Abstract
Summary
Background and objectives
Prognosis in nondialysis chronic kidney disease (CKD) patients under regular nephrology care is rarely investigated.
Design, setting, participants, & measurements
We prospectively followed from 2003 to death or June 2010 a cohort of 1248 patients with CKD stages 3 to 5 and previous nephrology care ≥1 year in 25 Italian outpatient nephrology clinics. Cumulative incidence of ESRD or death before ESRD were estimated using the competing-risk approach.
Results
Estimated rates (per 100 patient-years) of ESRD and death 8.3 (95% confidence interval [CI], 7.4 to 9.2) and 5.9 (95% CI 5.2 to 6.6), respectively. Risk of ESRD and death increased progressively from stages 3 to 5. ESRD was more frequent than death in stage 4 and 5 CKD, whereas the opposite was true in stage 3 CKD. Younger age, lower body mass index, proteinuria, and high phosphate predicted ESRD, whereas older age, diabetes, previous cardiovascular disease, ESRD, proteinuria, high uric acid, and anemia predicted death (P < 0.05 for all). Among modifiable risk factors, proteinuria accounted for the greatest contribution to the model fit for either outcome.
Conclusions
In patients receiving continuity of care in Italian nephrology clinics, ESRD was a more frequent outcome than death in stage 4 and 5 CKD, but the opposite was true in stage 3. Outcomes were predicted by modifiable risk factors specific to CKD. Proteinuria used in conjunction with estimated GFR refined risk stratification. These findings provide information, specific to CKD patients under regular outpatient nephrology care, for risk stratification that complement recent observations in the general population.
Introduction
The knowledge on the competing risk of the two main outcomes of chronic kidney disease (CKD), that is, ESRD and death, and on the risk factors underlying these outcomes is of paramount importance to put in place effective prevention strategies. Community studies and analyses made on large health insurance databases reported mortality rates remarkably larger than ESRD rates (1–5). However, information on cohorts referred to renal clinics, and particularly in patients under continuous nephrology care, is scarce.
Specific information on the prognosis and risk factors responsible for CKD progression and death in CKD patients followed in the setting of tertiary nephrology care is of major relevance for three reasons. First, these patients represent a selected population with peculiar clinical characteristics with respect to unreferred patients, including younger age, more advanced disease, higher burden of cardiovascular (CV) comorbidities, and higher BP (6–9). Second, ESRD and death are predicted by different risk factors, with age and comorbidities modifying the predictive role of main factors, BP in primis (10–13). Third, intensity of nephrology care modifies survival (14).
Previous studies in referred patients have shown ESRD rates similar or higher than mortality (10,11,15–20); however, the definition of risk factors for these outcomes still remains uncertain. Indeed, in most studies, information was retrospectively collected, and the duration of nephrology care and of CKD diagnosis, which are main modifiers of the competing risk of ERSD versus death (14,21), was fairly short or unspecified.
In 2003, we designed the TArget BP LEvels (TABLE) multicenter cohort study, aimed at identifying risk factors for ESRD, death, and CV complications in adult stage 3 to 5 CKD patients, attending Italian renal clinics for at least 1 year before the study. The cross-sectional evaluation revealed a high prevalence of patients not achieving main therapeutic goals (22). In this study, we report the prospective follow-up results to estimate the competing risks of ESRD and death and to assess the main determinants of these outcomes. The results can be helpful in refining the global risk profile in CKD patients receiving continuity of care in a nephrology clinic.
Study Population and Methods
This is a prospective observational study performed in 25 Italian outpatient nephrology clinics exclusively dedicated to the conservative care of CKD and with predefined clinical and laboratory protocols.
Patients
Eligible subjects were all of the consecutive patients attending the centers during a 9-month period of 2003 with diagnosis of CKD (low estimated GFR [eGFR] and/or proteinuria persisting for ≥3 months), eGFR <60 ml/min per 1.73 m2 (no substitutive treatment), and a first visit at the nephrology clinic dating back at least 1 year before the study visit. Patients with acute kidney injury during the 6 months preceding the study visit were excluded. All of the patients gave informed consent to the protocol, which was approved by the local ethical committee.
Data Collection and Definitions
The study visit in 2003 was the starting date of the follow-up study. The data were collected by participating nephrologists in anonymous case report forms, filled in at each center, and then sent back to the coordinating center for quality checks and analyses. At the study visit, information was collected on demographic, clinical, and laboratory data and medical history, including any previous CV event, defined as any event among coronary artery disease, congestive heart failure, and cerebrovascular and peripheral vascular disease. Twenty-four–hour urine collection was repeated if the value of the measured creatinine excretion rate was outside the 60% to 140% range of the value calculated according to Dwyer and Kenler (23). GFR was estimated by the four-variable Modification of Diet in Renal Disease (MDRD) study equation.
At the study visit, we also collected information on main modifiable risk factors that were defined as uncontrolled according to predefined thresholds (24–30): uncontrolled hypertension (BP, ≥130/80 mmHg), anemia (hemoglobin, <11 g/dl), high phosphate (serum phosphate, >4.6 mg/dl in CKD stage 3 to 4 or >5.5 mg/dl in CKD stage 5), proteinuria (protein excretion, >0.5 g/24 h), high cholesterol (total cholesterol, >190 mg/dl), high uric acid (serum uric acid, >6 in women and >7 mg/dl in men), and smoking habit (smoking in the last 6 months). All of the thresholds were accepted and shared by participating nephrologists in dedicated meetings of the study group.
The outcome measures were death and ESRD (either dialysis or renal transplant). The follow-up expiration date was June 30, 2010. Periodic updates were planned at 18, 24, 30, and 36 months after the study visit and yearly thereafter to collect information on outcome, BP, and eGFR. Information on other variables was not mandatory.
Statistical Analyses
Continuous variables were reported as either the means and SD or median and interquartile ranges (IQRs) according to their distribution, as assessed by the Shapiro–Wilk test. Categorical variables were reported as percentages. Differences in characteristics of patients among the three CKD stages were tested by means of one-way ANOVA or Kruskal–Wallis (according to their distribution) and Pearson chi-squared test for continuous and categorical variables, respectively. Cochran–Armitage trend test was used to compare prevalence of modifiable risk factors across stages. Possible heterogeneity of target prevalence among centers was investigated by means of intracluster correlation coefficient (31). Median follow-up was estimated by the inverse Kaplan–Meier approach (32).
To assess prognosis of CKD patients according to stage 3 to 5, we used ESRD and death before ESRD as outcomes. A further composite end point included ESRD and death, whichever occurred first. Because ESRD and death before ESRD are mutually exclusive events (i.e., the occurrence of either one prevents the occurrence of the other), Kaplan–Meier estimates of time to ESRD or death before ESRD are biased; we therefore calculated the cumulative incidence of ESRD or death before ESRD using the competing-risk approach (33), and stages were compared with the Gray test (34). Incidence of the composite outcome was estimated by standard Kaplan–Meier approach.
To assess the predictive role of the uncontrolled modifiable risk factors, we used ESRD and overall (before and after ESRD) death as outcomes. Multivariable Cox proportional-hazards models, stratified for CKD stage and center, were used to estimate event-specific hazard ratios (HRs) and 95% confidence intervals (CIs). When evaluating overall survival, ESRD was included as time-dependent covariate; when evaluating time to ESRD, dead subjects were censored at the date of death. For each modifiable risk factor, the heterogeneity of predictive role among CKD stages was assessed by likelihood ratio test of two CKD stage-stratified models: one with CKD stage-specific estimates and one with overall risk factor estimate. Under the null hypothesis of no heterogeneity, this statistic follows approximately a chi-squared distribution on J-1 (i.e., 3 to 1) degrees of freedom (35). The contribution of each covariate to the model fit was estimated as percentage reduction of R2 value of the model resulting, from omitting each variable in turn from the full model (36). We calculated R2 values according to the work of Nagelkerke (37).
Nonlinear association of the covariates with the end points was evaluated by restricted cubic splines and assessed by likelihood ratio test (36). Only for proteinuria was there evidence of a nonlinear association for either outcome, and a restricted cubic spline was used with four knots placed a priori at clinically relevant values (0, 0.5, 1, and 3 g/24 h of proteinuria).
Because the thresholds we used to categorize risk factors may not be universally accepted, we repeated the analyses by using continuous variables in the multivariable Cox models. The data were analyzed using SAS version 9.2 (SAS Inc., Cary, NC).
Results
Characteristics of the Cohort at Study Visit
We studied 1248 out of 1492 patients; 244 patients were excluded because of acute kidney injury (n = 189) or lack of follow-up information (n = 55). All of the patients were Caucasian. As reported in Table 1, the study cohort was characterized by advanced age (67 ± 14 years), high prevalence of diabetes (28%), and previous CV disease (32%). Median follow-up in the clinic before the study visit was 2.6 years (IQR, 1.4 to 5.6). Nutritional status was generally adequate, as documented by mean body mass index and mean serum albumin levels.
Table 1.
Demographics and clinical characteristics of patients at study visit by CKD stage
| Stage 3 (n = 609) | Stage 4 (n = 449) | Stage 5 (n = 190) | P | |
|---|---|---|---|---|
| Age, years | 66 (14) | 67 (13) | 67 (14) | 0.19 |
| Male gender, % | 65.1 | 51.7 | 46.3 | <0.01 |
| Previous FU, years | 2.3 (1.2 to 5.3) | 2.7 (1.5 to 5.2) | 3.2 (1.8 to 6.0) | <0.01 |
| BMI, kg/m2 | 27.4 (4.3) | 27.3 (4.9) | 27.0 (4.9) | 0.52 |
| sAlbumin, g/dl | 3.98 (0.47) | 3.86 (0.51) | 3.90 (0.50) | 0.01 |
| Diabetes, % | 24.0 | 31.4 | 31.1 | 0.01 |
| Previous CV event, % | 28.2 | 35.9 | 33.7 | 0.03 |
| Renal disease, % | 0.01 | |||
| diabetes | 11 | 19 | 15 | |
| hypertension | 26 | 23 | 20 | |
| GN/IN/PKD | 30 | 27 | 34 | |
| other | 32 | 32 | 31 | |
| Systolic BP, mmHg | 138 (18) | 141 (19) | 142 (18) | 0.01 |
| Diastolic BP, mmHg | 81 (11) | 81 (11) | 81 (9) | 0.86 |
| eGFR, ml/min per 1.73 m2 | 43.1 (8.8) | 22.3 (3.9) | 11.8 (2.8) | <0.01 |
| Calcium, mg/dl | 9.4 (0.7) | 9.2 (0.6) | 9.1 (0.7) | <0.01 |
| Phosphate, mg/dl | 3.6 (0.7) | 4.0 (0.8) | 4.5 (0.9) | <0.01 |
| Hemoglobin, g/dl | 13.6 (1.6) | 12.0 (1.7) | 11.4 (1.5) | <0.01 |
| Uric acid, mg/dl | 6.1 (1.5) | 6.2 (1.8) | 6.3 (1.9) | 0.35 |
| Cholesterol, mg/dl | 199 (36) | 201 (43) | 191 (43) | 0.01 |
| Proteinuria, g/24 h | 0.34 (0.10 to 0.90) | 0.70 (0.27 to 1.42) | 1.0 (0.56 to 2.0) | <0.01 |
Values are means (SD), medians (interquartile range), or percentages. The P values refer to the trend across stages. Previous FU, follow-up in the clinic before the study visit; BMI, body mass index; CV, cardiovascular; GN, glomerulonephritis; IN, interstitial nephritis; PKD, polycystic kidney disease; eGFR, GFR estimated by the four-variable Modification of Diet in Renal Disease equation; sAlbumin, serum albumin.
Therapy at study visit is reported in Table 2. Only one patient out of five had a salt intake <100 mEq/24 h, whereas a protein intake <0.8 g/kg per 24 h was detected in 52% of patients. Inhibition of renin angiotensin system (RAS) was a mainstay of pharmacologic intervention, but only a small fraction of patients were under dual RAS blockade. As to diuretic drugs, use of thiazides was sporadic (6.8% overall), whereas furosemide was given to more than one-third of patients with increasing frequency and dose from stages 3 to 5. Erythropoiesis-stimulating agents were generally used at low doses: median doses of darbepoetin and α or β epoetin were equal to 20 μg/wk (IQR, 15 to 30) and 4.000 IU/wk (IQR, 4000 to 6000), respectively. Phosphate binders were prescribed to 7.9% patients only.
Table 2.
Therapeutic regimens in patients at study visit by CKD stage
| Stage 3 (n = 609) | Stage 4 (n = 449) | Stage 5 (n = 190) | P | |
|---|---|---|---|---|
| LSD, % | 17.9 | 23.5 | 23.3 | 0.13 |
| LPD, % | 46.6 | 55.5 | 65.7 | 0.01 |
| BP-lowering drugs, n | 2.15 (0.98) | 2.39 (1.11) | 2.38 (1.05) | <0.01 |
| ACEi or ARB, % | 74.0 | 76.0 | 56.0 | <0.01 |
| ACEi + ARB, % | 6.2 | 5.1 | 4.2 | 0.51 |
| Furosemide use, % | 25.9 | 47.7 | 50.0 | <0.01 |
| Furosemide dose, mg/24 h | 25 (12.5 to 50) | 50 (25 to 125) | 50 (25 to 125) | <0.01 |
| Statin, % | 23.3 | 22.1 | 17.4 | 0.22 |
| ESA, % | 2.6 | 16.7 | 33.7 | <0.01 |
The values are the means (SD), medians (interquartile range), or percentages. The P values refer to the trend across stages. LSD, low salt diet (<100 mEq/24 h); LPD, low protein intake (<0.8 g/kg per 24 h); ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ESA, erythropoiesis-stimulating agents.
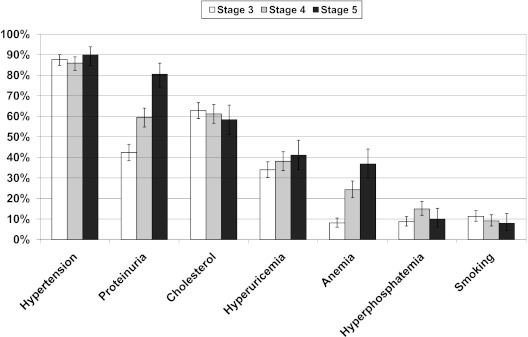
Figure 1 shows the distribution of the uncontrolled modifiable risk factors by CKD stage. The prevalence of proteinuria and anemia rose from stages 3 to 5, whereas poor hypertension control represented the main complication, with only 12.6% (95% CI, 10.8 to 14.5) of patients showing BP of <130/80 mmHg. There was no heterogeneity among the 25 participant clinics in the prevalence of patients with uncontrolled factors (intracluster correlation coefficient ranged from 0.036 to 0.066 for the seven factors examined).
Figure 1.
Prevalence of uncontrolled risk factors (as defined in the Study Population and Methods section) in CKD stages 3 (white), 4 (light gray), and 5 (dark gray). The bars show 95% confidence interval values. The P values for trends across stages are as follows: P = 0.69 for hypertension, P < 0.001 for proteinuria, P = 0.27 for high cholesterol, P = 0.05 for hyperuricemia, P < 0.001 for anemia, P = 0.12 for high phosphate, and P = 0.12 for smoking.
Survival Analysis
Median follow-up was 60 months (IQR, 55 to 64). Overall 363 ESRD events and 307 all-cause deaths (229 before and 78 after ESRD) were observed. Estimated rates (per 100 patient-years) were 8.3 (95% CI, 7.4 to 9.2) for ESRD and 5.9 (95% CI, 5.2 to 6.6) for all-cause death. Figure 2 shows the cumulative incidence of ESRD, death before ESRD, and the composite outcome by CKD stage. Risk of ESRD or death increased progressively from stages 3 to 5 (P < 0.0001 for either outcome). ESRD was a more frequent outcome than death in stages 4 and 5 CKD, whereas the opposite was true in stage 3 CKD.
Figure 2.
Cumulative incidence of ESRD, death before ESRD, and the composite outcome (first occurrence of ESRD or death) stratified by chronic kidney disease stage. A competing risk approach was used to evaluate the extent to which the cumulative probabilities of the overall composite end point reflected ESRD or death during follow-up. The number of patients at risk refers to the composite end point.
Multivariable Cox analysis is reported in Table 3. Increased proteinuria significantly interacted with CKD stage in the prediction of ESRD (P = 0.02), but not death, with HR progressively reducing from stage 3 to 5. No interaction with CKD stage was found for other modifiable risk factors for either outcome. Younger age, lower body mass index and high phosphate, as well as proteinuria, predicted ESRD, whereas older age, diabetes, CV disease, ESRD, proteinuria, high uric acid, and anemia predicted death. When estimating the hierarchy of prognostic factors (Table 3), age was the main predictor of death, and among modifiable risk factors, proteinuria accounted for the greatest contribution to the model fit. Additional analyses did not evidence significant interactions between high BP and proteinuria in the prediction of either outcome (data not shown). Adding the use of RAS inhibitors in the Cox models did not change HRs estimates (data not shown), and the use of these drugs did not contribute to explain the variability in ESRD (P = 0.17) or death (P = 0.11).
Table 3.
Multivariable Cox model of determinants of ESRD and death with estimated contribution of each determinant to model fit
| ESRD |
Death |
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | R2 Reduction (%) | HR (95% CI) | P | R2 Reduction (%) | |
| Age, 5 years | 0.94 (0.90 to 0.98) | <0.01 | 11.2 | 1.56 (1.46 to 1.66) | <0.01 | 77.7 |
| Male gender | 1.18 (0.94 to 1.48) | 0.16 | 2.4 | 1.29 (0.99 to 1.67) | 0.05 | 1.2 |
| Body mass index, kg/m2 | 0.97 (0.95 to 0.99) | 0.08 | 8.7 | 0.99 (0.97 to 1.02) | 0.61 | 0.1 |
| Diabetes mellitus | 1.19 (0.93 to 1.52) | 0.17 | 2.3 | 1.31 (1.01 to 1.69) | <0.05 | 1.3 |
| Previous CV event | 1.27 (1.00 to 1.62) | 0.05 | 4.4 | 1.35 (1.06 to 1.71) | 0.02 | 1.8 |
| Smoking | 1.01 (0.71 to 1.43) | 0.97 | <0.01 | 1.18 (0.80 to 1.74) | 0.41 | 0.2 |
| BP ≥130/80 mmHg | 1.10 (0.77 to 1.55) | 0.60 | 0.30 | 0.72 (0.50 to 1.04) | 0.08 | 0.9 |
| Hemoglobin <11 g/dl | 1.27 (0.99 to 1.63) | 0.05 | 4.0 | 1.43 (1.08 to 1.89) | 0.01 | 1.9 |
| Phosphate >4.6 to 5.5 mg/dl | 1.59 (1.18 to 2.15) | 0.02 | 10.2 | 1.35 (0.93 to 1.95) | 0.12 | 0.7 |
| Cholesterol >190 mg/dl | 0.90 (0.72 to 1.12) | 0.34 | 1.1 | 0.95 (0.75 to 1.20) | 0.67 | 0.1 |
| Uric Acid >6 to 7 mg/dl | 0.88 (0.70 to 1.11) | 0.27 | 1.5 | 1.30 (1.02 to 1.65) | 0.04 | 1.3 |
| Proteinuria ≥0.5 g/24 h | 39.2 | 1.41 (1.10 to 1.82) | 0.07 | 2.3 | ||
| stage 3 | 3.17 (1.76 to 5.72) | <0.01 | ||||
| stage 4 | 2.02 (1.41 to 2.88) | 0.01 | ||||
| stage 5 | 1.13 (0.73 to 1.76) | 0.59 | ||||
| ESRD | 1.51 (1.07 to 2.13) | 0.02 | 1.7 | |||
The model is stratified for center and CKD stage. CV, cardiovascular; HR, hazard ratio; CI, confidence interval.
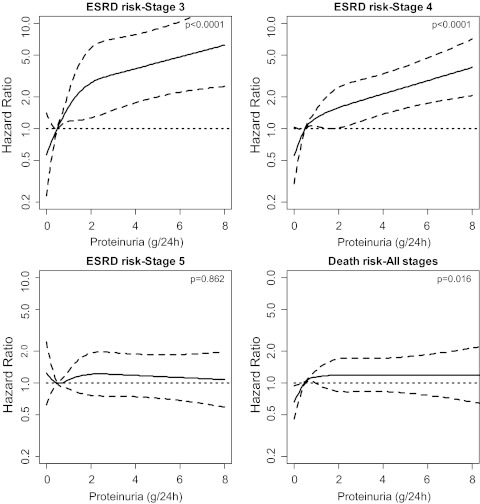
The adjusted HRs of proteinuria as continuous variable for ESRD (by CKD stages) and death in the whole cohort are shown in Figure 3. With mild to moderate proteinuria, a marked increase in the risk of ESRD was detected in CKD stage 3 and 4, whereas no predictive role of proteinuria on ESRD was detected in stage 5 even in the presence of severe proteinuria. On the other hand, a significant nonlinear association between proteinuria level and mortality was found in all of the three CKD stages (P = 0.02), the risk of death showing no further increment at levels greater than 0.5 g/24 h. Similar results were found when categorical variables were replaced by continuous variables (Supplemental Table), thus excluding the possibility that the prognostic role of dichotomized variables was mainly dependent on the choice of the cut off level.
Figure 3.
Plots of adjusted hazard ratios and 95% confidence intervals (as indicated by the curvilinear dash lines; the horizontal dash lines represent HR1) for ESRD in the three CKD stages and death for all stages by level of proteinuria as continuous variable (reference level, 0.5 g/24 h).
Discussion
The first main finding of this study is that in CKD patients under regular nephrology care, the incidence rate of ESRD outweighs that of death. This marks a fundamental difference in comparison with studies based on the general population (1–5). General healthcare and administrative databases do not provide information on the type of care, which is relevant information because mortality in CKD patients treated by nephrologists is lower than that of those treated by other specialists (14). In addition, our patients had a CKD diagnosis dating from at least 1 year before the study visit. This stringent criterion consistently identifies patients with true CKD. Indeed, prolonging the chronicity criterion for CKD definition from 3 to 12 months reduces the number of patients with CKD, by 16% to 51% according to the stage, and it is associated with higher rates of ESRD and lower mortality (21).
In the MDRD study cohort, a large group of referred patients who took part in a landmark clinical trial that started in the early 1990s (18), ESRD was the most common outcome with a low competing risk of death, a relationship independent of demographic factors, etiology of CKD, level of basal eGFR, and proteinuria. Notably, the incidence rate of ESRD (8.4 per 100 person-years) in the MDRD study was almost identical to that recorded in the TABLE cohort (8.3 per 100 person-years). The greater risk of ESRD was also found in a secondary analysis of another major clinical trial in referred African-American patients, the African American Study of Kidney Disease and Hypertension (AASK) study (19); this result suggests that racial differences should not play a major role on the competing risk of ESRD versus death in referred patients. In contrast with MDRD, with AASK, and with TABLE, three other studies with fairly short nephrology care reported a higher incidence of death than of ESRD in patients with moderate to severe CKD (10,15,20).
Remarkably, we found that the CKD stage is a relevant prognostic criterion for predicting the occurrence of ESRD as an event competing with the risk of death. Indeed, ESRD risk outweighed mortality in CKD stages 4 and 5, whereas the opposite occurred in CKD stage 3. This finding in our contemporary CKD cohort differs from observations in the MDRD study. In the context of that trial, at all stages of CKD and independently of proteinuria, the risk of ESRD was uniformly higher than that of death. MDRD study was on the basis of a relatively young population (average age 50 years) of nondiabetic patients with a low burden of cardiovascular comorbidities (8%), whereas our cohort, which reflects the population now commonly seen in nephrology clinics in Europe (38), had an average age of 67 years and included large fractions of diabetics (28%) and of patients with CV disease (32%). Therefore, the TABLE study, on the basis of patients reflecting today's CKD population on stable nephrology care, confirms that ESRD rather than death is the dominant clinical outcome in patients on long term follow-up in nephrology offices. Whether this finding depends on nephrology care or intrinsic features of the “prevalent” patients in nephrology clinics remains to be elucidated.
The second main objective of our study was to provide a global evaluation of the relationship between the main modifiable risk factors and the two outcomes of ESRD and death. This issue has never been tested in a cohort study of patients with a sufficiently long observation period in nephrology. A relevant finding of our study is that some modifiable determinants, more specific to CKD, are strong predictors of the risk of progression to ESRD and also contribute to modify the risk of death even though in a less relevant way. These CKD-specific risk factors were different for the two outcomes; in particular, proteinuria and hyperphosphatemia predicted the risk of ESRD, whereas proteinuria, anemia, and high uric acid levels predicted death. Notably, no predictive role on either outcome was observed for traditional modifiable determinants, such as uncontrolled hypertension, high cholesterol levels, and smoking. This finding supports the concept that CKD-specific modifiable factors progressively take stage as renal function deteriorates, whereas traditional risk factors are dominant in triggering the initial renal and CV damage (39). The lack of a predictive role for high BP is only apparently surprising when considering the growing evidence of a J-shaped relationship between BP and outcomes (12,13,40), as well as of the superior predictive role of ambulatory BP measurements versus office BP (41).
In our study, proteinuria strongly predicted ESRD at stages 3 and 4 but not at stage 5. Conversely, in the recent landmark collaborative meta-analysis, no such interaction was observed in the general populations cohorts (42). CKD patients under regular nephrology care are a highly selected, population and the reduced role of proteinuria when renal function declines might rather suggest that mechanisms triggered by renal function loss per se, like the accumulation of uremic toxins (43), may be of particular relevance at advanced stages of renal disease. Conversely, we did not find a greater mortality risk in patients with severe proteinuria (Figure 3). This finding is possibly accounted for by the low number of patients with elevated proteinuria and the predominant weight of age on mortality that blunts the role of the other factors, including proteinuria. In addition to proteinuria, other nontraditional, modifiable factors such as anemia, hyperphosphatemia, and hyperuricemia played minor but still relevant prognostic roles (Table 3), which is in keeping with previous observations (24,44–46).
Our study has limitations. First, the TABLE cohort was formed only by Caucasian patients. Second, CKD patients under regular nephrology care represent a selected population with survival and referral biases; thus, results cannot be extrapolated to patients not followed in nephrology clinics, which unfortunately is the rule rather than the exception (47). Third, even though we adjusted our analyses for several potential confounders, the possibility of residual confounding, including socioeconomic status and glycemic control, cannot be excluded. Fourth, analysis of risk factors was based on a single data collection, and we cannot exclude effects caused by changes over time that we were unable to assess. Finally, the observational nature does not allow interpretation of results in causal terms.
In conclusion, this prospective study points out that in CKD patients under regular nephrology care in Italy: (1) ESRD is more frequent than death in stage 4 and 5 CKD, but the opposite is true in stage 3; (2) among the main modifiable risk factors, proteinuria and high phosphate predict ESRD, whereas proteinuria, high uric acid, and anemia predict death; and (3) proteinuria must be considered in conjunction with eGFR to refine risk stratification.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was endorsed by the Italian Society of Nephrology (Gruppo di Studio sul Trattamento Conservativo della Insufficienza Renale Cronica). It was partially supported by a government grant from Ministero della Istruzione, Università, e Ricerca); Rome, Italy, to G.C. in 2007. Partial results have been presented in abstract form at the 2010 ERA-EDTA Congress in Munich (Germany).
Appendix
TArget BP LEvels in CKD (TABLE-CKD) Study Group:
L. De Nicola, R. Minutolo, P. Zamboli, F., C. Iodice, S. Borrelli, P. Chiodini, S. Signoriello, C. Gallo, G. Conte (Napoli-2° University Medical School); T. Materiale, B. Minale, C. Paglionico (Napoli-ASL NA 1); B. Cianciaruso, A. Pota (Napoli-University Medical School Federico II); F. Nappi, F. Avella (Nola-Hospital); B.R. Di Iorio, V. Bellizzi (Solofra-Hospital); R. Cestaro (Sapri-Hospital); V. Martignetti, L. Morrone (Benevento-Hospital); A. Lupo, C. Abaterusso (Verona-University Medical School); C. Donadio (Pisa-University Medical School); M. Bonomini, V. Sirolli (Chieti-University Medical School); F. Casino, T. Lopez (Matera-Hospital); F. Detomaso, M. Giannattasio (Putignano-Hospital); M. Virgilio, G. Tarantino (Molfetta-Hospital); C. Cristofano, S. Tuccillo, S. Chimienti (Manduria-Hospital); F. Petrarulo, V. Giancaspro (Bari-Di Venere Hospital); M. Strippoli (Bari-University Medical School); E. Laraia (Bari-S. Rita Hospital); M. Gallucci, B. Gigante (Galatina-Hospital); C. Lodeserto, D. Santese (Taranto-Hospital); A. Montanaro, R. Giordano (Martina Franca-Hospital); A. Caglioti, G. Fuiano (Catanzaro-University Medical School); C. Zoccali, G. Caridi, M. Postorino (Reggio Calabria-CNR); V. Savica, P. Monardo (Messina-Hospital); G. Bellinghieri, D. Santoro (Messina-University Medical School); and P. Castellino, F. Rapisarda, P. Fatuzzo, A. Messina, (Catania-University Medical School).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org/.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 3. O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II study. Arch Intern Med 167: 2490–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Foley R, Murray A, Herzog C, McBean A, Eggers P, Collins A: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 6. John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Patel UD, Young EW, Ojo AO, Hayward RA: CKD progression and mortality among older patients with diabetes. Am J Kidney Dis 46: 406–414, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Minutolo R, De Nicola L, Zamboli P, Chiodini P, Signoriello G, Toderico C, Arfè G, Boschi G, Brancati C, Iaccarino P, Conte G: Management of hypertension in patients with CKD: Differences between primary and tertiary care settings. Am J Kidney Dis 46: 18–25, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Conway B, Webster A, Ramsay G, Morgan N, Neary J, Whitworth C, Harty J: Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol Dial Transplant 24: 1930–1937, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Obi Y, Kimura T, Nagasawa Y, Yamamoto R, Yasuda K, Sasaki K, Kitamura H, Imai E, Rakugi H, Isaka Y, Hayashi T: Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin J Am Soc Nephrol 9: 1558–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE: Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 21: 1257–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Agarwal R: Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol 4: 830–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng CL, Kern EF, Miller DR, Tiwari A, Maney M, Rajan M, Pogach L: Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 168: 55–62, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH: Predicting renal replacement therapy and mortality in CKD. Am J Kidney Dis 50: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal R, Bunaye Z, Bekele DM, Light RP: Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 28: 569–575, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, Collins AJ, Kusek JW, Levey AS, Greene T: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Alves TP, Wang X, Wright JT, Appel LJ, Greene T, Norris K, Lewis J. for the AASK Collaborative Research Group: Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol 21:1361–1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoefield RA, Kalra PA, Baker P, Lane B, New JP, O'Donoghue DJ, Foley RN, Middleton RJ: Factors associated with kidney disease progression and mortality in a referred CKD population. Am J Kidney Dis 56: 1072–1081, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Eriksen BO, Ingebretsen OC: In chronic kidney disease staging the use of the chronicity criterion affects prognosis and the rate of progression. Kidney Int 72: 1242–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 22. De Nicola L, Minutolo R, Chiodini P, Zamboli P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F, Virgilio M, Laraia E, Di Iorio BR, Savica V. Conte G on behalf of the TArget Blood Pressure LEvels in Chronic Kidney Disease (TABLE in CKD) Study Group: Global approach to cardiovascular risk in chronic kidney disease: Reality and opportunities for intervention. Kidney Int 69:538–545, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Dwyer J, Kenler SR. Assessment of nutritional status in renal disease. In: Nutrition and the Kidney, edited by Mitch WE, Klahr S. 2nd Ed., Boston: Little, Brown and Company, 1993, pp 61–95 [Google Scholar]
- 24. Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG: Serum uric acid in essential hypertension: An indicator of renal vascular involvement. Ann Intern Med 93: 817–821, 1980 [DOI] [PubMed] [Google Scholar]
- 25. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 26. National Kidney Foundation: K/DOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: S17–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 27. K/DOQI Clinical Practice Guidelines for Bone Metabolism, & Disease in Chronic Kidney Disease. Am J Kidney Dis 42[Suppl 4]: S1–S201, 2003 [PubMed] [Google Scholar]
- 28. Ruggenenti P, Perna A, Remuzzi G. GISEN Group Investigators. Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Cianciaruso B, Italian Society of Nephrology: Conservative therapy guidelines for chronic renal failure. G Ital Nefrol 20 [Suppl 24]: 48–60, 2003 [PubMed] [Google Scholar]
- 31. McGraw KO, Wong SP: Forming inferences about some intraclass correlation coefficients. Psychol Methods l: 30–46, 1996 [Google Scholar]
- 32. Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343–346, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Kalbfleisch JD, Prentice RL: The statistical analysis of failure time data, New York: John Wiley, 1980 [Google Scholar]
- 34. Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16: 1141–1154, 1988 [Google Scholar]
- 35. Smith CT, Williamson PR, Marson AG: Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med 24: 1307–1319, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Harrell F: Regression modelling strategies with applications to linear models, logistic regression, and survival analysis, New York: Spinger-Varlag, 2001 [Google Scholar]
- 37. Nagelkerke NJD: A note on a general definition of the coefficient of determination. Biometrika 78: 691–692, 1991 [Google Scholar]
- 38. Sims RJ, Cassidy MJ, Masud T: The increasing number of older patients with renal disease. BMJ 327: 463–464, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26–33, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Lewis JB: Blood pressure control in chronic kidney disease: Is less really more? J Am Soc Nephrol 21: 1086–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Agarwal R, Andersen MJ: Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 1175–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80:17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J: European Uremic Toxin Work Group: A bench to bedside view of uremic toxins. J Am Soc Nephrol 19: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825–831, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Uric acid and long-term outcomes in CKD. Am J Kidney Dis 53: 796–803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levin A, Djurdjev O, Duncan J, Rosenbaum D, Werb R: Haemoglobin at time of referral prior to dialysis predicts survival: An association of haemoglobin with long-term outcomes. Nephrol Dial Transplant 21: 370–377, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Szczech LA: Where Is Walter? If He Isn't in My Office, Is He Really My Responsibility? The 2011 National Kidney Foundation Presidential Address. Am J Kidney Dis 57: 529–531, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.