Abstract
Summary
Background & objectives
Chronic kidney disease (CKD) is characterized by chronic inflammation, considered a nontraditional risk factor for cardiovascular disease, the major cause of death in CKD. Symmetric dimethylarginine (SDMA) was recently demonstrated to induce reactive oxygen species in monocytes. The present study further investigates the inflammatory character of SDMA compared with its structural counterpart asymmetric dimethylarginine (ADMA).
Design, setting, participants, & measurements
In vitro, the effect of SDMA on intracellular monocytic expression of IL-6 and TNF-α was studied followed by an evaluation of nuclear factor (NF)–κB activation. Additionally, an association of SDMA with inflammatory parameters in consecutive stages of CKD was evaluated in vivo.
Results
Monocytes incubated with SDMA showed increased IL-6 and TNF-α expression and a rise in active NF-κB. N-acetylcysteine abrogated both these effects. No significant effects were observed with ADMA. In vivo, 142 patients (67 ± 12 years) at different stages of CKD showed an inverse association between serum SDMA and ADMA and renal function. Correlations between SDMA and IL-6, TNF-α, and albumin were more significant than for ADMA, while multiple regression analysis only retained TNF-α at a high significance for SDMA (P < 0.0001). In receiver operating characteristic analysis for inflammation, defined as an IL-6 level above 2.97 pg/ml (median), the discriminative power of SDMA (area under the curve [AUC]: 0.69 ± 0.05) directly followed that of C-reactive protein (AUC: 0.82 ± 0.04) and albumin (AUC: 0.72 ± 0.05; for all, P < 0.0001) and preceded that of ADMA (P = 0.002).
Conclusions
The present study shows that SDMA is involved in the inflammatory process of CKD, activating NF-κB and resulting in enhanced expression of IL-6 and TNF-α, which is corroborated by the clinical data pointing to an in vivo association of SDMA with inflammatory markers in CKD at different stages.
Introduction
Cardiovascular disease (CVD) is the most important cause of death among people with chronic kidney disease (CKD) (1–3). Besides traditional risk factors, which insufficiently explain the high prevalence of CVD in CKD, nonclassic risk factors such as inflammation and oxidative stress play an at least as prominent role (4).
Together with a myriad of solutes normally cleared by the kidneys, and defined as uremic toxins, the endogenous methylated forms of L-arginine, symmetric dimethylarginine (SDMA) and asymmetric dimethylarginine (ADMA), accumulate in CKD. Vallance et al. measured elevated concentrations of both SDMA and ADMA in dialysis patients and were the first to describe ADMA as an inhibitor of nitric oxide synthase (NOS) (5). Nowadays, ADMA is generally accepted a marker of endothelial dysfunction and a strong predictor of CVD in general and specific patient populations, including those with CKD (6–9). A potential link between SDMA and CVD, however, had rarely been considered until some recent studies demonstrated the clinical importance of SDMA as an independent cardiovascular risk factor in general, as well as in patient groups, like those with CKD (10–13).
In a recent in vitro study, SDMA was shown to enhance reactive oxygen species (ROS) production in monocytic leukocytes by a mechanistic pathway involving Ca2+ influx (14). Next to this study, Bode-Böger et al. showed an increase in ROS production and an inhibition of NO synthesis in endothelial cells in the presence of SDMA (15). The latter effect was probably due to limiting the supply of the substrate L-arginine to NOS, as SDMA was described a potent competitor of L-arginine transport (16).
The present study investigates the proinflammatory properties of SDMA, with ADMA as a structurally related comparator. First, the impact of the dimethylarginines on cytokine expression was tested in an in vitro setting in whole blood monocytes, followed by an assessment of their effect on nuclear factor (NF)-κB activation. A cross-sectional study evaluated the correlation of the dimethylarginines with renal function and markers of inflammation in patients at different stages of CKD.
Materials and Methods
In Vitro Experiments
Materials.
The dimethylarginines SDMA and ADMA were purchased from Merck (Darmstadt, Germany). Separate stock solutions were prepared in 0.9% NaCl (Baxter, Lessines, Belgium) and were stored at −20°C. They were diluted in the cell culture medium or in heparinized whole blood (sodium heparin Vacutainer® tubes; Becton Dickinson, San Jose, CA), resulting in a maximal uremic concentration of 6.1 μM SDMA or 36.0 μM ADMA (17).
Intracellular Cytokine Quantification by Flow Cytometry.
Whole blood was incubated with saline (control) or different doses of ADMA (0.6, 3.6, and 36 μM) or SDMA (1.5, 3.1, and 6.1 μM) for 2 hours in a humidified atmosphere of 5% CO2 in air at 37°C, after which the protocol was performed, as described previously (18). Cells were finally stained for intracellular TNF-α (Fastimmune™Anti-Hu-TNF-α) or IL-6 (Fastimmune™Anti-Hu-IL-6). Samples were analyzed with a FACScan® flow cytometer (Becton Dickinson).
NF-κB Protocol.
Cell line culture
The role of the dimethylarginines on NF-κB activation was studied on the human monocytic THP-1 cell line (ATCC, Manassas, VA). Cells were maintained as a continuous culture in RPMI1640 medium with L-glutamine and 25 mM HEPES supplemented with 10% fetal bovine serum (Invitrogen), 50 μg/ml gentamycin (Schering-Plough, Kenilworth, NJ), 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol in a humidified atmosphere of 5% CO2 in air at 37°C. The medium was refreshed every 3 to 4 days.
Cell differentiation and stimulation
Initially, the THP-1 cells (106/ml) were differentiated toward the monocyte/macrophage phenotype in the presence of 10 ng/ml calcitriol for 72 hours (19). After a 24-hour rest period in the absence of calcitriol (20,21), THP-1 cells were incubated with the dimethylarginines for 18 hours to evaluate the effect on NF-κB activation. Unstimulated cells were included as a negative control.
Extraction of nuclear proteins
To quantify the active NF-κB, nuclear proteins were extracted from 107 THP-1 cells using the Nuclear Extract Kit from Active Motif (Carlsbad, CA), according to manufacturer‘s instructions. The extracted amount of nuclear proteins was quantified by the Bradford-based total protein determination assay (Sigma-Aldrich Co., St. Louis, MO).
NF-κB ELISA
The amount of the p65 NF-κB subunit, a parameter of immunological activation, was determined by the ELISA-based TransAM™ NF-κB p65 (Active Motif). Samples were analyzed using the EL808 Ultra Microplate Reader from Bio-Tek Instruments (Winooski, VT) at 450 nm (reference wavelength of 650 nm) using the KC4V3.0 Analysis Software.
Pharmacologic Intervention.
The impact of N-acetylcysteine (NAC; Sigma-Aldrich), an antioxidant, was tested to prevent induction of NF-κB activation and to inhibit induction of cytokine expression. A concentration of 30 mM was added to the culture medium 1 hour before incubation with saline, SDMA, or ADMA (22).
Clinical Study
Patient Selection.
Over an 18-month period (from January 2006 to June 2007), a total of 150 Caucasian prevalent CKD patients were recruited from the Nephrology Department's outpatient clinic at Amiens University Hospital, as described previously (23). All patients gave their informed, written consent. The study was approved by the local Investigational Review Board and performed in accordance with the ethical principles of the Declaration of Helsinki. One hundred forty-two patients who met all inclusion criteria and had available SDMA and ADMA serum level measurements were included in the present analysis.
Patients were hospitalized for the day to perform laboratory blood tests and BP measurement (23).
Laboratory Analyses.
Blood samples were collected before the other investigations were undertaken with patients in a nonfasting condition. Selected variables were measured after the samples had been frozen and stored at −80°C. Serum calcium, phosphate, albumin, cholesterol, hemoglobin, creatinine (Scr), and C-reactive protein (CRP) levels were assayed in an on-site biochemistry laboratory using standard auto-analyzer techniques (the Modular IIP® system, Roche Diagnostics, Basel, Switzerland). Serum cystatin C (CysC) levels were determined by immunonephelometry (N Latex Cystatin C®, Dade Behring, Marburg, Germany). Intact parathyroid hormone (PTH) was determined in a chemiluminometric immunoassay (Liaison N-tact PTH CLIA®, Diasorin, Stillwater, OK). To describe the true GFR as closely as possible, the estimated GFR combining Scr and CysC measurements (CKD-epi) was calculated for all nondialyzed patients according to the following equation: 177.6 × Scr−0.65 × CysC−0.57 × age−0.20 × (0.82 if female) (24). For descriptive purposes, patients were then classified into CKD stages according to the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (25).
IL-6 and TNF-α plasma levels were determined by ELISA (R&D Systems, Wiesbaden, Germany), with IL-6 normal value for 38 healthy volunteers of 1.32 pg/ml (range: nondetectable to 5.84 pg/ml) and for TNF-α of 2.88 pg/ml (range: nondetectable to 5.89 pg/ml).
Competitive ELISAs, obtained from DLD Diagnostika GmbH (Hamburg, Germany), were used for measuring ADMA and SDMA after acylation, according to manufacturer's guidelines. The reference value, obtained for 16 healthy controls, for ADMA is 0.55 ± 0.08 μmol/L and for SDMA 0.59 ± 0.21 μmol/L.
Statistical Analyses
For the in vitro assay, statistics were performed using a nonparametric paired Wilcoxon test. Clinical data are expressed as the mean ± SD or median and range or frequency, as appropriate. For analytical purposes, patients were divided according to median SDMA (1.12 μmol/L) or median ADMA serum values (0.67 μmol/L). Intergroup comparisons were performed using a chi-squared test for categorical variables and the t test or the Mann–Whitney test for continuous variables. Linear regression analyses were performed to better describe the relationship between serum SDMA/ADMA levels and selected demographic, clinical, and biochemical characteristics. A multiple linear regression analysis was then performed to identify the factors independently associated with SDMA and ADMA serum levels. For variables with non-Gaussian distribution, neperian logarithmic normalized values were used to replace tests that assume normally distributed variables. Sensitivity, specificity, and cut-off levels for CRP, creatinine, SDMA, ADMA, TNF-α, and albumin, as predictors of inflammation (defined as IL-6 >2.97 pg/ml, median), were analyzed by means of receiver operating characteristic (ROC).
A P value ≤0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
In Vitro Data
Intracellular TNF-α and IL-6 Expression in Monocytes.
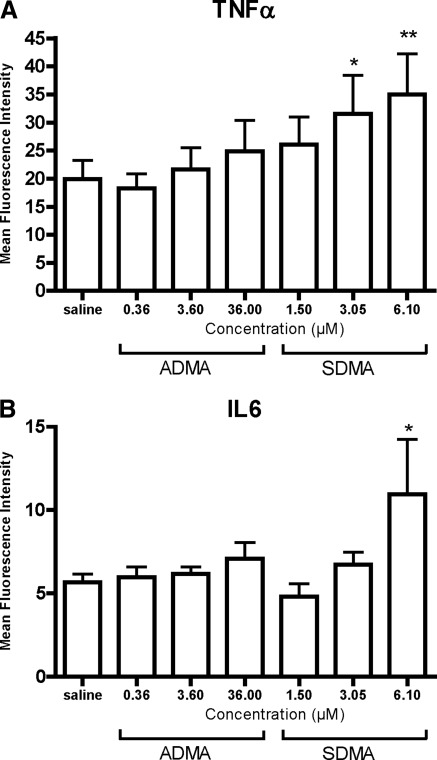
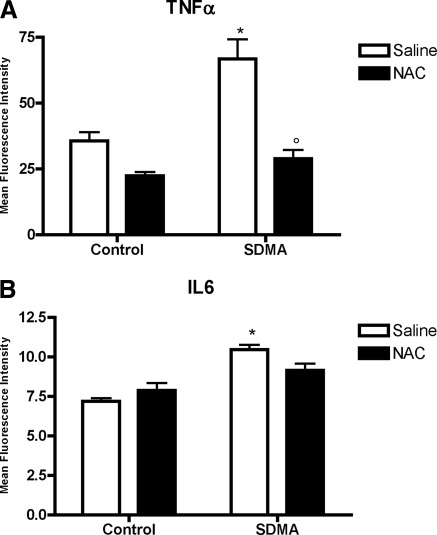
To investigate whether SDMA and ADMA are directly proinflammatory, both dimethylarginines were tested dose-dependently for their effect on the intracellular monocytic TNF-α and IL-6 expression. After a 4-hour incubation period, an increased expression of TNF-α from 3.05 μM SDMA on, and of IL-6 for 6.10 μM SDMA, could be observed (Figure 1). No such changes were observed for ADMA. Preincubation with NAC (30 mM), a mucolytic agent with direct and indirect antioxidant activity, as well as anti-inflammatory potential, neutralized this stimulatory effect of SDMA on TNF-α expression, while a similar trend was observed for IL-6 (Figure 2) (26).
Figure 1.
Dose-response effect of ADMA and SDMA on intracellular (A) TNF-α and (B) IL-6 expression in monocytes. Bars represent mean ± SEM; *P < 0.05; **P < 0.01 versus saline; n = 8.
Figure 2.
Effect of NAC on intracellular TNF-α and IL-6 expression in monocytes in the presence of SDMA (6.1 μM). Bars represent mean ± SEM; *P < 0.05 versus control; °P < 0.05 versus saline; n = 6. NAC, N-acetylcysteine; SDMA, symmetric dimethylarginine.
NF-κB Activation in Monocytic Cells
To elucidate a potential mechanism for increased cytokine expression, the dimethylarginines were tested for their role on NF-κB activation in a monocytic cell line. As shown in Table 1, and in parallel to the cytokine expression, only SDMA resulted in an increase of the NF-κB activation (P < 0.05). NAC inhibited the NF-κB activation in the presence of SDMA (Table 1). These results show that SDMA has a proinflammatory effect on monocytes, which could not be demonstrated for its structural analog ADMA.
Table 1.
Effect of the dimethylarginines on the activation of NF-κB in the monocytic THP-1 cell line
| Saline | Dimethylarginines |
||
|---|---|---|---|
| SDMA (6.1 μM) | ADMA (36.0 μM) | ||
| Control | 407.8 ± 232.2 | 568.8 ± 358.7a | 438.4 ± 236.7 |
| NAC | 347.3 ± 162.0 | 290.6 ± 177.2b | 302.4 ± 174.4 |
Values are means ± SD obtained from six experiments expressed as “ng active NF-κB per mg nuclear protein extract” isolated from 107 differentiated THP-1 cells. SDMA, symmetric dimethylarginine; ADMA, asymmetric dimethylarginine; NAC, N-acetylcysteine.
P < 0.05 versus saline.
P < 0.05 versus control.
Clinical Data
Relationship to Kidney Function.
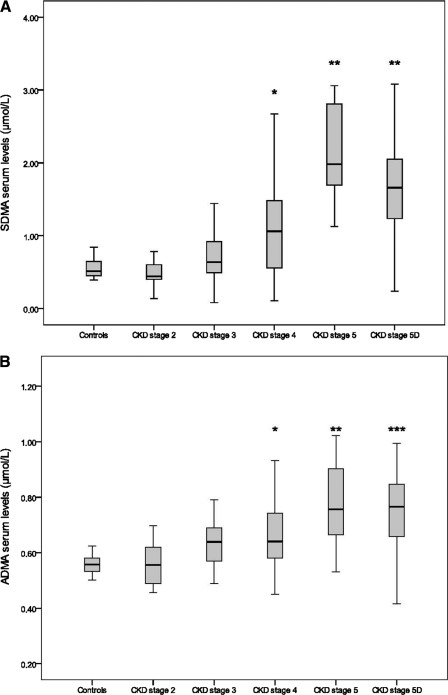
With progressive stages of CKD, SDMA levels increased gradually with values significantly higher versus controls and patients at CKD stages 2 and 3 from CKD stage 4 on (Figure 3). For ADMA, the relative rise was more moderate and reached significance versus controls from stage 4 on, and only at stage 5 and stage 5 on dialysis (stage 5D) versus CKD stage 2. This closer relationship of SDMA to renal function is further confirmed by a higher inverse correlation between SDMA and epi-GFR (GFR; R2 = 0.35; P < 0.0001) compared with the correlation between ADMA and epi-GFR (R2 = 0.15; P < 0.0001), in an analysis restricted to the nondialyzed subpopulation (n = 96). While both compounds have comparable normal values and their serum levels mutually correlate (n = 142; R2 = 0.33; P < 0.0001, data not shown), SDMA reaches relatively higher serum concentrations compared with controls than ADMA as CKD progresses.
Figure 3.
(A) Serum levels of SDMA in healthy controls and in CKD patients. *P < 0.02 versus controls and patients at CKD stages 2, 3, 5, and 5D; **P < 0.02 versus controls and patients at CKD stages 2, 3, and 4. (B) Serum levels of ADMA in healthy controls and in CKD patients. *P < 0.02 versus controls; **P < 0.02 versus controls and patients at CKD stage 2; ***P < 0.02 versus controls and patients at CKD stages 2 and 3. SDMA, symmetric dimethylarginine; ADMA, asymmetric dimethylarginine; CKD, chronic kidney disease.
Comparison of High Versus Low Concentration Strata.
When patients were stratified by median SDMA serum concentration, differences were found for 12 parameters (Table 2). The high SDMA stratum was characterized by a lower body mass index (BMI), diastolic BP (DBP), degree of statin use, hemoglobin, and albumin. On the other hand, CKD stages, phosphate, CRP, IL-6, and TNF-α were higher, as was serum ADMA. For ADMA, a similar stratification showed differences only for six parameters (Table 3). The high ADMA stratum was characterized by a lower hemoglobin and albumin, whereas CKD stages, phosphate, and IL-6 were higher, as was SDMA, per se.
Table 2.
Main clinical characteristics and baseline data as a function of the median of SDMA serum levels
| All n = 142 | SDMA ≤ 1.12 μM n = 71 | SDMA > 1.12 μM n = 71 | P | |
|---|---|---|---|---|
| Age (years) | 67 ± 12 | 66 ± 12 | 67 ± 13 | 0.66 |
| Male gender, n (%) | 86 (61) | 45 (63) | 41 (58) | 0.49 |
| BMI (kg/m2) | 28 ± 6 | 30 ± 7 | 27 ± 5 | 0.02 |
| SBP (mmHg) | 153 ± 26 | 153 ± 25 | 153 ± 27 | 0.93 |
| DBP (mmHg) | 81 ± 12 | 83 ± 11 | 79 ± 13 | 0.02 |
| Pulse pressure | 72 ± 23 | 69 ± 23 | 75 ± 24 | 0.20 |
| Diabetes, n (%) | 60 (42) | 34 (48) | 26 (37) | 0.17 |
| Smoking habit, n (%) | 56 (41) | 29 (43) | 27 (38) | 0.53 |
| Presence of CVD, n (%) | 45 (32) | 24 (34) | 21 (30) | 0.59 |
| Statin use, n (%) | 86 (61) | 53 (75) | 33 (46) | 0.01 |
| Iron use, n (%) | 33 (23) | 10 (14) | 23 (32) | 0.01 |
| EPO use, n (%) | 52 (37) | 14 (20) | 38 (53) | <0.01 |
| CKD stage, n (%) | <0.01 | |||
| 2 | 12 (8.5) | 12 (16) | 0 | |
| 3 | 37 (26.1) | 30 (42) | 7 (9) | |
| 4 | 37 (26.1) | 21 (29) | 16 (22) | |
| 5 | 10 (7) | 0 | 10 (14) | |
| 5D | 46 (32.4) | 8 (11) | 38 (53) | |
| Calcium (mmol/L) | 2.29 ± 0.18 | 2.30 ± 0.14 | 2.27 ± 0.21 | 0.32 |
| Albumin corrected calcium (mmol/L) | 2.36 ± 0.20 | 2.33 ± 0.15 | 2.38 ± 0.24 | 0.20 |
| Phosphate (mmol/L) | 1.29 ± 0.45 | 1.19 ± 0.35 | 1.38 ± 0.53 | 0.02 |
| Hemoglobin (g/L) | 12.1 ± 1.7 | 12.7 ± 1.7 | 11.5 ± 1.6 | <0.01 |
| Albumin (g/L) | 37 ± 6 | 39 ± 6 | 36 ± 6 | 0.01 |
| Intact-PTH (pg/ml) | 140.4 (47 to 190) | 128.4 (40 to 180) | 155.7 (52 to 206) | 0.16 |
| C-reactive protein (mg/L) | 3.5 (1.3 to 9.0) | 2.6 (1.3 to 5.1) | 4.1 (1.3 to 14.6) | 0.05 |
| IL-6 (pg/ml)a | 3.0 (1.5 to 6.1) | 2.1 (0.9 to 4.1) | 4.1 (1.9 to 7.2) | <0.01 |
| TNF-α (pg/ml)b | 3.8 (2.2 to 5.1) | 2.6 (2.2 to 4.6) | 4.2 (2.5 to 5.1) | 0.02 |
| Total cholesterol (mmol/L) | 4.9 ± 1.1 | 4.9 ± 1.1 | 4.8 ± 1.2 | 0.59 |
| LDL-cholesterol (mmol/L) | 2.6 ± 0.9 | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.19 |
| ADMA (μmol/L) | 0.69 ± 0.15 | 0.62 ± 0.11 | 0.76 ± 0.15 | <0.01 |
| SDMA (μmol/L) | 1.21 ± 0.76 | 0.61 ± 0.30 | 1.82 ± 0.59 | NA |
Data are means ± SD and median (interquartile range) for variables with non-Gaussian distribution, or number (frequency) for binary variables. Bold values represent significance. SDMA, symmetric dimethylarginine; BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; CVD, cardiovascular disease; EPO, erythropoietin; CKD, chronic kidney disease; PTH, parathyroid hormone; NA, not applicable.
n = 125.
n = 130.
Table 3.
Main clinical characteristics and baseline data as a function of the median of ADMA serum levels
| All n = 142 | ADMA ≤ 0.67 μM n = 71 | ADMA > 0.67 μM n = 71 | P | |
|---|---|---|---|---|
| Age (years) | 67 ± 12 | 68 ± 12 | 66 ± 13 | 0.49 |
| Male gender, n (%) | 86 (61) | 44 (62) | 42 (59) | 0.73 |
| BMI (kg/m2) | 28 ± 6 | 28 ± 6 | 27 ± 6 | 0.22 |
| SBP (mmHg) | 153 ± 26 | 153 ± 25 | 153 ± 28 | 0.95 |
| DBP (mmHg) | 81 ± 12 | 83 ± 13 | 80 ± 11 | 0.13 |
| Pulse pressure | 72 ± 23 | 71 ± 21 | 73 ± 25 | 0.47 |
| Diabetes, n (%) | 60 (42) | 32 (45) | 28 (39) | 0.61 |
| Smoking habit, n (%) | 56 (41) | 29 (43) | 27 (38) | 0.53 |
| Presence of CVD, n (%) | 45 (32) | 24 (34) | 21 (30) | 0.59 |
| Statin use, n (%) | 86 (61) | 46 (65) | 40 (56) | 0.30 |
| Iron use, n (%) | 33 (23) | 14 (20) | 19 (27) | 0.32 |
| EPO use, n (%) | 52 (37) | 18 (25) | 34 (48) | <0.01 |
| CKD stage, n (%) | <0.01 | |||
| 2 | 12 (8.5) | 10 (14) | 2 (3) | |
| 3 | 37 (26.1) | 25 (35) | 12 (17) | |
| 4 | 37 (26.1) | 21 (29) | 16 (22) | |
| 5 | 10 (7) | 3 (4) | 7 (10) | |
| 5D | 46 (32.4) | 12 (17) | 34 (48) | |
| Calcium (mmol/L) | 2.29 ± 0.18 | 2.28 ± 0.17 | 2.30 ± 0.19 | 0.54 |
| Albumin corrected calcium (mmol/L) | 2.36 ± 0.20 | 2.31 ± 0.19 | 2.4 ± 0.2 | 0.09 |
| Phosphate (mmol/L) | 1.29 ± 0.45 | 1.18 ± 0.37 | 1.39 ± 0.50 | 0.07 |
| Hemoglobin (g/L) | 12.1 ± 1.7 | 12.5 ± 1.7 | 11.7 ± 1.7 | 0.09 |
| Albumin (g/L) | 37 ± 6 | 39 ± 7 | 36 ± 5 | 0.05 |
| Intact-PTH (pg/ml) | 140.4 (47 to 190) | 147.1 (43 to 209) | 137.5 (48 to 164) | 0.87 |
| C-reactive protein (mg/L) | 3.5 (1.3 to 9.0) | 3.3 (1.1 to 9.4) | 3.7 (1.4 to 7.8) | 0.65 |
| IL-6 (pg/ml)a | 3.0 (1.5 to 6.1) | 2.2 (1.3 to 4.3) | 3.8 (1.9 to 7.0) | 0.01 |
| TNF-α (pg/ml)b | 3.8 (2.2 to 5.1) | 3.3 (2.2 to 5.1) | 4.2 (2.5 to 5.1) | 0.24 |
| Total cholesterol (mmol/L) | 4.9 ± 1.1 | 4.9 ± 1.05 | 4.8 ± 1.25 | 0.39 |
| LDL-cholesterol (mmol/L) | 2.6 ± 0.9 | 2.75 ± 0.91 | 2.50 ± 0.89 | 0.12 |
| ADMA (μmol/L) | 0.69 ± 0.15 | 0.57 ± 0.06 | 0.80 ± 0.11 | NA |
| SDMA (μmol/L) | 1.21 ± 0.76 | 0.89 ± 0.58 | 1.54 ± 0.79 | <0.01 |
Data are means ± SD and median (interquartile range) for variables with non-Gaussian distribution, or number (frequency) for binary variables. Bold values represent significance. ADMA, asymmetric dimethylarginine; BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; CVD, cardiovascular disease; EPO, erythropoietin; CKD, chronic kidney disease; NA, not applicable.
n = 125.
n = 130.
In brief, SDMA is more related to factors linked to the inflammatory status than ADMA. This difference is most striking for IL-6 (P < 0.0001 for SDMA versus 0.01 for ADMA) and TNF-α (P = 0.002 for SDMA versus NS for ADMA).
Regression Analyses.
In a linear regression analysis, SDMA correlated with erythropoietin use, CKD stage, hemoglobin, TNF-α, IL-6 (P < 0.0001 for all), phosphate, albumin, iron use, BMI, and statin use (Table 4). For ADMA, the correlating factors were CKD stage, phosphate (P < 0.0001), TNF-α, IL-6, hemoglobin, statin use, and albumin (Table 4).
Table 4.
Linear regression analysis—variables associated with the serum levels of SDMA and ADMA
| R2 | SDMA Difference (95% CI) | P | R2 | ADMA Difference (95% CI) | P | |
|---|---|---|---|---|---|---|
| EPO use | 0.35 | 0.35 (0.31 to 0.80) | <0.01 | |||
| CKD stage | 0.32 | 0.52 (0.40 to 0.65) | <0.01 | 0.11 | 0.06 (0.03 to 0.09) | <0.01 |
| Hemoglobin | 0.11 | −0.15 (−0.22 to −0.08) | <0.01 | 0.04 | −0.02 (−0.03 to 0.00) | 0.02 |
| Ln-normalized TNF-α | 0.10 | 0.57 (0.28 to 0.87) | <0.01 | 0.06 | 0.08 (0.02 to 0.14) | 0.06 |
| Ln-normalized IL-6 | 0.10 | 0.23 (0.11 to 0.36) | <0.01 | 0.06 | 0.03 (0.01 to 0.06) | 0.09 |
| Phosphate | 0.080 | 0.48 (0.21 to 0.75) | 0.01 | 0.08 | 0.10 (0.04 to 0.15) | <0.01 |
| Albumin | 0.07 | −0.03 (−0.05 to −0.01) | 0.01 | 0.03 | −0.04 (−0.01 to 0.00) | 0.03 |
| Iron use | 0.06 | 0.23 (0.13 to 0.72) | 0.05 | |||
| Body mass index | 0.05 | −0.03 (−0.05 to −0.01) | 0.01 | |||
| Statin use | 0.04 | −0.30 (−0.56 to −0.04) | 0.02 | 0.03 | −0.06 (−0.11 to 0.01) | 0.03 |
SDMA, symmetric dimethylarginine; ADMA, asymmetric dimethylarginine; CI, confidence interval; EPO, erythropoietin; CKD, chronic kidney disease.
When these parameters were entered into a multiple linear regression analysis, the CKD stage, TNF-α, hemoglobin, and BMI were independently associated with SDMA levels, with a global R2 of 0.43 (Table 5). Only the CKD stage and TNF-α were retained in the model for ADMA, with an R2 of only 0.23, as detailed in Table 5.
Table 5.
Multiple linear regression analysis—variables independently associated with the serum levels SDMA and ADMA
| SDMA |
ADMA |
|||
|---|---|---|---|---|
| Difference (95% CI) | P | Difference (95% CI) | P | |
| CKD stage | 0.35 (0.14 to 0.50) | 0.01 | 0.06 (0.03 to 0.09) | <0.01 |
| Ln-normalized TNF-α | 0.23 (0.16 to 0.66) | 0.01 | 0.06 (0.01 to 0.12) | 0.02 |
| Hemoglobin | −0.17 (−0.15 to −0.01) | 0.04 | ||
| Body mass index | −0.15 (−0.04 to −0.00) | 0.04 | ||
Variables entered in the model for SDMA: CKD stage, hemoglobin, ln-normalized TNF-α, ln-normalized IL-6, phosphate, albumin, body mass index, statin use, EPO use and Iron use; R2 = 0.44. Variables entered in the model for ADMA: CKD stage, phosphate, ln-normalized TNF-α, ln-normalized IL-6, hemoglobin, statin use, albumin; R2 = 0.23. SDMA, symmetric dimethylarginine; ADMA, asymmetric dimethylarginine; CI, confidence interval; CKD, chronic kidney disease.
Finally, although the univariate logistic regression analysis estimating the risk of having IL-6 above median was significant for both compounds, a multiple regression analysis with SDMA, as well as ADMA, resulted in a significant relative risk of 1.07 per increase of 0.1 μM SDMA concentration independently of ADMA.
ROC Curve Analysis.
ROC curves were made comparing SDMA, ADMA, CRP, TNF-α, and albumin as predictors of inflammation, defined as having IL-6 levels above the median (2.97 pg/ml; n = 125). Only CRP (positive inflammatory marker) and albumin (negative inflammatory marker) resulted in a higher area under the curve (0.82 ± 0.04 and 0.72 ± 0.05, respectively) than SDMA (0.69 ± 0.05), with the same degree of significance (P < 0.0001). ADMA was associated with a lower area under the curve at a lower significance level (0.66 ± 0.05; P = 0.002). The area under the curve, cut off levels, sensitivity and specificity, and cut off levels for ROC curves are shown in Table 6.
Table 6.
ROC curve coordinates for TNF-α, ADMA, SDMA, CRP, and albumin to inflammation (presence of IL-6> median, 2.97 pg/ml)a
| AUC Cutoff Level; Sensitivity/Specificity (%) | 95%CI | P | |
|---|---|---|---|
| CRP | 0.82 ± 0.04 | 0.74 to 0.89 | <0.01 |
| 3.75 mg/L; 71%/75% | |||
| Albumin | 0.72 ± 0.05 | 0.63 to 0.81 | <0.01 |
| 38.45 g/L; 71%/62% | |||
| SDMA | 0.69 ± 0.05 | 0.59 to 0.78 | <0.01 |
| 1.079 μmol/L; 71%/70% | |||
| ADMA | 0.66 ± 0.05 | 0.57 to 0.76 | 0.02 |
| 0.677 μmol/L; 64%/59% | |||
| TNF-α | 0.57 ± 0.05 | 0.47 to 0.67 | 0.20 |
| 3.4 pg/ml; 56%/53% |
Values expressed as AUC ± SE, cut-off level, sensitivity/specificity. For albumin, a lower AUC indicates a more positive test; for all other variables, higher AUC values indicate a more positive test. ROC, receiver operating characteristic; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; CRP, C-reactive protein; AUC, area under the curve; CI, confidence interval.
n = 125.
Discussion
The present study investigates the possible association of the uremic toxin SDMA and its structural counterpart ADMA to markers of inflammation. The effect of the dimethylarginines on intracellular cytokine expression and NF-κB activation were studied in monocytes, a key cell type in atherogenesis (27,28). An in vivo study assessed the association of SDMA and ADMA with inflammatory parameters in patients with different stages of CKD.
The main findings were as follows: (1) in vitro SDMA, unlike ADMA, induced an increased TNF-α and IL-6 expression, which can be linked to increased activation of NF-κB, as demonstrated in a THP-1 cell line; both effects could be abrogated by NAC (Figures 1 and 2; Table 1); (2) in the clinical trial, SDMA and ADMA concentrations increased as renal function deteriorated, be it more discriminatory for SDMA; (3) SDMA was consistently related to inflammatory parameters such as TNF-α and IL-6 but also to hemoglobin, BMI, and albumin; for ADMA, the relation to these parameters was, in general, less significant or absent (Tables 4 and 5); (4) in a ROC analysis, the AUC for an IL-6 >2.97 pg/ml (median) was higher for SDMA than ADMA, and immediately followed CRP and albumin.
Our in vitro data add arguments supporting the biologic relevance of SDMA, involving NF-κB as key transcription factor regulating genes encoding proinflammatory mediators such as cytokines, cell adhesion molecules, and acute-phase proteins (29). These data thus suggest a causal contribution of SDMA to the chronic inflammatory status characterizing the uremic condition and corroborating the previously observed increase in ROS production induced by SDMA (14,15,30). Moreover, in a holistic in vitro approach evaluating 10 guanidino compounds in several experimental test systems of vascular pathophysiology, SDMA exerted the highest number of proinflammatory and vascular damaging effects (31).
The clinical part of this study confirms the inflammatory character of SDMA by demonstrating a correlation between SDMA serum levels and several inflammatory parameters, such as IL-6 and TNF-α, next to clinical parameters, which are influenced by inflammation such as BMI, hemoglobin, and albumin (Tables 4 and 5). The inverse correlation between SDMA and BMI could be indirectly explained by the proinflammatory character of SDMA, namely, a higher degree of inflammation is known to be associated with denutrition, which might result in a lower BMI (32). But on the other hand, high BMI has been associated with inflammation as well (33). Also, ADMA shows a correlation with most of these parameters (IL-6, TNF-α, hemoglobin, and albumin), but at a markedly lower level of significance (Tables 4 and 5).
In previous studies from Oner-Iyidogan et al., in patients with consecutive stages of CKD, and from Caglar et al., in patients with proteinuria and normal GFR, a correlation respectively linking SDMA and both ADMA and SDMA to inflammation has been demonstrated (34,35). A partly discrepant finding was reported by Zoccali et al., who found normal ADMA in patients with acute inflammation and underlying chronic disease with a variable degree of renal insufficiency and a rise in ADMA once the source of inflammation was treated; no such association was found for SDMA (36). Our data, however, refer to the chronic moderate inflammation in CKD in contrast to the study by Zoccali et al., which refers to acute bacterial infection episodes.
The correlation found with kidney function confirm the data published by Bode-Böger et al., who found that if SDMA, ADMA, PTH, and scores of coronary artery disease were entered in a stepwise multiple linear regression model to predict GFR, only SDMA was retained and accounted for 36% of the GFR variance (15). Also, in a meta-analysis based on 18 studies (2006), SDMA was suggested a marker of renal function (37), as further confirmed in more recent studies (10–12,38). ADMA, on the other hand, is, according to our data, not well related to kidney function.
Irrespective of renal function, SDMA was predictive for total sequential organ failure in ICU patients with renal and hepatic failure and for total mortality after acute ischemic stroke (12,39). Additionally, both ADMA and SDMA were recently found to mediate the cardiovascular risk in the general population, and both compounds were shown as a predictor of survival in patients referred for coronary angiography, be it with different pattern of risk suggesting different underlying mechanims (LURIC-study) (10,11).
Throughout the literature, ADMA and SDMA concentrations vary widely, as already described by our own group and others, in both control as well as CKD patient groups (8,40,41). These differences might be due to different analyzing techniques, the type of sample submitted to analysis (serum, heparin plasma, EDTA plasma), the patient groups, the analytical grade of compound used for standardization, and so on. In accordance with our data, Fliser et al. stratified 227 patients with CKD, based on their GFR, and retrieved similar ADMA concentrations as the ones presented in Figure 3B (42). After a follow-up in progressors to a renal end point versus nonprogressors, ADMA rose in progressors with 30% to 0.55 ± 0.11 μM, while SDMA was doubled to 1.46 ± 0.67 μM, which are concentrations similar to the levels measured in our dialysis group. However, in other studies such as the one by Anderstam et al., the ratio of SDMA/ADMA concentration with a value of 5 exceeded the ratio of 3 found by Fliser et al. and in our own study (42,43).
Hence, despite their structural relation and synthesis by post-translational methylation of L-arginine in proteins, followed by a proteolytic release (44–46), the biologic effects and the removal pattern during progression of CKD of SDMA and ADMA differ. The discrepancy in relation to kidney function between ADMA and SDMA might be attributed to differences in removal from the body. While, in normal renal function, approximately 80% of ADMA is eliminated by the enzyme dimethylarginine dimethylaminohydrolase (DDAH), and only 20% by renal excretion, SDMA is completely eliminated by the kidneys (47). It is more difficult to explain the divergent biologic impact, but difference in steric structure might explain different affinities to receptors and/or uptakes by various cell types.
The removal of dimethylarginines during dialysis remains controversial and might be difficult with standard dialysis (48–51). Appropriate medical treatment can improve outcomes long before dialysis is needed. In the in vitro part of this study, NAC was shown to inhibit NF-κB activation induced by SDMA, which agrees with other studies demonstrating an inhibition of inflammation by NAC (52). Whether NAC also has a favorable impact on survival in patients with CKD has, to the best of our knowledge, not been investigated. Tepel et al. suggested a benefit in hemodialysis patients on composite cardiovascular end points (53). However, no effect was reported on total or cardiovascular mortality. In other processes affecting the kidneys, such as contrast-induced nephropathy, NAC plays, at least in some studies, a protective role (54,55). Although these are studies that were undertaken in a condition (acute kidney injury) that is only partially related to CKD, those results might still be worth mentioning, as they also very likely can be attributed to an anti-inflammatory effect related to the potential of NAC to scavenge ROS and its vasodilatory properties (56).
In our clinical study, a significantly higher statin use was observed in the population in the low SDMA group, and a negative correlation was found between statin use and the concentration of both dimethylarginines. A similar observation was found for IL-6 and statin use in a parallel analysis of the same patient cohort (23). Both the 4D study and the AURORA study demonstrated that statins lowered CRP levels in CKD 5D, however, without impact on outcome (57,58). It might be interesting to evaluate the effect of an intervention with statins on the SDMA and IL-6 concentration during progression of CKD. Nevertheless, another possibility to be taken into consideration is that as renal failure advances, cholesterol levels become lower (59), decreasing the need for statins.
Limitations of the present study include the small sample size, the lack of data on proteinuria, and the fact that we relied on a single blood sample. We also acknowledge that it is not possible to demonstrate a cause-effect relationship on the basis of a cross-sectional study. Nevertheless, both ADMA and SDMA were measured in parallel in patients at different stages of CKD, and both in vitro and in vivo data demonstrate the association of SDMA and inflammation.
In conclusion, the present study shows that SDMA, a dimethylarginine considered to be inert, activates NF-κB in monocytes in vitro, resulting in an increased intracellular expression of TNF-α and IL-6, while ADMA does not. This proinflammatory character was further confirmed in a clinical study in which SDMA was associated with inflammatory markers. Besides being a marker for renal function, SDMA correlates with several markers of inflammation, like IL-6 and TNF-α. Whether the interventional modification of the effect of SDMA will be associated with a change of the inflammatory status in CKD patients remains to be demonstrated.
Disclosures
None.
Acknowledgments
The clinical study was funded by a grant from Amiens University Hospital (PHRC: 2006/0100 [27/03/2006]). The in vitro work was supported by a governmental research grant from the Bijzonder Onderzoeksfonds (BOF, grant no.01105303) and a grant for working costs from the Fonds voorWetenschappelijk onderzoek (FWO, grant no. B/06941/03). D. V. Barreto and F. C. Barreto received postdoctoral grants from the Picardy Regional Council/University of Picardy Jules Verne and postdoctoral scholarschips from CNPq, Brazil. S. Eloot is working as postdoctoral fellow for FWO-Vlaanderen. The authors acknowledge the European Uraemic Toxin (EUTox) Work Group, a group of European researchers involved in studies and reviews related to uremic toxicity created under the auspices of the European Society for Artificial Organs (ESAO) and of the ERA-EDTA, composed of 20 research groups throughout Europe. More information about the European Uremic Toxin Work Group, supporting this publication, can be obtained at http://uremic-toxins.org.
E.S. and D.V.B. contributed equally to this work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R: The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 28: 478–483, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N: Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallance P, Leone A, Calver A, Collier J, Moncada S: Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Cooke JP: Asymmetrical dimethylarginine: The Uber marker? Circulation 109: 1813–1818, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Kielstein JT, Tsikas D, Fliser D: Effects of asymmetric dimethylarginine (ADMA) infusion in humans. Eur J Clin Pharmacol 62: 39–44, 2006 [Google Scholar]
- 8. Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W: Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study). Clin Chem 53: 273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R: Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 358: 2113–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Kiechl S, Lee T, Santer P, Thompson G, Tsimikas S, Egger G, Holt DW, Willeit J, Xu Q, Mayr M: Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis 205: 261–265, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Meinitzer A, Kielstein JT, Pilz S, Drechsler C, Ritz E, Boehm BO, Winkelmann BR, März W: Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: The Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem 57: 112–121, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Schulze F, Carter AM, Schwedhelm E, Ajjan R, Maas R, von Holten RA, Atzler D, Grant PJ, Böger RH: Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 208: 518–523, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Zeller M, Korandji C, Guilland JC, Sicard P, Vergely C, Lorgis L, Beer JC, Duvillard L, Lagrost AC, Moreau D, Gambert P, Cottin Y, Rochette L: Impact of asymmetric dimethylarginine on mortality after acute myocardial infarction. Arterioscler Thromb Vasc Biol 28: 954–960, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Schepers E, Glorieux G, Dhondt A, Leybaert L, Vanholder R: Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant 24: 1429–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Bode-Böger SM, Scalera F, Kielstein JT, Martens-Lobenhoffer J, Breithardt G, Fobker M, Reinecke H: Symmetrical dimethylarginine: a new combined parameter for renal function and extent of coronary artery disease. J Am Soc Nephrol 17: 1128–1134, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Closs EI, Basha FZ, Habermeier A, Forstermann U: Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1: 65–73, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W: Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Glorieux GL, Dhondt AW, Jacobs P, Van Langeraert J, Lameire N, De Deyn P, Vanholder R: In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int 65: 2184–2192, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Eperon S, Jungi TW: The use of human monocytoid lines as indicators of endotoxin. J Immunol Methods 194: 121–129, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Kim SH, Kang SN, Kim HJ, Kim TS: Potentiation of 1,25-dihydroxyvitamin D(3)-induced differentiation of human promyelocytic leukemia cells into monocytes by costunolide, a germacranolide sesquiterpene lactone. Biochem Pharmacol 64: 1233–1242, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Sokoloski JA, Sartorelli AC: Induction of the differentiation of HL-60 promyelocytic leukemia cells by nonsteroidal anti-inflammatory agents in combination with low levels of vitamin D3. Leuk Res 22: 153–161, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Foulds S: Novel flow cytometric method for quantifying nuclear binding of the transcription factor nuclear factor kappa B in unseparated human monocytes and polymorphonuclear cells. Cytometry 29: 182–186, 1997 [PubMed] [Google Scholar]
- 23. Barreto DV, Barretto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy Z: Plasma IL-6 is independently associated with mortality in both hemodialysis and pre-dialysis CKD patients. Kidney Int 77: 550–556, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 26. Sadowska AM, Keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, De Backer WA: Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: In vivo and in vitro study. Pharmacol Res 53: 216–225, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Libby P: Inflammation in atherosclerosis. Nature 420: 868–874, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Baeuerle PA, Henkel T: Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12: 141–179, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Stenvinkel P, Alvestrand A: Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin Dial 15: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Schepers E, Glorieux G, Dou L, Cerini C, Gayrard N, Louvet L, Maugard C, Preus P, Rodriguez-Ortiz M, Argiles A, Brunet P, Cohen G, Jankowski J, Jankowski V, Massy Z, Rodriguez M, Vanholder R: Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: An in vitro evaluation. Blood Purif 30: 277–287, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berlund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Ramos LF, Shintani A, Ikizler TA, Himmelfarb J: Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 19: 593–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oner-Iyidogan Y, Oner P, Kocak H, Gurdol F, Bekpinar S, Unlucerci Y, Caliskan Y, Cetinalp-Demircan P, Kocak T, Turkmen A: Dimethylarginines and inflammation markers in patients with chronic kidney disease undergoing dialysis. Clin Exp Med 9: 235–241, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Caglar K, Yilmaz MI, Sommez A, Cakir E, Kaya A, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Bilgi C, Oktenli C, Vural A, Zoccali C: ADMA, proteinuria and insulin resistance in non-diabetic stage I chronic kidney disease. Kidney Int 70: 781–787, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zoccali C, Maas R, Cutrupi S, Pizzini P, Finocchiaro P, Cambareri F, Panuccio V, Martorano C, Schulze F, Enia G, Tripepi G, Böger R: Asymmetric dimethyl-arginine (ADMA) response to inflammation in acute infections. Nephrol Dial Transplant 22: 801–806, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Kielstein JT, Salpeter SR, Bode-Böger SM, Cooke JP, Fliser D: Symmetric dimethylarginine (SDMA) as endogenous marker of renal function: A meta-analysis. Nephrol Dial Transplant 21: 2446–2451, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Tutarel O, Denecke A, Bode-Böger SM, Martens-Löbenhoffer J, Schieffer B, Westhoff-Bleck M, Kielstein JT: Symmetrical dimethylarginine outperforms CKD-EPI and MDRD-derived eGFR for the assessment of renal function in patients with adult congenital heart disease. Kidney Blood Press Res 34: 41–45, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MPC, Kuik DJ, Rauwerda JA, Van Leeuwen PAM: Asymmetrical dimethylarginine (ADMA) in critically ill patients: High plasma ADMA concentration is an independent risk factor of ICU mortality. Clinical Nutrition 22: 23–30, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Meert N, Schepers E, De Smet R, Argiles A, Cohen G, Deppisch R, Drueke T, Massy Z, Spasovski G, Stegmayr B, Zidek W, Jankowski J, Vanholder R: Inconsistency of reported uremic toxin concentrations. Artif Organs 31: 600–611, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, Böger R: Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol 13: 490–496, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E: Asymmetric dimethylarginine and progresssion of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol 16: 2456–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Anderstam B, Katzarski K, Bergstrom J: Serum levels of NG, NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol 8: 1437–1442, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Clarke S: Protein methylation. Curr Opin Cell Biol 5: 977–983, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Teerlink T: ADMA metabolism and clearance. Vasc Med 10[Suppl 1]: S73–S81, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Leiper JM: The DDAH-ADMA-NOS pathway. Ther Drug Monit 27: 744–746, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P: Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23: 1455–1459, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Kielstein JT, Böger RH, Bode-Böger SM, Martens-Löbenhoffer J, Lonnemann G, Frölich JC, Haller H, Fliser D: Low dialysance of asymmetric dimethylarginine (ADMA): In vivo and in vitro evidence of significant protein binding. Clin Nephrol 62: 295–300, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Kalousova M, Kielstein JT, Hodkova M, Zima T, Dusilova-Sulkova S, Martens-Löbenhoffer J, Bode-Böger SM: No benefit of hemodiafiltration over hemodialysis in lowering elevated levels of asymmetric dimethylarginine in ESRD patients. Blood Purif 24: 439–444, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Grooteman MP, Wauters IM, Teerlink T, Twisk JW, Nube MJ: Plasma dimethylarginine levels in chronic hemodialysis patients are independent of the type of dialyzer applied. Blood Purif 25: 281–289, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Schroder M, Riedel E, Beck W, Deppisch RM, Pommer W: Increased reduction of dimethylarginines and lowered interdialytic blood pressure by the use of biocompatible membranes. Kidney Int Suppl 78: S19–S24, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Witko-Sarsat V, Gausson V, Nguyen AT, Touam M, Drüeke T, Santangelo F, Descamps-Latscha B: AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int 64: 82–91, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W: The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 107: 992–995, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W: Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 343: 180–184, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Tepel M, Zidek W: N-Acetylcysteine in nephrology: Contrast nephropathy and beyond. Curr Opin Nephrol Hypertens 13: 649–654, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Safirstein R, Andrade L, Vieira JM: Acetylcysteine and nephrotoxic effects of radiographic contrast agents–a new use for an old drug. N Engl J Med 343: 210–212, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Krane V, Krieter DH, Olschewski M, Marz W, Mann JF, Ritz E, Wanner C: Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis 49: 267–275, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaille A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 360: 1395–1407, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]