Abstract
Summary
Background and objectives
Acute kidney injury (AKI) complicating cardiopulmonary bypass (CPB) results in increased morbidity and mortality. Urinary hepcidin-25 has been shown to be elevated in patients who do not develop AKI after CPB using semiquantitative mass spectrometry (SELDI TOF-MS). The goals of this study were to quantitatively validate these findings with ELISA and evaluate the diagnostic performance of hepcidin-25 for AKI.
Design, setting, participants, & measurements
A nested, case-control analysis of urinary hepcidin-25 in AKI (n = 22) and non-AKI (n = 22) patients was conducted to validate the SELDI TOF-MS data at the following times: preoperatively; the start of CPB; 1 hour on CPB; on arrival to the intensive care unit; and postoperative days (POD) 1 and 3 to 5. The diagnostic performance of hepcidin-25 was then evaluated in the entire prospective observational cohort (n = 338) at POD 1. AKI was defined as Cr >50% from baseline, within 72 hours postoperatively.
Results
Urinary hepcidin-25/Cr ratio was significantly elevated in all patients at POD 1 compared with baseline (P < 0.0005) and was also significantly elevated in non-AKI versus AKI patients at POD 1 (P < 0.0005). Increased log10 hepcidin-25/Cr ratio was strongly associated with avoidance of AKI on univariate analysis. On multivariate analysis, the log10 hepcidin-25/Cr ratio (P < 0.0001) was associated with avoidance of AKI with an area under the curve of 0.80, sensitivity 0.68, specificity 0.68, and negative predictive value 0.96.
Conclusions
Elevated urinary hepcidin-25 on POD 1 is a strong predictor of avoidance of AKI beyond postoperative day 1.
Introduction
Acute kidney injury (AKI) after cardiopulmonary bypass (CPB) is a serious complication of cardiac surgery, resulting in increased short-term mortality and hospitalization. It is also independently associated with increased mortality up to 10 years after surgery and even a transient postoperative decline in renal function is an independent predictor of developing chronic kidney disease (CKD) over the ensuing 5 years (1–6). Because of the elevated morbidity, mortality, and lack of specific therapeutic interventions, there is clearly a need to understand the pathophysiology of renal ischemia-reperfusion injury (IRI) and to develop novel diagnostic and prognostic markers for the early identification of AKI.
Hepcidin is a member of a cysteine-rich family of antimicrobial proteins of the innate immune system and plays a key role in maintaining iron homeostasis (7). Prepro-hepcidin (84aa) is cleaved to pro-hepcidin (64aa) and subsequently the active form, hepcidin-25. The cleavage forms of hepcidin-20 and hepcidin-22 forms are thought to be degradation products (8). Hepcidin acts by binding to the ferroportin receptor (an iron-exporting protein) in macrophages, hepatocytes, and enterocytes, resulting in the uptake and degradation of ferroportin. Removal of the iron-exporting protein, ferroportin, results in intracellular iron sequestration (7). Hepcidin-25 is synthesized primarily in the liver, freely filtered across the glomerulus, and almost completely reabsorbed, although it is unknown where this takes place in the tubules (9). In addition to the liver, there is renal expression of hepcidin that localizes to the apical pole of the thick ascending limb and collecting tubules, suggesting that hepcidin may be synthesized locally and then released apically into the urine (10).
In a prospective, nested case-control study of CPB patients, our group identified elevated urinary hepcidin-25 in patients who do not develop AKI after cardiac surgery (11). These urines were analyzed with surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI TOF-MS). This technique is only semiquantitative in nature and therefore the first goal of this study was to confirm the SELDI-based measurements using quantitative ELISA in the original cases and controls. The second goal of this study was to characterize the diagnostic performance of hepcidin-25 in the full cohort of 350 cardiac surgery patients who were prospectively enrolled for the original study.
Materials and Methods
The study protocol was approved by the institutional review boards of the University of Manitoba and St Boniface General Hospital.
Nested Case-Control Population, n = 44
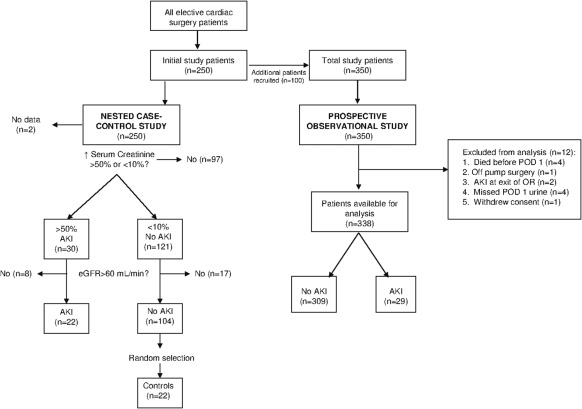
The original nested case-control study has been described in detail (11). Briefly, serial urine samples were collected at arrival to the operating room, start of CPB, 1 hour on CPB, arrival to the intensive care unit (ICU), postoperative day (POD) 1, and POD 3 to 5. An average of two to three preoperative serum creatinine values were used to establish a baseline for serum creatinine. AKI was defined as a >50% rise in serum creatinine from baseline within the first 72 hours postoperatively. Non-AKI was defined as a <10% increase in creatinine (11). Patients with a modification of diet in renal disease eGFR 1.73m2 <60 ml/min or protein:creatinine ratio >100 mg/mmol were excluded to minimize confounding from CKD. The nested case-control consisted of 22 patients with AKI and 22 randomly selected non-AKI patients (Figure 1). Urine from all six time points were used to validate the SELDI TOF-MS findings. The AKI and non-AKI groups were evenly matched with respect to age, gender, and comorbidity. The only differences between groups were pump, cross-clamp, and operating room times (11).
Figure 1.
Derivation of the nested case-control (n = 44) and prospective observational cohort (n = 338). The diagram for the nested case-control cohort is adapted from reference 11. AKI, acute kidney injury; eGFR, estimated GFR; POD, postoperative day; OR, operating room.
A second random sample of non-AKI patients (n = 23) was selected to confirm the kinetics of the hepcidin-25 across all six time points (data not shown). This confirmed that hepcidin-25 was significantly elevated only at POD 1. Therefore, hepcidin-25 was measured at POD 1 in the entire cohort (n = 338).
Prospective Observational Cohort, n = 338
In total, 350 adult cardiac surgery patients were enrolled with informed consent using the protocol described and 12 patients were excluded for the reasons summarized in Figure 1. In this part of the analysis, patients with CKD were included. The primary exposure variable was the urinary hepcidin-25/Cr level on POD 1. The primary outcome variable was AKI defined as a rise in serum creatinine >50% from baseline occurring within 72 hours of surgery. AKI occurring before POD 1 (n = 2) was excluded from the analysis, as it could not be meaningfully attributed to the POD 1 urinary hepcidin-25.
Urine Collection
Urine (10 ml) was collected by Foley catheter intraoperatively and in the ICU, and with a midstream clean catch thereafter. All samples were placed on ice and centrifuged at 2000G × 6 minutes, and the supernatant was stored at −80°C for subsequent analysis.
Urine Hepcidin-25 ELISA
Urinary hepcidin-25 was determined with a commercially available ELISA kit (Bachem S-1337 EIA) on a microplate reader (Biotek Synergy 4 microplate reader, Gen 5 software, Fisher Scientific). All samples that were out of range underwent serial dilution until they were within the linear range of the curve. The intra-assay and interassay coefficient of variation was 2.2% and 2.18%, respectively. Values are represented as a ratio of hepcidin-25/urine creatinine to correct for dilutional factors.
Statistical Analyses
All data were analyzed using SPSS Version 17 (SPSS, Chicago, IL). Descriptive statistics are presented as means ± SD or median [interquartile range]. Univariate differences in continuous variables were compared using either t tests or nonparametric tests (Mann–Whitney and Kruskal–Wallis) depending on the distribution. Categorical variables were compared using the chi-squared test. Linear mixed modeling was used to test for differences in hepcidin-25 between groups and over different time points.
Receiver operating characteristic (ROC) curves and logistic regression were used for the analyses of the association between hepcidin-25 and AKI in the entire cohort. Because of a markedly skewed distribution, the base 10 log of hepcidin-25 and hepcidin-25/Cr ratio was used in these analyses. Univariate logistic regression was used to define the odds ratio (OR) associated with each unit change in log hepcidin. ROC curves were generated and test performance characteristics calculated for a range of cutoff values of log hepcidin-25/Cr. In the multivariate analysis, a best-fit base logistic model for AKI was constructed using forward stepwise variable selection. All of the variables shown in Table 1 were considered for inclusion. Once the base model was defined, the log10 hepcidin-25/Cr was added to the base model to calculate the multivariate OR for AKI associated with hepcidin-25/Cr. The improvement in model discrimination (i.e., change in C-statistic) was calculated for the enriched model. In a secondary analysis, the effect of substituting log10 hepcidin-25 for log10 hepcidin-25/Cr was examined.
Table 1.
Comparison of demographic and perioperative variables in patients who did and did not develop AKI (Cr >50%) within 72 hours of operation (n = 338)
| Variable | Overall (n = 338) | No AKI (n = 310) | AKI (n = 28) | P |
|---|---|---|---|---|
| Age (years) | 63 ± 10 | 63 ± 10 | 66 ± 8 | 0.10 |
| Gender (women %) | 22 | 22 | 14 | 0.30 |
| Race (%) | 0.60 | |||
| white | 90 | 90 | 85 | |
| first nation | 5 | 5 | 9 | |
| other | 5 | 5 | 6 | |
| Comorbid conditions (%) | ||||
| diabetes mellitus | 27 | 27 | 28 | 0.90 |
| chronic obstructive pulmonary disease | 5 | 5 | 4 | 0.80 |
| angina | 88 | 89 | 86 | 0.70 |
| heart failure | 6 | 6 | 3 | 0.50 |
| myocardial infarction | 51 | 51 | 50 | 0.50 |
| prior revascularization | 8 | 8 | 7 | 0.60 |
| peripheral artery disease | 7 | 6 | 17 | 0.04 |
| cerebrovascular disease | 11 | 10 | 21 | 0.09 |
| ejection fraction (%) | 56 ± 16 | 56 ± 16 | 56 ± 16 | 0.30 |
| ejection fraction <30% (%) | 4 | 4 | 5 | 0.60 |
| APACHE | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.5 | 0.70 |
| EURO | 2.1 [1.3, 4.0] | 2.1 [1.2, 3.6] | 2.7 [1.5, 4.7] | 0.10 |
| Preoperative renal function | ||||
| baseline preoperative creatinine (μmol/L) | 87 ± 20 | 86 ± 19 | 95 ± 30 | 0.02 |
| GFR (ml/min per 1.73 m2) | 82 ± 20 | 82 ± 19 | 78 ± 28 | 0.30 |
| MDRD eGFR <60 ml/min per 1.73 m2 (%) | 14 | 13 | 24 | 0.08 |
| Type of surgery (%) | ||||
| coronary artery bypass graft (%) | 87 | 87 | 72 | |
| valve (%) | 13 | 13 | 28 | |
| Intraoperative variables | ||||
| pump time (minutes) | 102 ± 46 | 100 ± 46 | 118 ± 42 | 0.01 |
| cross-clamp time (minutes) | 66 ± 35 | 65 ± 36 | 76 ± 35 | 0.02 |
| total operating room time (minutes) | 229 ± 85 | 228 ± 83 | 255 ± 89 | 0.04 |
| urinary output (ml/h) | 209 [154, 289] | 223 [160, 298] | 172 [118, 233] | 0.02 |
| MAP at start of OR (mmHg) | 98 ± 15 | 97 ± 14 | 99 ± 16 | 0.01 |
| MAP at end of OR (mmHg) | 73 ± 11 | 73 ± 10 | 71 ± 11 | 0.40 |
| Postoperative variables | ||||
| percentage change in creatinine on exit from OR (%) | −10 ± 14 | −11 ± 14 | −1 ± 19 | <0.01 |
| postoperative day 1 hepcidin-25 | 2782 [1153, 4705] | 2912 [1371, 4799] | 849 [312, 3074] | <0.01 |
| postoperative day 1 hepcidin-25/creatinine | 198 [92, 303] | 201 [98, 319] | 68 [19, 206] | <0.01 |
| RBC transfusion in first 24 hours (% patients) | 28 | 28 | 31 | 0.40 |
| vasopressor requirement in first 24 hours (%) | 7 | 6 | 14 | 0.10 |
Continuous variables are expressed as mean ± SD or median [interquartile range] as appropriate depending on the distribution. Categorical values are expressed as %. Independent samples T test was used for Gaussian variables, Mann–Whitney U-test was used for non-Gaussian distributions, and chi-squared test for categorical variables. AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; EURO, European System for Cardiac Operative Risk Evaluation; MDRD, modification of diet in renal disease; eGFR, estimated GFR; OR, operating room; RBC, red blood cell MAP, mean arterial pressure.
Results
Nested Case-Control Analysis, n = 44
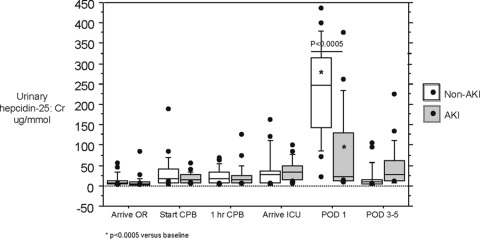
Urinary hepcidin-25 was significantly elevated at POD 1 in all patients undergoing CPB compared with baseline, regardless of whether or not they develop AKI (Figure 2). The principal finding was that hepcidin-25 was significantly elevated in those patients who did not develop AKI compared with those who did develop AKI at POD 1 (Figure 2). This quantitatively validates the semiquantitative results of the SELDI TOF-MS study (11).
Figure 2.
Urinary hepcidin-25/Cr ratio is elevated in all patients at postoperative day 1 (POD 1) compared with baseline (P < 0.0005) and is significantly increased in non–acute kidney injury versus AKI patients at POD 1 (P < 0.0005). OR, operating room; CPB, cardiopulmonary bypass; ICU, intensive care unit.
Prospective Observational Cohort Analysis, n = 338
Twenty-nine patients developed AKI at a median 3 days [interquartile range 2, 3] postoperatively. Table 1 presents the baseline and operative characteristics of patients who did and did not develop AKI. In the univariate comparisons, AKI was associated with peripheral arterial disease, elevated baseline creatinine, longer operative times, lower intraoperative urine output, a higher percentage change in creatinine immediately after surgery, and lower urinary hepcidin-25 levels (Table 1). Both log10 hepcidin-25/Cr and log10 hepcidin-25 demonstrated strong univariate associations with AKI in a linear regression model (Table 2). Discrimination was reasonable for a single predictor variable with an area under the ROC curve >0.7 for AKI defined as Cr >50%. The OR for AKI was <1, indicating that a higher hepcidin-25 level was associated with lower likelihood of AKI, congruent with the nested case-control findings. A cutoff of log10 hepcidin-25/Cr <2.1 yielded a sensitivity and specificity 0.68 for detecting AKI. The negative predictive value (NPV) at this threshold was excellent (0.96), virtually ruling out AKI for values higher than this threshold (Table 3).
Table 2.
Univariate logistic regression, AKI defined as Cr >50%
| Variable at POD 1 | Odds Ratio (95% CI) | P | AUC (95% CI) |
|---|---|---|---|
| Log hepcidin | 0.28 (0.15, 0.51) | <0.0001 | 0.70 (0.70, 0.88) |
| Log hepcidin/Cr | 0.20 (0.10, 0.41) | <0.0001 | 0.73 (0.64, 0.84) |
AKI, acute kidney injury; AUC, area under the curve; CI, confidence interval; POD, postoperative day.
Table 3.
Sensitivity and specificity of urinary log(hepcidin-25/Cr) for AKI defined as Cr >50%
| Urine log(hepcidin-25/Cr) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|
| <0.9 | 0.07 (0.01, 0.23) | 0.99 (0.97, 0.998) | 0.50 | 0.92 |
| <2.1 | 0.68 (0.58, 0.90) | 0.68 (0.48, 0.84) | 0.16 | 0.96 |
| <2.5 | 0.96 (0.82, 0.999) | 0.29 (0.24, 0.34) | 0.11 | 0.99 |
AKI, acute kidney injury; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
The multivariate analysis is summarized in Table 4. The small number of AKI events (n = 29) resulted in models with three or fewer independent variables. The combination baseline preoperative creatinine and percentage change in creatinine on arrival to ICU yielded the best prediction of AKI. Model discrimination of this two-variable model was similar to that of urinary hepcidin alone. When log10 hepcidin-25/Cr was added to this base model, it remained highly statistically significant (OR 0.26, 95% confidence interval 0.12 to 0.53, P < 0.0001) and produced a statistically significant improvement in the model area under the ROC curve, which increased from 0.75 to 0.80. In a secondary analysis, substitution of log10 hepcidin-25 gave very similar model performance, suggesting that correction for dilution factors with urinary creatinine makes minimal difference (Table 4).
Table 4.
Multivariate logistic regression
| Model and Variables | Odds Ratio (95% CI) | P | AUC (95% CI) |
|---|---|---|---|
| Model 0 (base) | <0.0001 | 0.75 (0.66, 0.84) | |
| baseline creatinine (per μmol/L) | 1.03 (1.01, 1.04) | ||
| percentage change in creatininea | 1.06 (1.03, 1.08) | ||
| Model 1 | <0.0001 | 0.80 (0.71, 0.89)b | |
| baseline creatinine (per μmol/L) | 1.02 (1.00, 1.04) | ||
| percentage change in creatininea | 1.06 (1.03, 1.08) | ||
| log hepcidin-25 | 0.30 (0.15, 0.60) | ||
| Model 2 | <0.0001 | 0.80 (0.70, 0.89)b | |
| baseline creatinine (per μmol/L) | 1.02 (1.00, 1.04) | ||
| percentage change in creatininea | 1.05 (1.02, 1.08) | ||
| log hepcidin-25/creatininec | 0.26 (0.12, 0.58) |
AUC, area under the curve; CI, confidence interval.
Percentage change in serum creatinine refers to the relative change in creatinine from baseline to exit from the operating room.
P < 0.05 compared to base model.
Hepcidin-25 corrected for urinary creatinine.
Discussion
Using semiquantitative methods, our group previously identified hepcidin-25 as a marker of preserved renal function in patients undergoing elective heart surgery with CPB. The present study has quantitatively validated these earlier results, showing that hepcidin-25 is upregulated in all patients by POD 1, but significantly more so in non-AKI versus AKI patients. Furthermore, the diagnostic performance of hepcidin-25 was evaluated in the entire prospective cohort of CPB patients (n = 338). The principal finding of this study is that hepcidin-25 is strongly and independently associated with avoidance of AKI after CPB (OR 0.26), and significantly improves discrimination of a multivariate base model of AKI. In this cohort, with a frequency of AKI of 8.6%, a high log10 hepcidin-25/Cr level (i.e., >2.1) largely excluded the risk of AKI (NPV 0.96). A low hepcidin level, however, was not useful for predicting AKI (positive predictive value 0.11 to 0.50). These results are promising and justify further validation of hepcidin-25 as a biomarker of preserved renal function in an independent cohort.
The search for biomarkers of early AKI has focused primarily on proteins associated with the progression or development of renal injury (e.g., NGAL, KIM-1, and IL-8). However, an unbiased proteomic approach has allowed identification of a unique protein, hepcidin-25, that may be associated with protection from AKI. Analysis of intraoperative urine samples demonstrates that all patients undergoing CPB experience renal tubular stress or injury (11,12). Despite this, only a minority go on to develop clinical AKI, suggesting that there may be downstream pathways that, when activated in response to renal tubular stress, result in either renoprotection or further injury. Our study demonstrates that all patients have an elevation in urinary hepcidin-25 after CPB compared with baseline, but it is much higher in those patients that do not subsequently develop AKI. We postulate that hepcidin-25 may play a role in renoprotection during IRI, and patients that cannot effectively upregulate hepcidin-25 in response to renal tubular stress/injury are more likely to develop clinical AKI. One mechanism by which this may occur is through hepcidin-25's role in promoting intracellular iron sequestration (7,8), thereby potentially limiting oxidative stress, free radical damage, and renal injury.
Tubular toxicity of iron was first observed 30 years ago when chelation of urinary iron prevented ischemic renal injury in rats (13–15). More recently, there has been accumulating evidence for the role of labile free iron in potentiating renal IRI in human models of CPB (16,17). Hemolysis is an unavoidable side effect of CPB and the release of plasma free hemoglobin is directly correlated with length of time on CPB (18). In an observational study of thoraco-abdominal and thoracic aortic repair, elevated plasma free hemoglobin was independently and significantly associated with renal tubular injury markers (N-acetyl-β-d-glucosaminidase) as well as clinical AKI (12). Perioperative red blood cell (RBC) transfusions may be another source of labile free iron. Indeed, RBC transfusions are strongly and independently associated with the development of AKI postcardiac surgery and the detrimental effects of storage on RBC deformability may result in further hemolysis with the release of plasma free hemoglobin (19,20).
Another potential source of labile iron in CPB contributing to postoperative AKI is serum myoglobin. It has been found to independently predict postoperative AKI after coronary artery bypass grafting after adjustment for confounding factors (21). These findings are supported by serum myoglobin being strongly associated with AKI after thoraco-abdominal and thoracic aortic repair (22). Heme protein breakdown, whether from hemolysis or rhabdomyolysis, are potential sources of labile iron that may contribute to AKI associated with CPB.
These observations are further supported by the protective role that iron sequestration plays in renal IRI. In a seminal study, exogenously administered NGAL was demonstrated to be renoprotective in mouse IRI via upregulation of heme-oxygenase 1 (23). It is important to note that the renoprotective effects were specifically dependent on iron being bound in the NGAL:siderophore:Fe complex. Furthermore, the upregulation of heme-oxygenase 1 suggests that iron trafficking and homeostasis plays a key role in renal IRI (23). The role of labile iron in potentiating renal injury may not be unique to IRI, as several iron-binding proteins have been detected in the urinary samples of children with human immunodeficiency virus nephropathy and urinary hepcidin-25 appears to be associated with renal relapse in lupus nephritis (24,25). This raises the possibility of a common renal injury pathway involving iron and iron-binding proteins. Altogether, accumulating evidence strongly suggests that free iron plays a key role in renal IRI and a clinical trial is currently underway to evaluate the utility of the iron chelator, deferoxamine, as a renoprotective agent for AKI (NCT00870883) (16).
Our study has several limitations that should be considered when interpreting the results. First, without concurrent serum and urine samples it is not possible to determine the source of the urinary hepcidin-25. Although prepro-hepcidin (84aa), hepcidin-20, and hepcidin-22 have been found to accumulate in renal insufficiency, serum hepcidin-25 has been demonstrated to be independent of GFR in CKD (26–28). Furthermore, recent data demonstrates that only 10% of hepcidin-25 may be freely circulating and serum hepcidin-25 is largely bound to α2-macroglobulin, thereby limiting renal clearance (29). This suggests that the elevated urinary hepcidin-25 observed in non-AKI patients may originate from increased renal expression as opposed to decreased filtration in the AKI group. Nevertheless, further studies are needed to definitively characterize the source and kinetics of hepcidin-25 in renal IRI.
Second, there is no standardization for quantification of the differences between immunoassays and mass spectrophotometric assays (30). A recent international study was done with the goal of harmonizing hepcidin-25 measurement, and it demonstrated up to a tenfold difference between assays (31). Although there were differences in absolute hepcidin-25 measurements, all assay results fell within a similar range and the same trends were identifiable (31). Nevertheless, our ELISA observations replicate our SELDI TOF-MS findings, so we feel these results accurately reflect the elevation of hepcidin-25 in non-AKI versus AKI patients after CPB.
Third, our study is observational and therefore a causal association between hepcidin-25 and renoprotection cannot be proved. Renal cross-clamp animal studies using exogenously administered hepcidin-25 would be helpful for determining whether hepcidin-25 is truly protective in IRI, or whether it is just an epiphenomenon. Fourth, this is a single-center study using a surrogate creatinine-based measure of AKI. The clinical utility of hepcidin has not been established by our data. Nevertheless, the present results justify further validation in independent cohorts large enough to allow analysis of hard outcomes such as need for dialysis and mortality. Moreover, it would be useful to determine how urinary hepcidin-25, as a marker for avoidance of AKI, performs in combination with other biomarkers of AKI (e.g., NGAL, KIM-1, and IL-18).
In summary, we have demonstrated that urinary hepcidin-25 is elevated at POD 1 in all patients undergoing CPB but it is significantly higher in those patients that do not subsequently develop AKI. Furthermore, hepcidin-25 is independently associated with lower AKI risk (OR 0.26) and may be useful for ruling out AKI (NPV 0.96). We postulate that hepcidin-25 is protective in renal IRI via degradation of the ferroportin receptor, resulting in decreased toxicity from labile iron. Further characterization of the role of hepcidin-25 is likely to provide important insights into the pathophysiology of AKI and potentially contribute to therapeutics for the early treatment of AKI. Finally, urinary hepcidin-25 may be useful as a biomarker for avoidance of AKI.
Disclosures
None.
Supplementary Material
Acknowledgments
Drs. Reslerova and Rigatto were funded by the Satellite Healthcare Coplon Grant. Dr. Ho was funded by the Bristol Myers Squibb Cardiovascular Fellowship. Drs. Nickerson and Rush are funded by the Canadian Institute of Health Research. The authors acknowledge the SBH CVT program.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Role of Catalytic Iron in Acute Kidney Injury,” on pages 2329–2331.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A: Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 119: 2444–2453, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Swaminathan M, Hudson CC, Phillips-Bute BG, Patel UD, Mathew JP, Newman MF, Milano CA, Shaw AD, Stafford-Smith M: Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thor Surg 89: 1098–1104, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Brown JR, Cochran RP, MacKenzie TA, Furnary AP, Kunzelman KS, Ross CS, Langner CW, Charlesworth DC, Leavitt BJ, Dacey LJ, Helm RE, Braxton JH, Clough RA, Dunton RF, O'Connor GT: Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg 86: 4–11, 2008 [DOI] [PubMed] [Google Scholar]
- 4. van Kuijk JP, Flu WJ, Chonchol M, Hoeks SE, Winkel TA, Verhagen HJ, Bax JJ, Poldermans D: Temporary perioperative decline of renal function is an independent predictor for chronic kidney disease. Clin J Am Soc Nephrol 5: 1198–1204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA: Immediate post-operative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Nemeth E, Ganz T: The role of hepcidin in iron metabolism. Acta Haematol 122: 78–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park CH, Valore EV, Waring AJ, Ganz T: Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EH, Tjalsma H: Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS One 3: e2706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W: The iron-regulatory peptide hormone hepcidin: Expression and cellular localization in the mammalian kidney. J Endocrinol 184: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M: Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: A nested case-control study. Am J Kidney Dis 53: 584–595, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen Windsant ICV, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ: Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int 77: 913–920, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Paller MS: Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol 255: F539–F544, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Shah SV, Walker PD: Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol 255: F438–F443, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Zager RA, Gamelin LM: Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 256: F446–F455, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Haase M, Bellomo R, Haase-Fielitz A: Novel biomarkers, oxidative stress and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol 55: 2024–2033, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Prowle JR, Westerman M, Bellomo R: Urinary hepcidin: An inverse biomarker of acute kidney injury after cardiopulmonary bypass? Curr Opin Crit Care 16: 540–544, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Vanek T, Snircova J, Spegar J, Straka Z, Horak J, Maly M: Increase in plasma free haemoglobin during cardiopulmonary bypass in heart valve surgery: Assessment of renal dysfunction by RIFLE classification. Perfusion 24: 179–183, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS: Acute kidney injury after cardiac surgery: Focus on modifiable risk factors. Circulation 119: 495–502, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ho J, Sibbald WJ, Chin Yee IH: Effects of storage on efficacy of red cell transfusion: When is not safe? Crit Care Med 31: S687–S697, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Benedetto U, Angeloni E, Luciani R, Refice S, Stefanelli M, Comito C, Roscitano A, Sinatra R: Acute kidney injury after coronary artery bypass grafting: Does rhabdomyolysis play a role? J Thorac Cardiovasc Surg 140: 464–470, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Miller CC, Villa MA, Sutton J, Lau D, Keyhani K, Estrera AL, Azizzadeh A, Coogan SM, Safi HJ: Serum myoglobin and renal morbidity and mortality following thoracic and thoraco-abdominal aortic repair: Does rhabdomyolysis play a role? Eur J Vasc Endovasc Surg 37: 388–394, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soler-Garcia AA, Johnson D, Hathout Y, Ray PE: Iron-related proteins: Candidate urine biomarkers in childhood HIV-associated renal diseases. Clin J Am Soc Nephrol 4: 763–771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X, Jin M, Wu H, Nadasdy T, Nadasdy G, Harris N, Green-Church K, Nagaraja H, Birmingham DJ, Yu CY, Hebert LA, Rovin BH: Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int 74: 799–807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taes YEC, Wuyts B, Boelaert JR, De Vriese AS, Delanghe JR: Prohepcidin accumulates in renal insufficiency. Clin Chem Lab Med 42: 387–389, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Malyszko J, Malyszko JS, Pawlak K, Mysliwiec M: Hepcidin, iron status, and renal function in chronic renal failure, kidney transplantation, and hemodialysis. Am J Hematol 81: 832–837, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Peters HPE, Laarakkers CMM, Swinkels DW, Wetzels JF: Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant 25: 848–853, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E, Huang ML, Rahmanto YS, Richardson DR, Vyoral D: Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood 113: 6225–6236, 2009 [DOI] [PubMed] [Google Scholar]
- 30. MacDougall IC, Malyszko J, Hider RC, Bansal SS: Current status of the measurement of blood hepcidin levels in chronic kidney disease. Clin J Am Soc Nephrol 5: 1681–1689, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, Hider RC, Koliaraki V, Mamalaki A, Olbina G, Tomosugi N, Tselepis C, Ward DG, Ganz T, Hendriks JC, Swinkels DW: Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: Need for standardization. Haematologica 94: 1748–1752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.