Abstract
Summary
Background and objectives
Peritoneal dialysis adequacy is typically assessed by urea clearance corrected for total body water (TBW) on the basis of anthropomorphic equations, which do not readily take into account changes in body composition, which may vary between ethnic groups. To determine whether ethnicity could affect estimates of peritoneal dialysis adequacy, we compared TBW estimated by anthropomorphic equations and that measured by multifrequency bioimpedance spectroscopy.
Design, setting, participants, & measurements
We calculated TBW in 600 healthy adult peritoneal dialysis outpatient attending two tertiary university hospitals serving an inner-city multiethnic population who had TBW measured by multifrequency bioimpedance spectroscopy performed.
Results
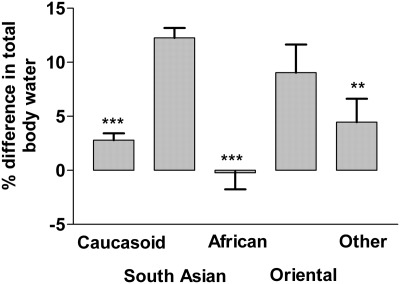
600 adult peritoneal dialysis patients were studied: mean age, 56.7 ± 0.6 years; 54.2% men; 29.7% diabetic; mean body mass index, 26.1 ± 0.2; 47.3% Caucasian; 29.2% South Asian; 12.8% African/Afro-Caribbean. Total body water was calculated using several anthropomorphic equations and was higher than that calculated MEASURED BY MF-BIS for all ethnic groups, apart from African/Afro-Caribbeans, with the greatest difference between Watson calculated TBW and multifrequency bioelectrical impedance spectroscopy 12.3 ± 0.6% for the South Asians, 9.0 ± 2.6% for Far Eastern Asians, 2.8 ± 0.6% Caucasians, and −0.2 ± 1.5% for African/Afro-Caribbeans.
Conclusions
In this United Kingdom–based multiethnic population, body composition differed particularly for the South Asian patients compared with Caucasians and African/Afro-Caribbeans. Overestimation of TBW by anthropomorphic-based equations would lead to a lower calculation of Kt/Vurea, which may lead to changes in peritoneal dialysis prescription to achieve clinical standard targets and also affect studies examining the relationship between Kt/V and survival.
Introduction
More than 100,000 patients with chronic kidney disease are treated by peritoneal dialysis. Although peritonitis remains the commonest complication and leading cause of transfer to hemodialysis (1), changes in peritoneal membrane function and structure occur with time, leading to changes in small solute clearances and ultrafiltration failure. Small solute clearance is one of the measurements used to determine adequate dialysis treatment. Although the relationship between small solute clearance and clinical outcome is somewhat more complex in peritoneal dialysis patients because of the relative contribution of residual renal function and peritoneal clearances (2–4), clinical guideline committees in both North America (5) and the United Kingdom (UK) (6) have advised a minimum weekly urea clearance of 1.7, adjusted for total body water by using anthropomorphic-based equations (7,8). Although it has been reported that there is a strong correlation between these anthropomorphic-based equations and total body water measured by bioimpedance techniques in healthy patients (9), body composition may vary with ethnicity (10,11) and also chronic disease (12,13). We therefore decided to compare total body water estimation by anthropomorphic equations and bioimpedance spectroscopy in an ethnically diverse peritoneal dialysis population.
Materials and Methods
Six hundred healthy adult patients treated by peritoneal dialysis under the care of two tertiary university hospitals who attended for routine outpatient peritoneal dialysis equilibration testing and bioimpedance assessments were studied between May 2007 and May 2010. Patients with amputations and cardiac pacemakers or defibrillators were excluded from study because bioimpedance measurements were not made. Bioelectrical impedance measurements were performed in a standardized manner as previously reported, as part of established routine clinical care (14,15). Direct multifrequency bioelectrical impedance spectroscopy (MF-BIS) analysis method was used using hand and feet tactile electrode system (Biospace in body 720, Seoul, South Korea; BCM, Fresenius Medical Care, Bad Homberg, Germany). Height was measured by a standard wall mounted measure (Sigmeas 1, Doherty signature range, www.mediclick.co.uk). Serum albumin was measured by the bromcresol green method. Racial origins were self-reported and checked against National Health Service records, and if discrepant National Health Service records were used. Calculation of total body water was performed using the equations of Watson et al. (7), Hume and Weyers (8), Lee et al. (10), Chumlea et al. (11), Chertow et al. (13), Johansson et al. (16), and the HEMO Study (17) (Appendix). Ethical approval was granted by the local ethical committee as audit and clinical service development.
Statistical Analyses
Statistical analysis was by ANOVA with Tukey post-analysis correction; if variables were not normalized by log transformation, then Dunnett's correction was used for post-analysis. Simple correlation was by Pearson correlation, and multiple linear regression analysis was performed in a step backward fashion, using all variables that were statistically significant on simple correlation analysis and then removed if confidence limits crossed zero or did not improve the fit of the model. Because both dialysis vintage and urine output were not normally distributed, both were log transformed, with anuric patients given a urine output of 1 ml. The data are expressed as means ± SD, median and interquartile range, or percentages. Statistical analysis was undertaken with Prism version 4.0 (Graph Pad, San Diego, CA) SPSS software for Windows version 15.0 (SPSS Inc., University of Chicago, Chicago, IL), and Bland Altman analysis (18) by Analyze-It (Leeds, UK). Statistical significance was taken at or below the 5% level.
Results
Total body water was calculated from 600 adult peritoneal dialysis patients: mean age, 56.7 ± 15.4 (range, 18 to 89.8) years; 54.2% men; 29.7% diabetic; mean body mass index, 26.1 ± 0.2; median peritoneal dialysis vintage, 15.3 months (range, 3 to 37 months); median daily urine output, 38 ml (range, 7 to 250 ml); mean 4-hour dialysate effluent to plasma creatinine ratio, 0.69 ± 0.13; serum albumin, 38.5 ± 7.4 g/L. 47.3% of patients were of Caucasian ethnicity, 29.2% were from the South Asian subcontinent, 12.8% were African or Afro-Carribbean, 2.8% were Asian, and 7.8% were of other or mixed races. The African/Afro-Caribbean group were younger than most other groups (Table 1). There were more diabetics in the Asian groups (Table 1), and South Asian patients were shorter than the Caucasians.
Table 1.
Patient demographics according to ethnicity
| Characteristic | Caucasian | South Asian | African | East Asian | Other |
|---|---|---|---|---|---|
| n | 284 | 175 | 77 | 17 | 47 |
| Age (years) | 58.1 ± 16.1a | 57.2 ± 14.4a | 47.9 ± 14.3 | 65.7 ± 7.9a | 57.5 ± 16.6b |
| Male (%) | 53.9 | 60.6 | 39 | 52.9 | 57.4 |
| Diabetic (%) | 21.2c | 40.5 | 26.3 | 62.9 | 42.6 |
| Weight (kg) | 73.3 ± 16.5 | 69.3 ± 13.4 | 70.7 ± 17.2 | 70.6 ± 13.8 | 72.2 ± 16.6 |
| Height (cm) | 167.0 ± 10c | 163.1 ± 10 | 166.5 ± 8 | 161.8 ± 8 | 164.5 ± 10 |
| BMI (kg/m2) | 26.2 ± 4.7 | 26.0 ± 4.3 | 25.4 ± 4.8 | 27.0 ± 5.1 | 26.5 ± 5.2 |
| Vintage (months) | 14 (3.4 to 36.5) | 17 (3 to 36.2) | 18.8 (5 to 40) | 29 (7.9 to 56) | 7.2 (18 to 7) |
| Urine volume (ml) | 29 (4 to 77)d | 63 (11 to 450) | 41 (16 to 250) | 46 (29 to 122) | 54 (12 to 387) |
| Alb (g/L) | 38.5 ± 4.4 | 38.8 ± 11.8 | 38.9 ± 4.9 | 38.9 ± 4.3 | 37.8 ± 4.6 |
| D4/PCreat | 0.69 ± 0.13 | 0.67 ± 0.12 | 0.70 ± 0.12 | 0.78 ± 0.13 | 0.67 ± 0.13 |
Patients were divided according to ethnicity: Caucasian, South Asian subcontinent (India, Pakistan, Bangladesh), African (sub-Saharan African, Afro-Caribbean); East Asian (far Eastern Asian, China, Korea, Japan, Thailand), and Other (including mixed racial origins). The data are presented as means ± SD, median (interquartile range), or percentage. BMI, body mass index; Vintage, peritoneal dialysis vintage months; Alb, 24-hour urine volume, serum albumin; D4/PCreat, 4-hour peritoneal dialysis equilibration test dialysate to plasma creatinine ratio.
P < 0.01 versus African.
P < 0.001 versus African.
P < 0.01 versus South Asian.
P < 0.001 versus South Asian.
Total body water was estimated using anthropomorphic-based equations, for each of the ethnic groups (Table 2), and the only difference was between the Caucasians and South Asians using the Johansson equation for all patients (16). The HEMO study–derived equation on the basis of hemodialysis patients postdialysis at their dry weight (17) calculated lower body water compared with the other anthropomorphic equations. Total body water measured by MF-BIS was lower than that calculated for most ethnicities, apart from African/Afro-Caribbeans (Tables 2 and 3). Because the Watson formula is recommended by both Kidney Disease Outcomes Quality Initiative (KDOQI) and the United Kingdom Renal Association for calculating total body water in peritoneal dialysis patients, we compared the percentage difference between the ethnic groups (Figure 1), showing greater difference with South Asians. There were significant differences in measured total body water and intracellular water between the South Asian cohort compared with the Caucasians and African/Afro-Caribbeans (Table 2).
Table 2.
Assessment of total body water by anthropomorphic-based equations and bioimpedance spectroscopy (MF-BIS)
| Caucasian | South Asian | African | East Asian | Other | |
|---|---|---|---|---|---|
| Watson et al. | 37.0 ± 7.6 | 35.8 ± 6.3 | 35.8 ± 6.1 | 35.0 ± 5.3 | 36.5 ± 6.2 |
| Hume and Weyers | 38.1 ± 7.7 | 36.3 ± 6.7 | 36.7 ± 7.8 | 35.9 ± 5.5 | 37.3 ± 6.3 |
| J-A | 37.4 ± 7.4a | 34.9 ± 6.4 | 36.8 ± 7.6 | 34.5 ± 5.5 | 36.3 ± 6.5 |
| J-S | 37.3 ± 7.9 | 35.6 ± 6.8 | 36.1 ± 8.3 | 35.0 ± 5.6 | 36.4 ± 4.5 |
| Lee et al. | 37.5 ± 8.4b | 35.3 ± 7.0 | 36.0 ± 8.7 | 35.0 ± 5.8 | 36.4 ± 6.5 |
| Chumlea et al. | 38.3 ± 8.5 | 37.0 ± 7.2 | 36.6 ± 8.8 | 36.2 ± 5.9 | 37.6 ± 6.7 |
| Chertow et al. | 40.8 ± 8.6 | 38.9 ± 7.2 | 39.5 ± 9.0 | 38.3 ± 6.1 | 39.8 ± 6.9 |
| HEMO | 30.5 ± 5.7 | 29.5 ± 4.5 | 31.5 ± 7.1 | 27.4 ± 3.3 | 28.2 ± 4.2 |
| MF-BIS | 36.4 ± 8.3c | 32.3 ± 6.9 | 36.5 ± 9.8a | 32.6 ± 6.9 | 35.6 ± 8.1 |
| ICW (L) | 21.0 ± 5.4c | 17.9 ± 4.3 | 21.3 ± 6.2c | 18.6 ± 4.4 | 20.2 ± 5.2 |
| ECW (L) | 15.4 ± 3.8b | 14.3 ± 3.2 | 15.2 ± 4.2 | 14.0 ± 3.1 | 15.4 ± 3.6 |
| ECW/TBW (%) | 42.3 ± 4.0c | 44.4 ± 5.0 | 41.7 ± 4.0c | 43.2 ± 4.0 | 43.4 ± 5.0 |
Watson et al. (7), Johansson et al. equation for all patients (J-A) and adjusted for sexes (J-S) (16), Hume and Weyers (7), Lee et al. (10), Chumlea et al. (11), Chertow et al. (13), and HEMO (17) equations were used. Intracellular water (ICW), extracellular water (ECW), and total body water (TBW) all measured by multi-frequency bioelectrical impedance spectroscopy (MF-BIS). Ratio ECW/TBW − 100. Patients were divided according to ethnicity: Caucasian, South Asian, African (including Afro-Caribbean), East Asian, and Other. The data are presented as means ± SEM or percentages.
P < 0.01 versus South Asian.
P < 0.05 versus South Asian.
P < 0.001 versus South Asian.
Table 3.
Comparison of total body water assessed by multiple frequency bioimpedance spectroscopy and anthropomorphic-based equations and bioimpedance spectroscopy (MF-BIS)
| Bias l | 95% CI | T Stat |
r2 |
|||
|---|---|---|---|---|---|---|
| Value | P | Value | P | |||
| Caucasian | ||||||
| Watson et al. | 0.67 | 0.22 to 1.12 | 2.92 | <0.001 | 0.77 | <0.001 |
| Hume and Weyers | 1.73 | 1.29 to 2.18 | 7.63 | <0.001 | 0.79 | <0.001 |
| J-A | 1.07 | 0.61 to 1.52 | 4.63 | <0.001 | 0.77 | <0.001 |
| J-G | 0.90 | 0.47 to 1.33 | 4.13 | <0.001 | 0.81 | <0.001 |
| Lee et al. | 1.13 | 0.67 to 1.59 | 4.81 | <0.001 | 0.79 | <0.001 |
| Chumlea et al. | 1.93 | 1.46 to 2.41 | 7.99 | <0.001 | 0.77 | <0.001 |
| Chertow et al. | 4.45 | 3.99 to 4.9 | 19.34 | <0.001 | 0.81 | <0.001 |
| HEMO | −5.79 | −6.28 to −5.3 | −23.3 | <0.001 | 0.76 | <0.001 |
| South Asian | ||||||
| Watson et al. | 3.56 | 3.02 to 4.1 | 13.06 | <0.001 | 0.72 | <0.001 |
| Hume and Weyers | 4.05 | 3.47 to 4.62 | 13.97 | <0.001 | 0.71 | <0.001 |
| J-A | 2.68 | 2.08 to 3.27 | 8.89 | <0.001 | 0.67 | <0.001 |
| J-G | 3.35 | 2.8 to 3.91 | 11.9 | <0.001 | 0.72 | <0.001 |
| Lee et al. | 3.11 | 2.53 to 3.69 | 10.56 | <0.001 | 0.71 | <0.001 |
| Chumlea et al. | 4.73 | 4.12 to 5.33 | 15.47 | <0.001 | 0.71 | <0.001 |
| Chertow et al. | 6.7 | 0.11 to 7.28 | 22.57 | <0.001 | 0.82 | <0.001 |
| HEMO | −2.71 | −3.49 to −1.94 | −6.94 | <0.001 | 0.58 | <0.001 |
| African/Afro-Caribbean | ||||||
| Watson et al. | −0.73 | −1.86 to 0.4 | −1.29 | 0.20 | 0.74 | <0.001 |
| Hume and Weyers | 0.18 | −0.88 to 1.23 | 0.739 | 0.74 | 0.77 | <0.001 |
| J-A | 0.3 | −0.76 to 1.36 | 0.57 | 0.57 | 0.79 | <0.001 |
| J-G | −0.43 | −1.47 to 0.61 | −0.83 | 0.41 | 0.77 | <0.001 |
| Lee et al. | −0.54 | −1.6 to 0.5 | −1.04 | 0.30 | 0.77 | <0.001 |
| Chumlea et al. | 0.09 | −1.0 to 1.19 | 0.17 | 0.87 | 0.76 | <0.001 |
| Chertow et al. | 2.9 | 1.86 to 3.95 | 5.55 | <0.001 | 0.77 | <0.001 |
| HEMO | −4.94 | −5.36 to −4.52 | −23.15 | <0.001 | 0.64 | <0.001 |
Watson et al. (7), Johansson et al. equation for all patients (J-A) and adjusted for gender (J-G) (16), Hume and Weyers (7), Lee et al. (10), Chumlea et al. (11), Chertow et al. (13), and HEMO (17) equations were used. Bias 1, Bland Altman bias (anthropomorphic total body water-MF-BIS); 95% CI, 95% confidence interval for bias; r2, Pearson correlation.
Figure 1.
The percent difference in total body water between that calculated by the Watson equation and that measured by multifrequency bioimpedance spectroscopy in different ethnic groups. The groups were Caucasian, South Asian, African/Afro-Caribbeans (African), Far East Asian (Oriental), and other. **, P < 0.01 versus South Asian; ***, P < 0.001 versus South Asian.
To explore the differences in calculated and measured total body water, Bland Altman analysis was performed (Table 3), which showed the least bias for African/Afro-Caribbeans and the greatest bias for South Asians. To look at the effects of gender, we analyzed bias for the three main ethnic groups (Table 4). All anthropomorphic-based equations overestimated body water for both male and female South Asian peritoneal dialysis patients. The least bias was observed for African/Afro-Caribbeans.
Table 4.
Comparison of total body water assessed by multiple frequency bioimpedance spectroscopy and anthropomorphic-based equations and bioimpedance spectroscopy (MF-BIS) in males and females
| Equation | Caucasian |
African/Afro-Caribbean |
South Asian |
|||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Watson et al. | 0.85a | 0.54a | −0.79 | −0.69 | 3.11a | 3.11b |
| (0.14 to 1.56) | (0.01 to 1.07) | (−2.93 to 1.36) | (−1.99 to 0.61) | (3.4 to 4.61) | (2.47 to 3.75) | |
| J-A | 0.21 | 2.12b | −1.65 | 1.55a | 3.49b | 3.49b |
| (0.46 to 0.89) | (1.58 to 2.68) | (−3.52 to 0.22) | (0.37 to 2.72) | (0.38 to 2.92) | (2.74 to 4.24) | |
| J-G | 1.42b | 0.38 | −0.27 | −0.54 | 2.2b | 2.2b |
| (0.74 to 2.11) | (−0.11 to 0.86) | (−2.31 to 1.78) | (−1.7 to 0.63) | (2.44 to 4.87) | (1.35 to 2.86) | |
| Lee et al. | 1.82b | 0.40 | −0.49 | −0.58 | 2.32b | 2.32b |
| (1.08 to 2.56) | (−0.1 to 0.89) | (−2.54 to 1.56) | (−1.77 to 0.61) | (1.94 to 4.42) | (1.67 to 2.97) | |
| Hume and Weyers | 2.24b | 1.22b | −0.19 | 0.41 | 2.88b | 2.88b |
| (1.52 to 2.97) | (0.75 to 1.69) | (−2.32 to 1.94) | (−0.73 to 1.55) | (3.13 to 5.58) | (2.2 to 3.55) | |
| Chumlea et al. | 3.47b | 0.22 | 1.42 | −0.76 | 2.54b | 2.54b |
| (2.77 to 4.18) | (−0.27 to 0.70) | (−0.64 to 3.5) | (−1.99 to 0.47) | (4.49 to 6.95) | (1.92 to 3.17) | |
| Chertow et al. | 5.42b | 3.40b | 3.54b | 2.5b | 5.42b | 5.42b |
| (4.72 to 6.13) | (2.92 to 3.88) | (1.57 to 5.52) | (1.29 to 3.7) | (5.83 to 8.83) | (4.77 to 6.08) | |
Watson et al. (7), Johansson et al. equation for all patients (J-A) and adjusted for gender (J-G) (16), Hume and Weyers (7), Lee et al. (10), Chumlea et al. (11), and Chertow et al. (13) equations were used. The values are the Bland Altman bias (anthropomorphic total body water-MF-BIS), 95% confidence interval of bias in parentheses, and Pearson correlation (r2).
P < 0.05
Simple correlation analysis was performed, and the difference was between calculated total body water by Watson equations and that measured by MF-BIS; there were positive correlations with body mass index (r = 0.14, P < 0.01) and age (r = 0.1, P = 0.01) and negative correlations with ICW (r = 0.10, P = 0.01) and TBW (0.09, P = 0.16). Multiple regression analysis was then performed, and age, female gender, body mass index, Caucasian racial origin, and greater intracellular and extracellular volumes were associated with smaller differences in bias (Table 5). Although diabetes improved the fit of the regression model, the 95% confidence limits for diabetes crossed the line of unity.
Table 5.
Factors associated with difference between total body water measured by multi-frequency bioimpedance spectroscopy and the Watson equation
| Variable | Change | 95% Confidence Limits | P | Power |
|---|---|---|---|---|
| Age (per 5 years) | −0.54 | −0.65 to −0.41 | <0.001 | 1.0 |
| Gender (male) | 13.64 | 12.69 to 14.58 | <0.001 | 1.0 |
| Caucasian | −1.44 | −2.18 to −0.92 | <0.001 | 1.0 |
| BMI (per kg/m2) | −1.10 | −1.28 to −0.92 | <0.001 | 1.0 |
| ECW (per 5 L) | −5.5 | −2.96 to −2.61 | <0.001 | 1.0 |
| ICW (per 5 L) | −13.93 | −2.70 to −2.49 | <0.001 | 1.0 |
| DM | 0.89 | −0.74 to 1.7 | 0.03 | 0.57 |
The r2 value for the model is 0.887, and the adjusted r2 value is 0.885. BMI, body mass index; ECW, extracellular water; ICW, intracellular water; DM, diabetes mellitus.
Discussion
Although the Watson (7) and Hume (8) equations are recommended by KDOQI for estimating total body water, several groups developed their own equations on the basis of Caucasian patients (11) and Southeast Asian patients (10). However, these standard anthropomorphic equations do not necessarily take into account changes in body composition, and others added factors for the presence of diabetes, because many patients with type 2 diabetes have greater adipose tissue (13). Johansson et al. measured total body water in a small cohort of peritoneal dialysis patients and developed a further series of anthropomorphic equations (16).
As with several previous studies, we found that total body water was overestimated by anthropomorphic-based equations (19). However, the bias between equations varied, with greatest positive bias generated by the Chertow equation (13). Because the HEMO equation was derived from hemodialysis patients post-dialysis at dry or target weight, this equation tended to underestimate total body water (17). Looking at different racial groups, bias was least for our African/Afro-Caribbean cohort, but was greatest for those from the South Asian subcontinent. In the UK, there is increasing recognition of different body compositions between Caucasians and UK-based South Asians, in particular to the increased relative amount of body fat in this group (20). The newer equations, from Johansson, Lee, Chertow, and Chumlea, did not appear to offer any benefit compared with the older Watson and Hume equations that have been recommended by KDOQI (7).
Apart from race causing a difference between estimated and measured total body water, we found that younger age, male gender, increasing body weight, and lower intracellular and extracellular volumes were all associated with greater differences between estimated and measured total body water. On the other hand diabetes, residual urine output, serum albumin, or peritoneal transplant status did not appear to affect the difference in estimated and measured total body water. Diabetes may not have been such a significant factor in our study as compared with previous anthropomorphic-based studies, because bioimpedance can readily estimate the differences between body fat and muscle because of the differences in tissue hydration.
Anthropometric equations are largely dependent on body weight, but even adding in height and gender, these adjustments do not take into account body composition. Taking a theoretical example of two subjects of identical weight, gender, and height, where one subject is lean and the other is obese, the estimated total body water using the Watson equation would be identical for both subjects. However, the obese subject will have increased adipose tissue, which contains approximately 20% water, whereas the lean subject will have relatively more lean tissue, which contains around 70%. As such, the Watson formula would tend to overestimate the true water volume in obese patients and underestimate the water volume in lean subjects (21). This explains why the difference between estimated and measured total body water increased in the South Asian group, who have relatively more adipose tissue (20) and are more prone to type 2 diabetes (22). These differences would be apparent using an isotope gold-standard dilution such as deuterium-labeled water. However, this option is not practical in routine clinical practice. However, MF-BIS has been validated against deuterium-labeled water in both healthy subjects and dialysis patients (10,23,24). Because MF-BIS relies on the electrical resistance, the resistance differs between adipose and muscle tissue because of the different water content, and as such MF-BIS can also be used to assess body composition (25).
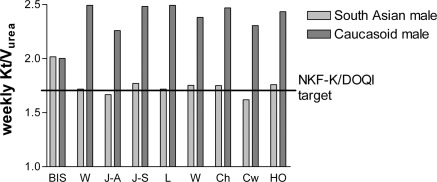
To put this into clinical context, we show the effect of calculating peritoneal dialysis adequacy in two patients, one Caucasian and one South Asian (Figure 2) who have similar body weight but marked differences in body composition. All of the anthropomorphic-based equations overestimated delivered weekly peritoneal Kt/Vurea compared with that from MF-BIS, whereas peritoneal KT/Vurea was underestimated in the South Asian patient, with some equations suggesting that this patient failed to achieve clinical practice target guidelines (6,7). These data may help to explain in part reports of peritoneal dialysis technique and patient survival in Asian patients with relatively low calculated Kt/Vurea (26).
Figure 2.
Calculated weekly peritoneal Kt/Vurea for two patients. The patients were a South Asian man (weight, 80.4 kg; fat mass, 40.1 kg; skeletal muscle mass, 20.8 kg) and a Caucasian man (weight, 80.4 kg; fat mass, 20.8 kg; skeletal muscle mass, 33.9 kg). Body water was measured by multifrequency bioimpedance spectroscopy (BIS) or calculated using Watson (W), Johansson all patients (J-A), Johansson gender-adjusted (J-G), Lee (L), Hume (H), Chumlea (Ch), Chertow (Cw), and HEMO (HO) equations. Target Kt/Vurea was from NKF-K/DOQI Clinical practice guidelines for peritoneal dialysis adequacy (5).
In this study MF-BIS showed that there were significant differences in total body water in UK-based patients from the South Asian subcontinent. As such, estimations of peritoneal dialysis adequacy on the basis of total body water using standard anthropomorphic would underestimate Kt/Vurea delivered, because the estimate of body water was much greater for anthropomorphic equations than that measured by MF-BIS. Because variation in body composition can lead to differences between actual delivered Kt/Vurea and that estimated, this may in part account for some of the paradoxical relationship reported between Kt/Vurea and survival in peritoneal dialysis patients (27).
Disclosures
Dr. S. Fan has been paid honoraria by Fresenius Medical Company for speaking on topics related to hemodialysis and peritoneal dialysis but not directly linked to this work.
Acknowledgments
We wish to thank Dr. Joe Chilcot for statistical advice.
Appendix
Watson et al. (7)
Men
total body water (TBW) = 2.477 − (0.09156 × age) + (0.1074 × height cm) + (0.3362 × weight kg)
Women
TBW = −2.097 + (0.1069 × height cm) + (0.2466 × weight kg)
Hume and Weyers (8)
TBW = (0.194786 × height cm) + (0.296785 × weight kg) − 14.012934
Women
TBW = (0.34454 × height cm) + (0.183809 × weight kg) − 35.270121
Lee et al. (10)
Men
TBW = −28.3497 × (0.243057 × height cm) + (0.366248 × weight kg)
Women
TBW = −26.6224 × (0.26513 × height cm) + (0.232948 × weight kg)
Chumlea et al. (11)
Men
TBW = 23.04 − (0.03 × age years) + (0.5 × weight kg) − (0.62 × body mass index)
Women
TBW = −10.5 − (0.01 × age years) + (0.2 × weight kg) + (0.18 × height cm)
Chertow et al. (13)
TBW = −(0.07493713 × age years) − (1.01767792 × 1 if male/0 if female) + (0.12703384 × height cm) − (0.04012056 × weight kg) + (0.57894981 × 1 if diabetic/0 if not diabetic − (0.00067247 × weight kg × weight kg) − (0.03486146 × age years × 1 if male/0 if female) + (0.11262857 × weight kg × 1 if male/0 if female) + (0.00104135 × age years × weight kg) + (0.00186104 × height cm × weight kg)
Johansson et al. (16)
All patients
TBW = −42.879 − (0.03368 × age years) + (0.274 × weight kg) + (0.372 × height cm)
Men
TBW = −10.759 − (0.078 × age years) + (0.312 × weight kg) + (0.192 × height cm)
Women
TBW = −29.994 − (0.0004 × age years) + (0.214 × weight kg) + (0.294 × height cm)
HEMO Equation (17)
TBW = 0.824 × Watson TBW × (1 + 0.033 if diabetic) × (1 + 0.033 if female) × (1 + 0.043 if of African descent) × (1 − (0.002 × (age − 50)/10 if male)) × (1 − (0.015 × (age − 50)/10 if female))
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Davenport A: Peritonitis remains the major clinical complication of peritoneal dialysis: The London UK peritonitis audit 2002–2003. Perit Dial Int 29: 297–302, 2009 [PubMed] [Google Scholar]
- 2. Bargman JM, Thorpe KE, Churchill DN: CANUSA Peritoneal Dialysis Study Group: Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. NECOSAD Study Group: The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 41: 1293–1302, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Vonesh E: On small solute clearance and patient outcomes: Evidential practice or observational trepidation? Perit Dial Int 29: 623–629, 2009 [PubMed] [Google Scholar]
- 5. NKF-K/DOQI Clinical practice guidelines for peritoneal dialysis adequacy: Clinical practice recommendations for peritoneal dialysis adequacy Am J Kid Dis 48[Suppl 1]: S98–S158, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Woodrow G, Davies S: Peritoneal dialysis in chronic kidney disease www.renal.org/Clinical/GuidelinesSection/PeritonealDialysis.aspx#Rationale3 Accessed July 2011
- 7. Watson PE, Watson ID, Batt RD: Total body water volume for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Hume R, Weyers E: Relationship between total body water and surface area in normal and obese subjects. J Clin Pathol 24: 234–238, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campos AC, Chen M, Meguid MM: Comparisons of body composition derived from anthropometric and bioelectrical impedance methods. J Am Coll Nutr 8: 484–489, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Lee SW, Song JH, Kim GA, Lee KJ, Kim M: Assessment of total body water from anthropometry based equations using bioelectrical impedance as reference in Korean adult control and haemodialysis subjects. Nephrol Dial Transplant 16: 91–97, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Chumlea WC, Guo SS, Zeller CM, Reo NV, Baumgartner RN, Garry PJ, Wang J, Pierson RN, Jr, Heymsfield SB, Siervogel RM: Total body water reference values and prediction equations for adults. Kidney Int 59: 2250–2258, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Fürstenberg A, Davenport A: Comparison of multifrequency bioelectrical impedance analysis and dual-energy x-ray absorptiometry assessments in outpatient haemodialysis patients. Am J Kidney Dis 57: 123–129, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Chertow GM, Lazarus JM, Lew NL, Ma L, Lowrie EG: Development of a population-specific regression equation to estimate total body water in haemodialysis patients. Kidney Int 51: 1578–1582, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Davenport A, Willicombe M: Comparison of fluid status in patients treated by different modalities of peritoneal dialysis using multi-frequency bioimpedance. Int J Artif Organs 32: 779–786, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Davenport A, Willicombe M: Hydration status does not influence peritoneal equilibration test ultrafiltration volumes. Clin J Am Soc Nephrol 4: 1207–1212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansson AC, Samuelsson O, Attman PO, Bosaeus I, Haraldsson B: Limitations in anthropometric calculations of total body water in patients on peritoneal dialysis. J Am Soc Nephrol 12: 568–573, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Daugirdas JT, Greene T, Chertow GM, Depner TA: Can rescaling dose of dialysis to body surface area in the HEMO Study explain the different responses to dose in women versus men? Clin J Am Soc Nephrol 5: 1628–1636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 19. Woodrow G, Oldroyd B, Wright A, Coward WA, Truscott JG, Tutney JH, Brownjohn AM, Smith MA: Comparison of anthropomorphic equations for estimation of total body water in peritoneal dialysis patients. Nephrol Dial Transplant 18: 384–389, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Whincup PH, Nightingale CM, Owen CG, Rudnicka AR, Gibb I, McKay CM, Donin AS, Sattar N, Alberti KG, Cook DG: Early emergence of ethnic differences in type 2 diabetes precursors in the UK: The Child Heart and Health Study in England (CHASE Study). PLoS Med 20:e1000263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spalding EM, Chandna SM, Davenport A, Farrington K: Kt/V underestimates the haemodialysis dose in women and small men. Kidney Int 74: 348–355, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Davenport A, Willicombe MK: Does diabetes mellitus predispose to increased fluid overload in peritoneal dialysis patients? Nephron Clin Pract 114: c60–c66, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, Fuller NJ: A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85: 80–89, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller N: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Fürstenberg A, Davenport A: Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy x-ray absorptiometry. Am J Nephrol 33: 150–156, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Lo WK, Jiang Y, Cheng SW, Cheng IK: Survival of CAPD patients in a center using three two-liter exchanges as standard regime. Perit Dial Int 16[Suppl 1]: S163–S166, 1999 [PubMed] [Google Scholar]
- 27. Rumpsfeld M, McDonald SP, Johnson DW: Peritoneal small solute clearance is nonlinearly related to patient survival in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 29: 637–646, 2009 [PubMed] [Google Scholar]