inherited renal disease had associated retinal abnormalities. These were two patients with macular drusen due to dense deposit disease, and one with MELAS syndrome and retinal atrophy (Figure 1). All three had impaired vision. In all cases, the renal diagnosis was known but the retinal complications were not recognized previously. No coloboma or crystals, no tortuous vessels typical of Fabry disease or Hereditary Angiopathy, Nephropathy, and muscle Cramps syndrome, and no infarcts or calcification were seen in any of the other patients. There were no significant retinal findings in the patients with CKD stages 1 to 2 apart from those described below.
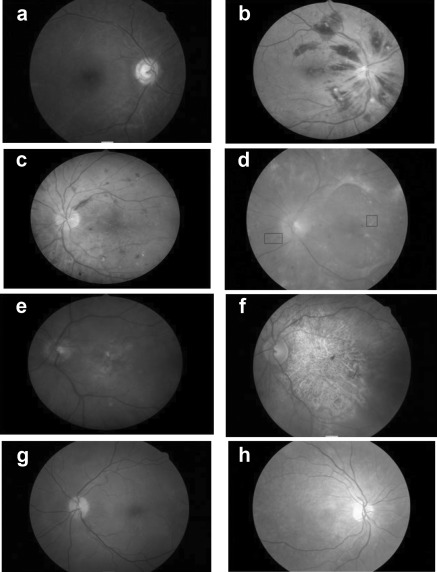
Figure 1.
Retinal appearances in patients with chronic kidney disease stages 3 to 5: (a) thrombosed vessel and retinal atrophy secondary to severe hypertension in focal segmental glomerulosclerosis, (b) multiple hemorrhages from retinal vein thrombosis, (c) undiagnosed diabetic retinopathy, (d) fibrous change in diabetic retinopathy with hemorrhage and exudates, (e) central drusen in macular degeneration, (f) severe geographic atrophy in macular degeneration, (g) macular drusen in a young patient with dense deposit disease, and (h) retinal atrophy in patient with focal segmental glomerulosclerosis and Myopathy, Encephalopathy, Lactic Acidosis, Stroke-like episodes syndrome.
Retinal Abnormalities
Retinal hemorrhage, microvascular and diabetic retinopathy, and macular degeneration were all more common in patients with CKD stages 3 to 5 than in those with CKD stages 1 to 2 (Table 1, Figure 1). Retinal images were gradeable for microvascular retinopathy in all patients, but in patients with CKD stages 3 to 5 they were ungradeable for diabetic changes in one patient and for macular degeneration in 10 patients. Images were ungradeable in two patients with CKD stages 1 to 2 for macular degeneration. Poor images were generally due to coincidental cataract.
Table 1.
Retinal abnormalities in patients with CKD 3 to 5 or CKD 1 to 2
| Retinal Abnormalities | CKD Stages 1 to 2 (n = 150) | CKD Stage 3 (n = 48) | CKD Stage 4 (n = 40) | CKD Stage 5 (n = 62) | CKD Stages 3 to 5 (n = 150) | OR, CI, P (CKD stages 3 to 5 compared with CKD stages 1 to 2) | P for Trend |
|---|---|---|---|---|---|---|---|
| Microvascular retinopathy | 66 (44%) | 25 (52%) | 30 (75%) | 46 (74%) | 101 (67%) | 2.62, 1.64 to 4.20, <0.01 | <0.01 |
| Moderate-severe microvascular retinopathy | 19 (13%) | 12 (25%) | 18 (45%) | 29 (47%) | 59 (39%) | 4.47, 2.50 to 8.00, <0.01 | <0.01 |
| Any diabetic retinopathy | 16 (11%) | 9 of 47 (19%) | 14 (35%) | 18 (29%) | 41 of 149 (28%) | 3.18, 1.69 to 5.98, <0.01 | <0.01 |
| Proliferative diabetic retinopathy | 2 (1%) | 2 of 47 (4%) | 8 (20%) | 8 (13%) | 18 of 149 (12%) | 10.17, 2.32 to 44.65, <0.01 | <0.01 |
| Macular degeneration | 43 of 148 (29%) | 18 of 45 (40%) | 23 of 36 (64%) | 21 of 59 (36%) | 62 of 140 (44%) | 1.94, 1.19 to 3.16, 0.01 | 0.08 |
| Severe macular degeneration (grades 4 to 5) | 1 of 148 (1%) | 3 of 45 (7%) | 3 of 36 (8.3%) | 4 of 59 (6.8%) | 10 of 140 (7%) | 11.31, 1.43 to 89.54, <0.01 | 0.02 |
CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval.
Hemorrhage
Retinal hemorrhage was present in 59 (39%) patients with CKD stages 3 to 5, microvascular retinopathy in 101 (67%) patients, diabetic retinopathy in 41 (28%) patients, and macular degeneration (drusen within 2 disc diameters of the macula) in 62 (44%) patients.
Retinal hemorrhage was increased in patients with CKD stages 3 to 5 (59, 39%) compared with CKD stages 1 to 2 (19, 13%) (OR 4.47, CI 2.5 to 8.0, P < 0.0001). Hemorrhage varied from microaneurysms to multiple, large bleeds including retinal vein occlusion in one patient (Figure 1). Most bleeds were small, transient, and of no visual consequence.
The odds of hemorrhage in CKD stages 3 to 5 were increased with hypertension (OR 4.34, CI 1.97 to 9.55, P < 0.0001), diabetes (OR 4.63, CI 1.82 to 11.75, P = 0.01), and low hemoglobin level (mean difference −10.70, CI −15.91 to 05.49, P < 0.001) but not with aspirin use (P = 0.63) on univariate analysis. On multivariate analysis, hemorrhage was increased with renal impairment (OR 4.22, CI 2.08 to 8.56, P < 0.001), diabetes (OR 4.29, CI 2.31 to 7.99, P < 0.001), and smoking (OR 2.35, CI 1.20 to 4.59, P = 0.01) after adjusting for age, gender, hypertension, dyslipidemia, and anemia.
Microvascular Retinopathy
Microvascular retinopathy was more common in CKD stages 3 to 5 than CKD stages 1 to 2. One hundred one (67%) patients with CKD stages 3 to 5 had a mild or moderate microvascular retinopathy (focal or generalized arteriolar narrowing, arteriovenous nicking, hemorrhage, exudates, etc.; Table 1) compared with 66 (44%) patients with CKD stages 1 to 2 (OR 2.62, CI 1.64 to 4.20, P < 001). Fifty-nine (39%) patients with CKD stages 3 to 5 had moderate-severe microvascular changes (hemorrhage, exudates) compared with 19 (13%) patients with CKD stages 1 to 2 (OR 4.47, CI 2.50 to 8.00, P < 0.0001). Moderate-severe microvascular retinopathy became more common as the hypertension worsened as assessed by the number of antihypertensive agents needed for treatment. Thus, 10 of the 40 patients (25%) with CKD stages 3 to 5 who were using two antihypertensives had a moderate-severe retinopathy compared with 16 of the 31 (51.6%) who were treated with at least four agents (P = 0.027). Moderate-severe microvascular retinopathy was just as common in patients with CKD stage 5 on dialysis as not (22 of 48, 46% and 7 of 18, 39%, P = 0.78). None of the patients had severe microvascular retinopathy with papilledema. Moderate microvascular changes became more common as renal function deteriorated (P < 0.001).
Multivariate analysis demonstrated that reduced eGFR (OR 2.85, CI 1.63 to 5.00, P < 0.001) as well as diabetes (OR 1.83, CI 1.06 to 3.16, P = 0.03), and a smoking history (OR 1.71, CI 1.00 to 2.93, P = 0.05) were independent risk factors for microvascular retinopathy after adjusting for age, gender, hypertension, and dyslipidemia.
Diabetic Retinopathy
Sixty-four (47%) patients with CKD stages 3 to 5 and 35 (23%) patients with CKD stages 1 to 2 had diabetes. Diabetic retinopathy was more common in CKD stages 3 to 5 than CKD stages 1 to 2. Forty-one (41 of 149, 28%) patients with CKD stages 3 to 5 had a moderate-severe diabetic retinopathy (grades 37 to 65, with at least microaneurysms and exudates) compared with 16 (11%) patients with CKD stages 1 to 2 (OR 3.18, CI 1.69 to 5.98, P < 0.001). In addition, diabetic retinopathy became more common as renal function deteriorated in CKD stages 3 to 5 (P < 0.001). Hemoglobin A1c was not different in patients with diabetic retinopathy with CKD stages 3 to 5 compared with CKD stages 1 to 2 (7.9 ± 1.81 and 8.5 ± 2.34%, respectively, P = 0.30).
Proliferative changes (grades 60 to 65, with new vessels, photocoagulation scars, or fibrous proliferation) were also more common in CKD stages 3 to 5 than CKD stages 1 to 2 (18 of 149, 12% compared with 2 of 150, 1%, OR 10.17, CI 2.32 to 44.65, P = 0.001) and similarly became more common as renal function deteriorated (P ≤ 0.001). Two patients without known diabetes had a proliferative diabetic retinopathy.
Multivariate analysis confirmed that diabetes (OR 5.94, CI 3.04 to 11.62, P < 0.001) and reduced eGFR (OR 2.18, CI 1.02 to 4.66, P = 0.04) were independent risk factors for diabetic retinopathy after adjusting for age, gender, hypertension, dyslipidemia, and smoking history.
Macular Degeneration
Macular degeneration (large, soft perimacular drusen, geographic atrophy, hypo- and hyperpigmentation) was increased in patients with CKD stages 3 to 5 compared with patients with CKD stages 1 to 2. Sixty-two (62 of 140, 44%) patients with CKD stages 3 to 5 had these changes compared with 43 (43 of 148, 29%) patients with CKD stages 1 to 2 (OR 1.94, CI 1.19 to 3.16, P = 0.010). Severe changes (grades 4 to 5) were increased in patients with CKD stages 3 to 5 (OR 11.31, CI 1.43 to 89.54, P ≤ 0.005) and became more common as renal function deteriorated (P = 0.02). Four patients with CKD stages 3 to 5 were technically blind from undiagnosed macular degeneration and were referred for urgent ophthalmologic review.
Multivariate analysis confirmed that age (OR 1.04, CI 1.02 to 1.07, P < 0.0001) and CKD stages 3 to 5 (OR 1.79, CI 1.00 to 3.20, P < 0.05) were independent determinants of macular degeneration after adjusting for gender, hypertension, diabetes, dyslipidemia, and smoking history.
Patients Requiring Urgent Ophthalmologic Review
One hundred nineteen (79%) patients with CKD stages 3 to 5 were found in this study to have pathologic retinal abnormalities. These included 82 (55%) patients with changes related to diabetes, macular degeneration, or other significant disease (MELAS syndrome, retinal vein thrombosis, etc.). Sixty-seven (45%) patients with normal renal function had pathologic abnormalities: 16 (11%) with diabetic retinopathy and 1 with late macular degeneration.
Eleven patients with CKD stages 3 to 5 (7%) with a previously undetected retinal abnormality were referred for urgent ophthalmologic review. This was for the retinal complications of dense deposit disease (n = 2) or MELAS syndrome (n = 1), proliferative diabetic retinopathy (n = 3), macular degeneration (n = 4), or retinal vein thrombosis (n = 1). One person with CKD stage 1 to 2 was referred for ophthalmologic management of a diabetic proliferative retinopathy. Thus, patients with CKD stages 3 to 5 were more likely to have retinal abnormalities that required urgent ophthalmologic review than other hospital patients (χ2 with Yates correction = 7.03, P < 0.001).
Discussion
Hospital patients from the wards and clinics with CKD stages 3 to 5 were more likely to have retinal hemorrhage, microvascular and diabetic retinopathy, and macular degeneration than patients with CKD stages 1 to 2. In general, retinal abnormalities were also more severe in patients with CKD stages 3 to 5 and worsened as renal function deteriorated. Retinal changes that threatened vision were also more common in CKD stages 3 to 5 than in other hospital patients, and resources should focus on this patient group.
Retinal Screening for the Diagnosis of Renal Disease
The demonstration of associated retinal features potentially aids in the diagnosis of some forms of renal disease. This is particularly true of inherited renal disease including Alport syndrome. Eighteen patients with CKD stages 3 to 5 in this study were known to have inherited renal disease, and three were demonstrated to have previously unrecognized retinal abnormalities (i.e., retinal atrophy in MELAS syndrome and macular drusen in dense deposit disease). Vision was already impaired at the time of examination and was likely to continue to deteriorate in all three patients. Although there is currently no treatment for these diseases, regular ophthalmologic monitoring may prevent complications such as retinal hemorrhage or detachment and help preserve vision.
This study did not identify any previously undiagnosed renal disease on the basis of the retinal features, possibly because the renal diagnosis was likely to be known by adulthood, many abnormalities in acquired disease resolve after presentation, and some diagnostic abnormalities are demonstrated only with peripheral retinal photographs or special techniques such as optical coherence tomography.
Retinal Hemorrhage
Retinal hemorrhage was found in nearly 40% of patients with CKD stages 3 to 5. This was because hemorrhage is present in hypertension, diabetes, and macular degeneration, all of which were increased in patients in CKD stages 3 to 5, in addition to the tendency for uremic bleeding. Hemorrhage typically did not affect vision, and many small bleeds were only evident using red-free images. Hemorrhage alone did not warrant ophthalmologic review except where it reflected moderate or severe forms of diabetic retinopathy or macular degeneration. Retinal hemorrhage in patients with CKD stages 1 to 2 probably occurred because of associated patient comorbidities.
Microvascular Retinopathy
Microvascular retinopathy occurred in two thirds of the patients with renal failure, and nearly 40% had moderate-severe changes. The retinal vein occlusion may have occurred as a complication of hypertension (24). Although many retinal microvascular features are also present in hypertension, multivariate analysis of patients in this study demonstrated an effect independent of hypertension. This may involve the nontraditional pathways for vascular risk such as inflammation, disturbed calcium homeostasis, and endothelial dysfunction (10–12). We did not assess hypertension on a single BP reading but on a previous expert diagnosis. The relationship demonstrated here between hypertension and microvascular retinopathy presumably occurred because the hypertension was relatively poorly controlled.
In other high-risk populations, retinal microvascular abnormalities predict an increased likelihood of coronary heart disease, stroke, and death (25–27) and correlate with declining renal function (28,29).
Diabetic Retinopathy
Diabetic retinopathy was also more common and more severe in renal failure. Patients with undiagnosed diabetes and unrecognized proliferative retinopathy were identified among the patients with CKD stages 3 to 5. The increased severity of retinal complications in diabetic renal failure emphasizes the need for regular monitoring because the retinopathy is asymptomatic, may progress rapidly if untreated, and visual loss can be prevented or limited with treatment. The presence of a diabetic retinopathy is also a marker of increased risk of cardiac disease and death (30).
Macular Degeneration
Macular degeneration was also more common and more severe in patients with CKD stages 3 to 5. The definition of macular degeneration included drusen within 2 disc diameters of the foveola. These must be distinguished from the smaller, more widespread drusen sometimes seen in glomerulonephritis (31) and other systemic inflammatory conditions.
Most patients with macular degeneration including those with impaired vision were unaware of their diagnosis. We identified four patients with reduced eGFR and previously unrecognized late-stage macular degeneration that should have been monitored to prevent complications.
This study found age and renal failure, but not hypertension, were major determinants of macular degeneration, although it remains difficult to dissect out the independent effects of renal failure and hypertension. Age is a consistent risk factor in other series and hypertension in some, possibly through reducing the ability of the choroidal circulation to clear drusen (32,33). The maculopathy in renal failure is probably mainly inflammatory in nature (34). Again, patients with reduced eGFR should be screened for macular degeneration because of its consequences for vision and the possibility of preventing complications.
Screening to Preserve Vision
In general, microvascular retinopathy is considered nonvision-threatening, but this is not so for diabetic retinopathy and macular degeneration. Patients with diabetes should undergo retinal screening every 2 years and more often if abnormalities are present. Patients with macular degeneration grades 4 and 5 require an ophthalmologic assessment. Interventions preserve vision in diabetes, and monitoring may prevent complications in macular degeneration and other retinal diseases.
In this study, 11 patients with reduced eGFR required urgent ophthalmologic review using current international guidelines. Some had visual loss that could be halted, if not reversed, by interventions such as laser therapy. For others, review and treatment might prevent further complications. None of the four patients with diabetic retinopathy had attended for their routine ophthalmologic screening despite hospital visits for dialysis and renal review.
In conclusion, moderate-severe microvascular retinopathy, proliferative diabetic retinopathy, and late-stage macular degeneration are common, severe, and threaten vision in hospital patients with stages CKD stages 3 to 5. These changes occur more often and are more severe than in patients with CKD stages 1 to 2. Early recognition, monitoring, and intervention will result in a better visual outcome for these patients.
Disclosures
None.
Acknowledgments
We thank the many patients who took part in these studies. This material was presented in abstract form at the annual meeting of the American Society of Nephrology; October 31 through November 5, 2007; San Francisco, CA. An analysis of the retinal vascular caliber in a subset of these patients has been submitted for publication independent of this manuscript. Medical students undertook this work in their B Medical Science year at the University of Melbourne (R.D., A.A., K. N.-F.H.T., Q.L., S.C., and N.A.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Huynh SC, Kifley A, Strippoli GF, Mitchell P: Is renal impairment a predictor of the incidence of cataract or cataract surgery? Findings from a population-based study. Ophthalmology 112: 293–300, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bajracharya L, Shah DN, Raut KB, Koirala S: Ocular evaluation in patients with chronic renal failure—A hospital-based study. Nepal Med Coll J 10: 209–214, 2008 [PubMed] [Google Scholar]
- 3. Liew G, Mitchell Wong TY, Iyengar SK, Wang JJ: CKD increases the risk of age-related macular degeneration. J Am Soc Nephrol 19: 806–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans RD, Rosner M: Ocular abnormalities associated with advanced kidney disease and hemodialysis. Semin Dial 18: 252–257, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Haddad R, Witzmann K, Braun O: Metastatic calcification to the peripheral fundus in chronic-renal-failure. Ophthalmologica 179: 178–183, 1979 [DOI] [PubMed] [Google Scholar]
- 6. Patel DV, Snead MP, Satchi K: Retinal arteriolar calcification in a patient with chronic renal failure. Br J Ophthalmol 86: 1063, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong TY, Mitchell P: Hypertensive retinopathy. N Engl J Med 351: 2310–2317, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Early Treatment Diabetic Retinopathy Study Research Group: Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98: 786–806, 1991 [PubMed] [Google Scholar]
- 9. Seddon JM, Sharma S, Adelman RA: Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 113: 260–266, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Arici M, Walls J: End-stage renal disease, atherosclerosis, and cardiovascular mortality: Is C-reactive protein the missing link? Kidney Int 59: 407–414, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Obialo CI: Cardiorenal consideration as a risk factor for heart failure. Am J Cardiol 99: 21D–24D, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26–33, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP: Smoking and age-related macular degeneration: A review of association. Eye 19: 935–944, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Klein R, Klein BE, Tomany SC, Cruickshanks KJ: The association of cardiovascular disease with long-term incidence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 110: 1273–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Izzedine H, Bodaghi B, Launay-Vacher V, Deray G: Eye and kidney: From clinical findings to genetic explanations. J Am Soc Nephrol 14: 516–529, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Walker P, Welsh M, Wurzner R, Zipfer PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Watnick T, Germino G: From cilia to cyst. Nat Genet 34: 355–356, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Savige J, Liu J, Cabrera Fernandez D, Handa JT, Hageman GS, Wang YY, Vote B, Fassett R, Sarks S, Colville D: The pathogenesis of the perimacular dot and fleck retinopathy in Alport syndrome. Invest Ophthalmol Vis Sci 51: 1621–1627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Savige J, Ratnaike S, Colville D: Retinal characteristics of inherited renal disease in adults and children. J Am Soc Nephrol 2011, in press [DOI] [PubMed] [Google Scholar]
- 20. Duvall-Young J, MacDonald MK, McKechnie NM: Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: A histopathological report. Br J Ophthalmol 73: 297–302, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haynes BF, Fishman ML, Fauci AS, Wolff SM: The ocular manifestations of Wegener's granulomatosis. Fifteen years experience and review of the literature. Am J Med 63: 131–141, 1977 [DOI] [PubMed] [Google Scholar]
- 22. Kraus A, Cervantes G, Barojas E, Alarcón Segovia D: Retinal vasculitis in mixed connective tissue disease. A fluoroangiographic study. J Rheumatol 12: 1122–1124, 1985 [PubMed] [Google Scholar]
- 23. Jampol LM, Lahav M: Ocular clinical findings and basement membrane changes in Goodpasture's syndrome. Am J Ophthalmol 79: 452–463, 1975 [DOI] [PubMed] [Google Scholar]
- 24. Yau JW, Lee P, Wong TY, Best J, Jenkins A: Retinal vein occlusion: An approach to diagnosis, systemic risk factors and management. Int Med J 38: 904–910, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD: Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 287: 1153–1159, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD: Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol 86: 1002–1006, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong TY, McIntosh R: Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 73-74: 57–70, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, Klein BE, Heiss G, Hubbard LD, Duncan BB: Retinal microvascular abnormalities and renal dysfunction: The Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 15: 2469–2476, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Edwards MS, Wilson DB, Craven TE, Stafford J, Fried LF, Wong TY, Klein R, Burke GL, Hansen KJ: Associations between retinal microvascular abnormalities and declining renal function in the elderly population: The Cardiovascular Health Study. Am J Kidney Dis 46: 214–224, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY: Diabetic retinopathy and the risk of coronary heart disease. The Atherosclerosis Risk in Communities Study. Diabetes Care 30: 1742–1746, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Mullins RF, Aptsiaur N, Hageman GS: Structure and composition of drusen associated with glomerulonephritis: Implications for the role of complement activation in drusen biogenesis. Eye 15: 390–395, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Hyman L, Schacht AP, He Q, Leske MC: Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol 118: 351–358, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Friedman E: The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol 130: 658–663, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N: Association between C-reactive protein and age-related macular degeneration. JAMA 291: 704–710, 2004 [DOI] [PubMed] [Google Scholar]