Abstract
Summary
Background and objectives
The incidence and prevalence of metabolic acidosis increase with declining kidney function. We studied the associations of both low and high serum bicarbonate levels with all-cause mortality among stage 3 and 4 chronic kidney disease (CKD) patients.
Design, setting, participants, & measurements
We examined factors associated with low (<23 mmol/L) and high (>32 mmol/L) serum bicarbonate levels using logistic regression models and associations between bicarbonate and all-cause mortality using Cox-proportional hazard models, Kaplan–Meier survival curves, and time-dependent analysis.
Results
Out of 41,749 patients, 13.9% (n = 5796) had low and 1.6% (n = 652) had high serum bicarbonate levels. After adjusting for relevant covariates, there was a significant association between low serum bicarbonate and all-cause mortality (hazard ratio [HR] 1.23, 95% CI 1.16, 1.31). This association was not statistically significant among patients with stage 4 CKD and diabetes. The time-dependent analysis demonstrated a significant mortality risk associated with a decline from normal to low bicarbonate level (HR 1.59, 95% CI 1.49, 1.69). High serum bicarbonate levels were associated with death irrespective of the level of kidney function (HR 1.74, 95% CI 1.52, 2.00). When serum bicarbonate was examined as a continuous variable, a J-shaped relationship was noted between serum bicarbonate and mortality.
Conclusions
Low serum bicarbonate levels are associated with increased mortality among stage 3 CKD patients and patients without diabetes. High serum bicarbonate levels are associated with mortality in both stage 3 and stage 4 CKD patients.
Introduction
Metabolic acidosis is a well recognized and common complication of chronic kidney disease (CKD) consequent to impairment in ammoniagenesis and decreased bicarbonate reabsorption with progressive kidney disease (1). Its prevalence and severity increase with the decline in kidney function (2). Animal and human studies have shown that chronic metabolic acidosis associated with CKD has several deleterious effects that include increased protein catabolism, uremic bone disease, muscle wasting, accumulation of β-2 microglobulin, chronic inflammation, and impaired glucose homeostasis and cardiac function (3–8). These conditions, in turn, may contribute to the higher cardiovascular burden among CKD patients. Patients with CKD have a higher prevalence of heart failure, other comorbid conditions, and diuretic use that contribute to high bicarbonate levels, which might impart independent detrimental effects (9,10).
The associations between serum bicarbonate and mortality in nondialysis-dependent CKD have been examined in previous studies. A U-shaped association between serum bicarbonate levels and all-cause mortality was noted among U.S. veterans with nondialysis-dependent CKD (11). A secondary analysis of the African American Study of Kidney Disease and Hypertension (AASK) trial reported a lower risk for the composite endpoint of death, dialysis, or GFR event with increasing bicarbonate level, even within the normal range of bicarbonate levels (12). However, ethnic and gender exclusivity of these studies may limit the generalization of the associations between serum bicarbonate levels and mortality to a diverse CKD population.
Therefore, in the present study, we investigated the association of serum bicarbonate levels with all-cause mortality in a diverse, large population of stage 3 and stage 4 CKD patients (eGFR 15 to 59 ml/min per 1.73 m2) followed in our health care system.
Materials and Methods
We conducted an analysis using our preexisting Electronic Health Record (EHR)-based CKD registry. The development and validation of our EHR-based CKD registry at Cleveland Clinic have been described in detail elsewhere (13).
Study Population
Patients who met the following criteria as of January 1, 2005, were included in the study population: (1) had at least one face-to-face outpatient encounter with a Cleveland Clinic health care provider, (2) had two estimated GFR (eGFR) values <60 ml/min per 1.73 m2 (the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation [14]) more than 90 days apart, and (3) had bicarbonate measured with the confirmatory eGFR as an outpatient using a standard assay at various laboratories in our health system. Patients aged <18 years old and those who were diagnosed with end-stage renal disease needing dialysis or renal transplant before CKD diagnosis were excluded.
Definitions and Outcome Measures
Renal Function.
We applied the CKD-EPI equation to patients in our health system who had two outpatient serum creatinine levels between January 1, 2005, and April 4, 2011, to calculate eGFR. All creatinine measurements were performed by the modified kinetic Jaffe reaction, using a Hitachi 747 to 200 Chemistry Analyzer (1996 to 2001) or a Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics, Indianapolis, IN) in our laboratory. CKD was defined according to current guidelines as follows: stage 3 CKD (eGFR 30 to 59 ml/min per 1.73 m2) and stage 4 CKD (eGFR 15 to 29 ml/min per 1.73 m2). We further categorized stage 3 into CKD stage 3a (eGFR 45 to 59 ml/min per 1.73 m2) and stage 3b (eGFR 30 to 44 ml/min per 1.73 m2) (15).
Serum Bicarbonate.
Serum bicarbonate was measured using an enzymatic procedure using phosphenolpyruvate carboxylase (PEPC) on the Roche Modular platform. Within- and between-run precision of human serum is stated at 1.3% and 1.9%, respectively. Only outpatient laboratory measures were included in this analysis. Serum bicarbonate levels measured on the day of CKD diagnosis (second eGFR <60 ml/min per 1.73 m2 at least 90 days after the first eGFR), as described, were used for the main analysis. The serum bicarbonate levels measured after CKD diagnosis were used for the time-dependent analysis.
Comorbid Conditions and Laboratory Parameters.
Demographic details were extracted from the EHR. Diabetes mellitus, hypertension, coronary artery disease, and other comorbid conditions were defined using prespecified criteria and validated. These conditions existed before CKD diagnosis (13). Serum bicarbonate levels and other relevant outpatient laboratory details were obtained from our electronic laboratory records.
Mortality.
The primary outcome of interest, all-cause mortality, was ascertained from our EHR and linkage of our CKD registry with the Social Security Death Index. Patients were followed from their date of inclusion in the registry (date of second qualifying eGFR) until April 2011.
Statistical Analyses
We compared baseline characteristics between CKD patients with and without measured serum bicarbonate levels using chi-squared and two-sample t tests for categorical and continuous variables, respectively. Based on the cutoffs used in our laboratory, CKD patients with measured outpatient serum bicarbonate values at the time of CKD diagnosis were further classified into three groups: <23 mmol/L (low), 23 to 32 mmol/L (normal), and >32 mmol/L (high). Associations of the baseline characteristics and these three groups were assessed using chi-squared and ANOVA tests for categorical and continuous variables, respectively.
Two separate logistic regression analyses were conducted to examine factors associated with abnormal serum bicarbonate levels. For the first model, the outcome was low serum bicarbonate level (<23 mmol/L) versus normal serum bicarbonate level (23 to 32 mmol/L), and for the second model, the outcome was high serum bicarbonate (>32 mmol/L) versus normal serum bicarbonate level (23 to 32 mmol/L). Covariates were based on information known before CKD diagnosis and chosen a priori based on factors previously shown or thought to be related to both serum bicarbonate and mortality. These include age, gender, race, body mass index (BMI), eGFR, diabetes, hypertension, hyperlipidemia, coronary artery disease, heart failure, chronic obstructive pulmonary disease (COPD), smoking, year of entry into our CKD registry, serum albumin, and hemoglobin levels. Nine percent of patients had missing covariate data; 4% and 6% of patients were missing hemoglobin and albumin data, respectively, and they were excluded from the multivariable model.
To evaluate whether survival among persons with CKD was associated with serum bicarbonate levels, we used Kaplan–Meier plots and log-rank tests with entry into the registry as the time of origin. We used Cox proportional hazards models to assess the association between the baseline serum bicarbonate levels and mortality, while adjusting for the covariates mentioned above (factors adjusted for in the logistic regression analyses), and use of renin-angiotension blockers and diuretics. We also fit the adjusted model with continuous serum bicarbonate as a cubic effect to estimate the log hazard of mortality for each patient, and plotted a cubic regression curve for serum bicarbonate versus the linear predictor.
We tested all two-way interactions between serum bicarbonate and the covariates included in the adjusted Cox proportional hazards model. To evaluate the associations between serum bicarbonate changes during follow-up and mortality, we fit a Cox proportional hazards model on patients with normal serum bicarbonate (23 to 32 mmol/L) at baseline and entered a time-dependent effect for reaching serum bicarbonate <23 mmol/L during follow-up, as well as a time dependent effect for reaching 25% decrease in eGFR, while adjusting for all baseline covariates previously mentioned. We did not censor patients at ESRD/renal transplantation, due to the lack of relevant data.
All data analyses were conducted using Unix SAS version 9.2 (SAS Institute, Cary, NC), and graphs were created using R 2.11.1 (The R Foundation for Statistical Computing, Vienna, Austria). The CKD registry and this study were approved by the Cleveland Clinic Institutional Review Board.
Results
Patient Characteristics
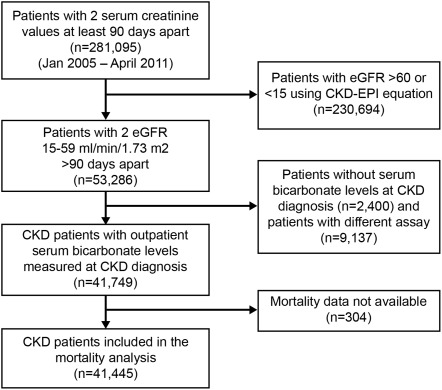
We identified patients with stage 3 and 4 CKD (n = 53,286) from our CKD registry. Of those, 41,749 (78%) had serum bicarbonate levels measured at CKD diagnosis with reference ranges 23 to 32 mmol/L and constituted the study population (Figure 1). An additional 9137 (17%) patients had measurements with different assays, and 2400 (4.5%) had missing values. Mean age of the study cohort was 72 ± 11.9 years, with 54% being females and 13% African Americans. Patients with and without serum bicarbonate levels measured were significantly different on all variables compared except hemoglobin, although some of the absolute differences were small (Table 1).
Figure 1.
Flow chart showing how patients were selected for this analysis from the electronic-health-record-based registry. CKD, chronic kidney disease; eGFR, estimated GFR.
Table 1.
Characteristics of patients serum bicarbonate levels measured at CKD diagnosis
| Variablea | Not Measured (n = 2400) | Low Bicarbonate (<23 mmol/L) (n = 5796) | Normal Bicarbonate (23–32 mmol/L) (n = 35,301) | High Bicarbonate (>32 mmol/L) (n = 652) |
|---|---|---|---|---|
| Ageb (mean [SD]) | 73.7 (9.8) | 68.9 (13.8) | 72.3 (11.5) | 71.9 (12.2) |
| eGFRb (mean [SD]) | 47.0 (10.0) | 42.3 (12.2) | 48.3 (9.7) | 46.4 (10.9) |
| eGFR stage | ||||
| Stage 3a (GFR 45 to 59 [%]) | 63.8 | 48.1 | 69.7 | 63.7 |
| Stage 3b (GFR 30 to 44 [%]) | 28.0 | 32.4 | 24.2 | 26.5 |
| Stage 4 (GFR 15 to 29 [%]) | 8.2 | 19.5 | 6.1 | 9.8 |
| Male gender (%) | 52.6 | 49.6 | 45.5 | 43.3 |
| African American (%) | 8.9 | 18.5 | 12.2 | 16.1 |
| Diabetes (%) | 12.9 | 27.7 | 22.6 | 21.6 |
| Hypertension (%) | 79.3 | 87.6 | 89.7 | 84.8 |
| Coronary artery disease (%) | 14.8 | 20.9 | 23.2 | 26.1 |
| Cerebrovascular disease (%) | 7.8 | 9.3 | 9.5 | 8.6 |
| Congestive heart failure (%) | 2.5 | 9.1 | 8.6 | 21.9 |
| Malignancy (%) | 29.2 | 26.1 | 23.6 | 23.2 |
| Hyperlipidemia (%) | 72.9 | 71.7 | 79.5 | 75.2 |
| COPD (%) | 6.3 | 7.8 | 9.0 | 20.1 |
| Smoking (%) | ||||
| nonsmoker | 84.0 | 83.5 | 88.7 | 86.5 |
| smoker | 9.0 | 10.2 | 7.0 | 7.7 |
| missing information | 7.0 | 6.3 | 4.3 | 5.8 |
| BMI kg/m2 (%) | ||||
| <18.5 | 0.96 | 1.8 | 1.1 | 1.2 |
| 18.5 to 24.9 | 23.8 | 24.3 | 23.1 | 28.1 |
| 25 to 29.9 | 34.5 | 32.8 | 35.6 | 28.5 |
| 30 to 34.9 | 19.7 | 20.3 | 21.1 | 15.6 |
| 35 to 39.9 | 9.1 | 9.4 | 8.8 | 12.1 |
| > 40 | 4.7 | 6.9 | 5.8 | 9.2 |
| missing information | 7.4 | 4.5 | 4.3 | 5.2 |
| Albumin (g/dl)b (mean [SD]) | 4.0 ± 0.51 | 4.0 ± 0.53 | 4.2 ± 0.41 | 4.1 ± 0.48 |
| Hemoglobin (g/dl) (mean [SD]) | 12.9 ± 1.8 | 12.1 ± 2.0 | 13.0 ± 1.7 | 12.8 ± 1.9 |
Overall serum bicarbonate groups <23, 23 to 32, and >32 significantly different with P < 0.001 for all variables except cerebrovascular disease. The chi-squared test was used to assess categorical variables and ANOVA used for continuous variables. CKD, chronic kidney disease; eGFR, estimated GFR; COPD, chronic obstructive pulmonary disease; BMI, body mass index.
Chi-squared test, P < 0.05, unless otherwise noted, comparing patients with serum bicarbonate measured versus all those not measured.
t-test, P < 0.05, comparing patients with serum bicarbonate measured versus all those not measured.
All demographic variables and comorbid conditions were also significantly different among patients with serum bicarbonate <23 mmol/L, 23 to 32 mmol/L and >32 mmol/L, with the exception of cerebrovascular disease (Table 1). Most notably, patients with lower serum bicarbonate levels (<23 mmol/L) were younger and were more likely to be African American, have diabetes, and have a lower eGFR than those with normal levels. Ten percent of stage 3a CKD (eGFR 45 to 59 ml/min per 1.73 m2) patients had serum bicarbonate <23 mmol/L, while 18% of stage 3b (eGFR 30 to 44 ml/min per 1.73 m2) and 34% of stage 4 (eGFR 15 to 29 ml/min per 1.73 m2) CKD patients had serum bicarbonate <23 mmol/L.
Factors Associated with Low and High Serum Bicarbonate Levels
Low Serum Bicarbonate Levels (<23 mmol/L).
In the multivariable analysis, males had 32% higher odds than females, African Americans had 24% higher odds than Caucasians, and diabetics had 20% higher odds than nondiabetics of having low serum bicarbonate levels (Table 2). Higher eGFR, higher hemoglobin and albumin levels, older age, and the presence of hyperlipidemia and congestive heart failure were associated with lower odds of having low serum bicarbonate levels.
Table 2.
Factors associated with low serum bicarbonate and high serum bicarbonate at CKD diagnosis
| Effect | Model 1 (n = 37,346) |
Model 2 (n = 32,623) |
||
|---|---|---|---|---|
| Low Bicarbonate (<23 mmol/L) OR (95% CI) | P | High Bicarbonate (>32 mmol/L) OR (95% CI) | P | |
| Age quintiles | <0.01 | 0.09 | ||
| 62.4 to 70.1 versus 18 to 62.4 | 0.72 (0.66, 0.79) | 0.79 (0.61, 1.02) | ||
| 70.2 to 76.4 2 versus 18 to 62.4 | 0.63 (0.58, 0.70) | 0.71 (0.55, 0.93) | ||
| 76.4 to 82.3 versus 18 to 62.4 | 0.57 (0.52, 0.63) | 0.82 (0.63, 1.06) | ||
| 82.3 to 104 versus 18 to 62.4 | 0.50 (0.45, 0.55) | 0.73 (0.56, 0.96) | ||
| Male gender | 1.32 (1.23, 1.40) | <0.01 | 0.94 (0.79, 1.11) | 0.46 |
| African-American race versus others | 1.24 (1.14, 1.35) | <0.01 | 1.09 (0.86, 1.37) | 0.49 |
| BMI group (kg/m2) | 0.17 | <0.01 | ||
| <18.5 versus 18.5 to 24.9 | 1.28 (1.00, 1.63) | 0.82 (0.40, 1.68) | ||
| 25 to 29.9 versus 18.5 to 24.9 | 0.96 (0.89, 1.04) | 0.71 (0.57, 0.88) | ||
| 30 to 34.9 versus 18.5 to 24.9 | 0.96 (0.87, 1.05) | 0.67 (0.51, 0.86) | ||
| 35 to 39.9 versus 18.5 to 24.9 | 0.94 (0.84, 1.07) | 1.17 (0.88, 1.56) | ||
| 40+ versus 18.5 to 24.9 | 0.88 (0.77, 1.02) | 1.16 (0.83, 1.62) | ||
| missing versus 18.5 to 24.9 | 1.03 (0.87, 1.21) | 0.97 (0.65, 1.46) | ||
| eGFR (per 10-ml/min per 1.73 m2 increase) | 0.66 (0.64, 0.67) | <0.01 | 0.92 (0.85, 0.998) | 0.046 |
| Diabetes | 1.20 (1.11, 1.29) | <0.01 | 0.79 (0.64, 0.98) | 0.03 |
| Hypertension | 1.03 (0.93, 1.14) | 0.54 | 0.71 (0.56, 0.90) | 0.04 |
| Hyperlipidemia | 0.79 (0.73, 0.85) | <0.01 | 0.84 (0.69, 1.03) | 0.10 |
| Coronary artery disease | 0.94 (0.87, 1.02) | 0.12 | 1.13 (0.92, 1.38) | 0.25 |
| Congestive heart failure | 0.82 (0.73, 0.91) | <0.01 | 2.57 (2.08, 3.16) | <0.01 |
| COPD | 0.88 (0.78, 0.98) | 0.019 | 2.52 (2.05, 3.09) | <0.01 |
| Smoking | <0.01 | 0.77 | ||
| yes versus no | 1.38 (1.24, 1.54) | 0.92 (0.67, 1.27) | ||
| missing versus no | 1.28 (1.12, 1.47) | 1.10 (0.75, 1.60) | ||
| Hb per 1 g/dl increase | 0.83 (0.82, 0.85) | <0.01 | 1.01 (0.96, 1.06) | 0.83 |
| Albumin per 1 mg/dl increase | 0.79 (0.74, 0.84) | <0.01 | 0.71 (0.59, 0.86) | <0.01 |
Model was additionally adjusted for the year of inclusion in the registry. CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval; BMI, body mass index; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease.
High Serum Bicarbonate Levels (>32 mmol/L).
Presence of heart failure and COPD were associated with 2.6- and 2.5-fold higher odds of having high bicarbonate levels, respectively (Table 2). Higher eGFR, higher albumin, and presence of hypertension were associated with lower odds of having serum bicarbonate >32 mmol/L.
Serum Bicarbonate Levels and All-Cause Mortality
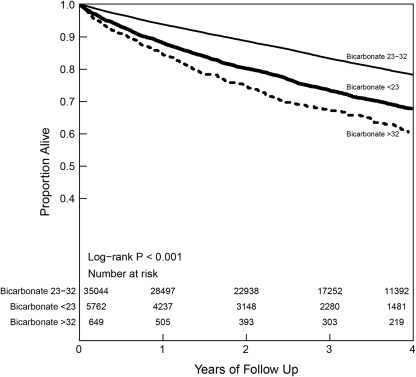
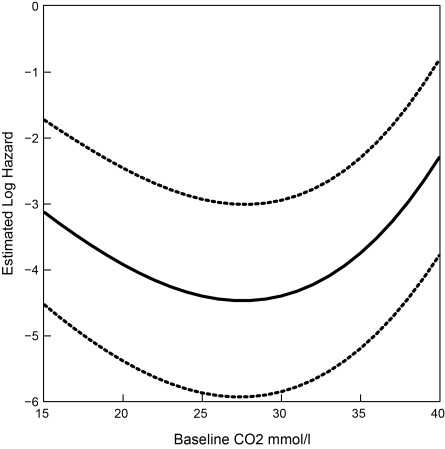
Among our study population, 41,445 patients had mortality data and 7997 of them died during an average follow-up of 2.9 years. The Kaplan–Meier analysis showed significant differences in all-cause mortality for CKD patients in the different serum bicarbonate groups (log-rank P < 0.001; Figure 2). Both low serum bicarbonate (<23 mmol/L; HR 1.23, 95% CI 1.16, 1.31) and high serum bicarbonate (>32 mmol/L; HR 1.74, 95% CI 1.52, 2.00) at baseline were associated with an increased hazard for death, after adjusting for demographics, comorbid conditions, renal function, and relevant medications (Table 3). There was a J-shaped association between serum bicarbonate and mortality when serum bicarbonate was examined as a continuous variable (Figure 3). This association was consistent across the various stages of CKD (Supplementary Figure 1).
Figure 2.
Kaplan–Meier survival curve based on serum bicarbonate levels among chronic kidney disease patients.
Table 3.
Associations between serum bicarbonate levels and all-cause mortality among chronic kidney disease patients
| Bicarbonate Group | Unadjusted HR [95% CI] (n = 41,445) | Model A HR [95 %CI] (n = 41,445) | Model B HR [95 %CI] (n = 37,687) | Model C HR [95% CI] (n = 37,687) |
|---|---|---|---|---|
| <23 mmol/L versus 23 to 32 mmol/L | 1.65 (1.56, 1.75) | 1.75 (1.65, 1.85) | 1.35 (1.27, 1.44) | 1.23 (1.16, 1.31) |
| >32 mmol/L versus 23 to 32 mmol/L | 2.12 (1.86, 2.42) | 2.08 (1.83, 2.37) | 1.76 (1.54, 2.02) | 1.74 (1.52, 2.00) |
Model A adjusted for age, gender, and race. Model B adjusted variables included in Model A, plus body mass index, diabetes, hypertension, hyperlipidemia, malignancy, congestive heart failure, cerebrovascular disease, coronary artery disease, chronic obstructive pulmonary disease, history of angiotensin-converting enzyme/angiotensin receptor blocker prescription, history of diuretic prescription at chronic kidney disease diagnosis, and hemoglobin and albumin. Model C adjusted variables included in Model B, plus baseline estimated GFR. HR, hazard ratio; CI, confidence interval.
Figure 3.
Associations (log hazard and 95% confidence interval) between all-cause mortality and baseline serum bicarbonate levels. Model adjusted for age, gender, African-American race, body mass index, diabetes, hypertension, hyperlipidemia, malignancy, congestive heart failure, cerebrovascular disease, and coronary artery disease at chronic kidney disease diagnosis and estimated GFR, use of renin-angiotensin system blockers and diuretics, hemoglobin and albumin.
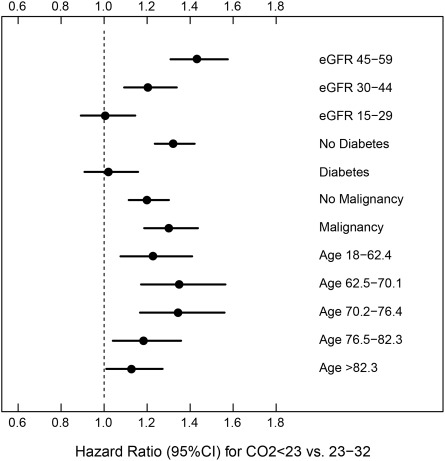
We found four significant two-way interactions with serum bicarbonate, which indicated that the increased mortality hazard associated with abnormal levels of serum bicarbonate was different at different levels of eGFR (P < 0.001), presence or absence of diabetes (P < 0.001) and malignancy (P = 0.03), and age (P < 0.001). When examined based on the stage of CKD, the association between low serum bicarbonate and mortality was only significant among the subgroup of patients with stage 3 CKD but not in the subgroup of patients with stage 4 CKD (Figure 4). The increased mortality hazard associated with serum bicarbonate <23 mmol/L was present for nondiabetic patients but absent for diabetics, stronger for patients with history of malignancy when compared with those without malignancy, and evident for patients aged 62 to 76 years, but less pronounced at other age levels (P = 0.08) (Figure 4).
Figure 4.
Hazard ratio and 95% confidence interval (CI) of all-cause mortality associated with low serum bicarbonate levels among select subgroups. eGFR, estimated GFR.
Of the 35,044 patients with serum bicarbonate 23 to 32 mmol/L at baseline and mortality information, 9297 reached bicarbonate <23 mmol/L at some point during follow-up. When we fit a time-dependent Cox model on 31,781 patients with baseline serum bicarbonate 23 to 32 mmol/L and complete covariate data, reaching serum bicarbonate <23 mmol/L during follow-up was independently associated with a hazard ratio of 1.59 (95% CI 1.49, 1.69), while also adjusting for reaching a 25% decrease in eGFR during follow-up. Of 35,044 patients with normal serum bicarbonate at baseline, only 1509 (<5%) reached levels >32 mmol/L during follow-up, and we did not construct a time-dependent Cox model to assess the mortality risk due to the small sample size.
Discussion
Among stage 3 and 4 CKD patients, male gender, African American race, and diabetes were associated with low serum bicarbonate (<23 mmol/L), while the presence of heart failure and COPD was associated with high serum bicarbonate (>32 mmol/L) levels. After adjusting for relevant covariates, low and high serum bicarbonate levels were associated with a 23% and 74% higher risk for all-cause mortality, respectively. The relationship between serum bicarbonate and all-cause mortality differed based on age, level of kidney function, and presence or absence of diabetes and malignancy. A time-dependent analysis suggested a decline in bicarbonate level was also significantly associated with increased all-cause mortality in this population.
Compared with the prior studies that examined the relationship between bicarbonate and mortality, our study has a large number of stage 3 and 4 CKD patients, with and without diabetes, as well as strong representation of African Americans, thereby enhancing the generalizability of the results. This allowed us to examine the interactions between serum bicarbonate and other clinically relevant variables that provided us some novel findings. Strengths of our CKD registry include the prior validation of the CKD and included comorbid conditions using standard definitions and availability of data to conduct a time-dependent analysis. These results emphasize the need for close monitoring of serum bicarbonate in patients diagnosed with CKD because patients with normal serum bicarbonate levels at baseline who develop lower bicarbonate levels as the renal function declines over time seem to be at higher risk for death.
We found a J-shaped association between serum bicarbonate as a continuous variable and mortality. As renal function declined, the effects of lower bicarbonate levels on mortality seemed to become less relevant. Results were consistent when we examined eGFR as a categorical (different stages of CKD) or as a continuous variable (Figure 4 and Supplementary Figure 1). Patients with lower bicarbonate levels also have rapid decline in renal function, and recent clinical trials suggest that bicarbonate supplementation seems to have renoprotective effects in both earlier and later stages of CKD (17–20). Our results suggest that, in contrast to patients with mild-to-moderate CKD, the uremic effects of advanced CKD per se may outweigh the effects of lower bicarbonate levels on mortality. In addition, patients with rapid decline in eGFR (possibly owing to lower bicarbonate levels) have higher rates of cardiovascular disease, which may explain these findings (21,22). We did not have cause-specific mortality data to address whether these deaths were cardiovascular-related or due to other causes. Previous studies that examined the associations between serum bicarbonate and mortality included estimated and/or measured GFR, an important confounding factor in the analysis (11,12,16). We adjusted for eGFR using the CKD-EPI equation and found similar positive associations between low and high serum bicarbonate levels and mortality among CKD patients.
Diabetic patients had higher odds of having lower bicarbonate levels than nondiabetics. However, the associations of low serum bicarbonate levels with mortality were of significance only in nondiabetics. This finding, again, may be attributed to the higher cardiovascular risk profile of diabetic patients that of nondiabetics with CKD and may outweigh the effects of low bicarbonate levels. The relationship between low bicarbonate and mortality seems to be compounded by the presence of malignancy. Exact mechanisms that may explain these findings are unclear but could be attributed to the hypoxia that may worsen acidosis in cancerous states and to the higher use of antineoplastic agents in sicker populations (23). Although these subgroup analyses should be interpreted with caution, these results may highlight subgroups most at risk for mortality associated with low bicarbonate levels, and these also serve as baseline estimates for designing future interventional studies on this topic.
We explored the associations between low and high serum bicarbonate and mortality separately as the patient characteristics that predispose to these conditions differ. Our study showed an increased risk for death with high bicarbonate levels irrespective of the level of kidney function. This augmented death risk might be a reflection of the presence of higher comorbid conditions (especially heart failure and COPD) in this population (Table 2), or due to the harmful effects of metabolic alkalosis per se on the myocardium (mediated through electrolyte disturbances), skeletal muscle, and the central nervous system (9). Experimental evidence suggests that lower GFR might maintain an alkalotic state, thereby conferring a persistent risk for these patients (24).
Our study is not without limitations. Apart from being a single-center study, this analysis was limited to patients with eGFR <60 ml/min per 1.73 m2, and these findings may not be applicable to patients with less severe forms of kidney disease (eGFR ≥60 ml/min per 1.73 m2 with albuminuria and/or other structural abnormalities). Even although we were able to adjust for several relevant covariates in the analyses, we did not have complete details about use of sodium bicarbonate, data relating to albuminuria, and mineral and bone disorder parameters to be adjusted for in the analyses. Furthermore, we lacked measured GFR data. Despite the strong associations between mortality and low and high bicarbonate levels, as well as J-shaped association in this cohort of CKD patients, we cannot infer causality based on the observational data.
In summary, low serum bicarbonate levels are associated with death among stage 3 CKD, while high serum bicarbonate levels are associated with death among both stage 3 and stage 4 CKD patients. Further studies should examine the mechanisms that may underlie such associations and differential causes of death in these patients. Importantly, future interventional studies may explore whether bicarbonate supplementation would decrease mortality in high-risk patients, such as those with low serum bicarbonate levels and higher renal function, and among the nondiabetic population.
Disclosures
This publication was made possible by Grant Number RR024990 (to S.D.N.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. S.E.J. is supported by NIH Diversity Supplement 3U01HL064244–10S1. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview. The authors have no relevant financial interest in the study. The creation of the CCF CKD registry was funded by an unrestricted grant from Amgen, Inc., to the Department of Nephrology and Hypertension Research and Education Fund.
Supplementary Material
Acknowledgments
Part of the work in this manuscript was presented at the World Congress of Nephrology in Vancouver, Canada, on April 10, 2011. The authors wish to thank Welf Saupe, Donna Rumley, and John Sharp of Cleveland Clinic, who aided in data extraction during the development of the registry.
Concept and design of the study, data analysis, and interpretation of data and critical revision for intellectual content: S.D.N., J.D.S., S.A. Writing the final manuscript and final approval of version to be published: S.D.N., J.D.S., S.A., E.W., R.R., S.E.J., A.J., J.F.S., T.R.S., M.J.S., J.V.N.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate online is available for additional clinical information at www.cjasn.org.
References
- 1. Kraut JA, Kurtz I: Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am J Kidney Dis 45: 978–993, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M'rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B: Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, Stitchantrakul W, Leeprasert V, Ongphiphadhanakul B, Radinahamed P, Rajatanavin R: Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int 62: 2160–2166, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE: The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97: 1447–1453, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitch WE, Price SR: Mechanisms activated by kidney disease and the loss of muscle mass. Am J Kidney Dis 38: 1337–1342, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ayus JC, Krothapalli RK: Effect of bicarbonate administration on cardiac function. Am J Med 87: 5–6, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Mak RH: Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int 54: 603–607, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls JL: Nutrition in CAPD: Serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 61: 1286–1292, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Khanna A, Kurtzman NA: Metabolic alkalosis. Respir Care 46: 354–365, 2001 [PubMed] [Google Scholar]
- 10. Laski ME, Sabatini S: Metabolic alkalosis, bedside and bench. Semin Nephrol 26: 422–433, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navaneethan SD, Jolly SE, Schold JD, Arrigain S, Saupe W, Sharp J, Lyons J, Simon JF, Schreiber MJ, Jr, Jain A, Nally JV, Jr: Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol 6: 40–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 16. Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ: Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56: 907–914, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yaqoob MM: Acidosis and progression of chronic kidney disease. Curr Opin Nephrol Hypertens 19: 489–492, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Shlipak MG, Katz R, Kestenbaum BF, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiche JF, Brahimi-Horn MF, Pouyssegur J: Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J Cell Mol Med 14: 771–794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cogan MG, Liu FY: Metabolic alkalosis in the rat: Evidence that reduced glomerular filtration rather than enhanced tubular bicarbonate reabsorption is responsible for maintaining the alkalotic state. J Clin Invest 71: 1141–1160, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.