Abstract
Summary
Background and objectives
Autosomal dominant polycystic kidney disease (ADPKD), a frequent cause of end-stage renal disease, has no cure. V2-specific vasopressin receptor antagonists delay disease progression in animal models.
Design, setting, participants, and measurements
This is a prospectively designed analysis of annual total kidney volume (TKV) and thrice annual estimated GFR (eGFR) measurements, from two 3-year studies of tolvaptan in 63 ADPKD subjects randomly matched 1:2 to historical controls by gender, hypertension, age, and baseline TKV or eGFR. Prespecified end points were group differences in log-TKV (primary) and eGFR (secondary) slopes for month 36 completers, using linear mixed model (LMM) analysis. Sensitivity analyses of primary and secondary end points included LMM using all subject data and mixed model repeated measures (MMRM) of change from baseline at each year. Pearson correlation tested the association between log-TKV and eGFR changes.
Results
Fifty-one subjects (81%) completed 3 years of tolvaptan therapy; all experienced adverse events (AEs), with AEs accounting for six of 12 withdrawals. Baseline TKV (controls 1422, tolvaptan 1635 ml) and eGFR (both 62 ml/min per 1.73 m2) were similar. Control TKV increased 5.8% versus 1.7%/yr for tolvaptan (P < 0.001, estimated ratio of geometric mean 0.96 [95% confidence interval 0.95 to 0.97]). Corresponding annualized eGFR declined: −2.1 versus −0.71 ml/min per 1.73 m2/yr (P = 0.01, LMM group difference 1.1 ml/min per 1.73 m2/yr [95% confidence interval 0.24 to 1.9]). Sensitivity analyses including withdrawn subjects were similar, whereas MMRM analyses were significant at each year for TKV and nonsignificant for eGFR. Increasing TKV correlated with decreasing eGFR (r = −0.21, P < 0.01).
Conclusion
ADPKD cyst growth progresses more slowly with tolvaptan than in historical controls, but AEs are common.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder which, over decades, results in progressive development of multiple renal cysts, urinary concentration defects, hypertension, and ultimately ESRD (1–4). Kidney and back pain from cyst hemorrhage, stones, infection, biomechanical stresses, stretching of the renal capsule, or pressure on other organs can impact quality of life (5). Less common extrarenal manifestations such as cerebral aneurysms may be life-threatening.
Studies in animal models have implicated arginine vasopressin and its second messenger cAMP as important promoters of cyst cell proliferation and fluid secretion into cysts (6). Suppression of vasopressin release by forced hydration, genetic crosses between cyst-prone animals and those lacking vasopressin, and vasopressin V2 receptor blockade consistently reduce cyst burden and protect renal function (6). These compelling preclinical studies provided a rationale for vasopressin V2 receptor antagonism as a preventive therapy for human ADPKD.
Total kidney volume (TKV) is a practical, intermediate end point of later outcomes in ADPKD including pain, hypertension, renal insufficiency, and ESRD. The Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) and other studies are establishing the relationship between TKV growth and important clinical outcomes (7–9). However, it will take years of study to prove whether a treatment that slows TKV expansion will positively affect estimated GFR (eGFR), ESRD, or death. The current study explores the potential use of TKV as a surrogate for ADPKD therapies targeting vasopressin V2 signaling.
Materials and Methods
Consenting ADPKD subjects of prior trials were enrolled in two multicenter open-label tolvaptan-treatment studies, in North America (156-04-250 TEMPO42, ClinicalTrials.gov; NCT00413777) and Japan (156-05-002, NCT00841568). Protocols and informed consents for both studies were approved by local ethics committees as outlined by the Declaration of Helsinki. De-identified data for matched controls were provided, after ethics committee approval, by the National Institutes of Health-sponsored Modification of Diet in Renal Disease (MDRD) and CRISP (NCT01039987) studies (10).
Eligibility for the TEMPO42 study required the following: men or women age >18 years fulfilling Ravine's diagnostic criteria (11), prior participation in a phase 1 tolvaptan ADPKD trial, and willingness to adhere to contraceptive precautions. Exclusion criteria included the following: inability to comply with study procedures, eGFR <30 ml/min per 1.73 m2, anticipation of renal replacement therapy within 1 year, and active treatment that would affect end point measures (e.g., diuretic administration). The 156-05-002 trial inclusions were similar except for age >20 years without an upper limit. This study excluded subjects with serum creatinine ≥2.5 mg/dl, uncontrolled hypertension, systolic BP <90 mmHg, serious cardiac or hepatic disease, or a history of significant bleeding or bleeding tendency.
The primary objective of TEMPO42 and 156-05-002 studies was to confirm the long-term safety and tolerability of tolvaptan. Subject safety was assessed by regular monitoring of adverse events (AEs), directed physical examinations, vital signs, laboratory, and electrocardiogram measurements. The secondary objective of these trials was to acquire pilot efficacy data. Efficacy was assessed by changes in urine osmolality (Uosm), TKV, eGFR, and hypertension status. Pharmacokinetic/pharmacodynamic analyses were also performed. This report focuses on comparisons of tolvaptan to historical matched-control trajectories of TKV and eGFR. These slope comparisons were analyzed as a linear mixed model of annualized change in log-transformed TKV or eGFR over 3 years. Assessing slope reduces the variability associated with eGFR and facilitates projection over time (12).
Control data were gathered from participants in the CRISP and MDRD studies (7,10,13). CRISP included 241 ADPKD subjects, 15 to 46 years old with eGFR >70 ml/min by Cockcroft-Gault at entry. MDRD included 200 ADPKD subjects, 18 to 70 years old, segregated by iothalamate GFR (study A 25 to 55 or study B 13 to 24 ml/min per 1.73 m2) and randomized to low or usual BP targets and a usual or low-protein diet. All matched controls for eGFR were from study A, an observational cohort equivalent because interventions did not influence the rate of renal function decline.
Longitudinal TKV were available only in the CRISP study. MDRD subjects were needed to match the lower GFR values in some subjects in the tolvaptan group. Therefore for matched control pairing, the pool of matches was restricted to CRISP subjects for TKV, but included both CRISP and MDRD subjects for eGFR. Sets of potential controls were first identified by matching gender and hypertensive status for each tolvaptan-treated subject. Then tolvaptan-treated subjects were ordered randomly, and in this order they were matched to subjects in their set who had the smallest sum of percent absolute differences in age and baseline value of the parameter of interest (TKV divided by height or eGFR using ethnicity-adjusted CKD-EPI equation) from their potential matches (14,15). Once identified, each control subject was used only once. Matching proceeded in a randomly selected order for tolvaptan-treated subjects. The first control subject was matched to the first tolvaptan-treated subject, and then the second control was matched to the second tolvaptan-treated subject, and so on until all had one match. The process then proceeded in reverse, selecting a second control subject for the tolvaptan-treated subject who was last matched, progressing to the first tolvaptan-treated subject in this order until all had two matches.
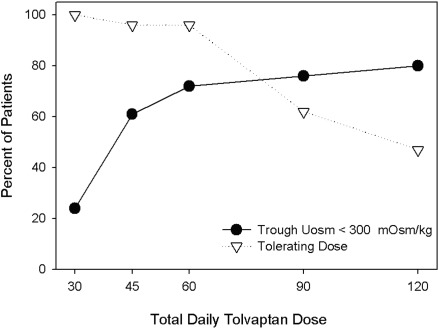
In the initial 2 months of the TEMPO42 study, a split-dose regimen of oral tolvaptan (8 a.m./4 p.m.) was up-titrated (15/15, 30/15, 45/15, 60/30, 90/30 mg/d) until tolerability was reached. Subjects were then randomized to one of two doses (45/15 and 60/30 mg) chosen after analysis of efficacy (Uosm <300 mOsm/Kg in 70% and 77% of patients, respectively) and self-reported tolerability (tolerable for the rest of life in 96% and 61% of patients, respectively; Figure 1). After the titration period, those on the higher dose were allowed to down-titrate as needed. A 15/15-mg/d split dose regimen was used in study 156-05-002.
Figure 1.
Tolerability and efficacy during titration phase. In the initial 2 months of the TEMPO42 study a split-dose regimen of oral tolvaptan (8 a.m./4 p.m.) was up titrated (15/15, 30/15, 45/15, 60/30, 90/30 mg/d) until tolerability was reached. Tolerability was defined as self-reported tolerance of a specific dose regimen by responding yes to the question: “Could you tolerate taking this dose of tolvaptan for the rest of your life?” Efficacy was defined by the capacity to suppress the action of vasopressin on the kidney reflected by sustained urine hypotonicity (Uosm <300 mOsm/kg).
Tolvaptan subjects were evaluated at predose baseline, weekly during titration, at 2, 6, 9, and 12 months, and every 4 months thereafter for 36 months in TEMPO42 and at predose baseline, 1, 2, and 4 weeks, and every 4 weeks thereafter for 36 months in study 156-05-002. Serum creatinine data for CRISP and/or MDRD were available at screening, baseline, at 2, 4, 8, and 12 months, and every 4 months thereafter and were available annually for CRISP. Additional values were available between 36 and 42 months for some subjects. TEMPO42 and 156-05-002 study measurements of serum creatinine tests were performed centrally at Quest Laboratories and SRL Inc., respectively. Serum creatinine measurements in 156-05-002 used an enzymatic assay, whereas TEMPO42, CRISP, and MDRD used kinetic alkaline picrate assays.
TKV in CRISP, TEMPO42, and 156-05-002 studies was determined by magnetic resonance imaging at baseline and at months 12, 24, and 36 using comparable methods. Computed tomography was used in one 156-05-002 subject. TKV was also measured at month 2 for TEMPO42 and at month 6 for 156-05-002. The TEMPO42 magnetic resonance imaging acquisition protocol included T2-weighted single-shot fast spin-echo images with fat saturation and three-dimensional spoiled gradient interpolated T1-weighted images without fat saturation. T2- and T1-weighted images were similar for the 156-05-002 study. Gadolinium enhancement used at baseline and some 1-year visits in TEMPO42 were abandoned due to safety concerns. Gadolinium-enhanced images were not used for measuring TKV. Coronal 3- to 5-mm (TEMPO42) and 5- to 7-mm (156-05-002)-thick slices covering the entire kidneys were acquired during a breath-hold and sent to central reading facilities (Perceptive Informatics, Inc., Billerica, MA, or Biovisiq Japan, Inc., Osaka, Japan) for quality control and TKV measurement using Alic software (Perceptive Informatics, Inc.) or Virtual Place Advance version 2.01 (AZE Ltd., Tokyo, Japan). Serial kidney outlines were verified by independent radiologists familiar with ADPKD, blinded to patient name and acquisition sequence.
The statistical analysis plan prespecified a primary end point of percent rate of change in TKV over 3 years in tolvaptan-treated subjects and matched controls (1:2) receiving standard care. Secondary end points included rate of change in eGFR, by the four-variable MDRD equations. The formula was adjusted for Japanese ethnicity for the 156-05-002 subjects using a coefficient of 0.808 (15). Summary statistics of baseline characteristics were evaluated to ensure adequate balance between cohorts.
For the primary end point, comparison of tolvaptan-treated month 36 completers with their TKV control matches was performed using a linear mixed model on the log-transformed TKV slope analysis procedure with group and group time interaction as fixed effect, baseline as fixed covariate, intercept and time as both fixed and random effect, and unknown variance-covariance structure for the random effects. Time is a continuous variable. Sandwich estimate of the variance-covariance matrix was used to test the group time interaction. This analysis was based on all available data. For the secondary analysis, all available data for tolvaptan-treated completers were compared with eGFR control matches using a linear mixed model on eGFR, using a similar slope analysis procedure to the primary analysis, except that eGFR was not log-transformed. A sensitivity analysis of primary and secondary end points included all available TKV and eGFR data from TEMPO42 and 156-05-002 noncompleters to assess impact of withdrawn subjects.
In addition to the primary and sensitivity analyses described above, mixed model repeated measures (MMRM) sensitivity analyses were applied to change from baseline for eGFR and percent changes from baseline in TKV. Least-squares mean differences of the two treatment groups at each yearly visit under the MMRM were used to estimate group differences at years 1, 2, and 3. The MMRM included group, visit, and group visit interaction as class variables and baseline TKV or eGFR as covariates. MMRM analysis used all available data at baseline and years 1, 2, and 3.
Correlation of annualized percent change in TKV with annualized change in eGFR was assessed using available data for the tolvaptan completers and TKV-matched CRISP control subjects. This comparison was a prospective objective of the protocol; however, specific use of Pearson correlation was decided upon post hoc. Data analysis was performed by one of the authors (J.O.) according to protocol-specified statistical analysis plan prepared in collaboration with the authors. The sponsor holds the data, which are freely available.
Results
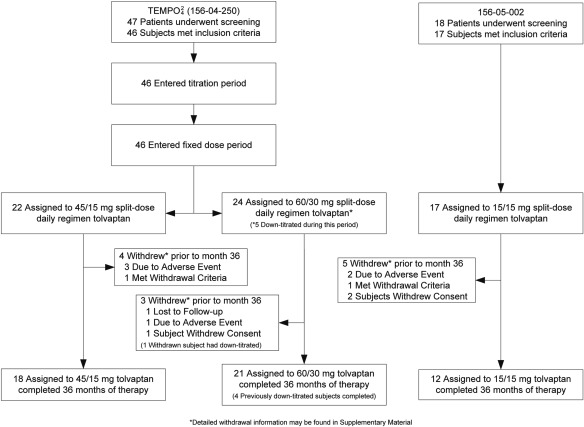
Subject disposition is described in Figure 2. Overall compliance was good for those completing 36 months' treatment. For the TEMPO42 study the average dose reported after 36 months as taken in the 18 subjects assigned 45/15 (i.e., 60 mg/d) group was 59.2 mg/d; for the 21 subjects in the 60/30 (i.e., 90 mg/d) group, including five subjects who had down-titrated (thus an expected exposure of 82.9 mg/d after adjustment after down-titration to 45/15), was 80.7 mg/d. Compliance in all but one subject in the 45/15 mg/d group was confirmed by measurements of tolvaptan metabolite levels. Eighty percent of completers for the 156-05-002 study were more than 90% compliant by pill count, whereas the remaining two maintained more than 50% compliance.
Figure 2.
Subject disposition in TEMPO42 and 156-05-002 trials. Of 48 eligible subjects for the TEMPO42 study, 47 entered screening and 46 participated; seven subjects withdrew early (including one of six in the high-dose group who permanently down-titrated), leaving 39 who completed 3 years' therapy. Exposure verified by tolvaptan metabolite levels confirmed compliance in all but one subject in the 45/15-mg/d group. Of 18 eligible subjects for the 156-05-002 study, 17 participated, five withdrew, and 12 completed 3 years' therapy. Subjects completing 36 months were used in the primary analysis; analyses including data from withdrawn subjects who received at least one dose of study drug and who had posttreatment data were also performed.
The observed AEs were consistent with the mechanism of action of tolvaptan and the natural history of ADPKD. Most were mild or moderate in severity. Table 1 lists very common AEs (reported in >10% of patients; Supplemental Table 1 lists all AEs). Small mean increases from baseline were seen for serum creatinine and uric acid, starting at the earliest time points. No clinically meaningful trends were seen for any hematology, urinalysis, or ECG parameter.
Table 1.
Very common (>10%) adverse events reported with tolvaptan in North American and Japanese subjects
| MedDRA Adverse Event Term | Number of Subjects Reporting AEsa; 156-05-002bor TEMPO42c |
||
|---|---|---|---|
| 15/15 mg/d (n = 17) | 45/15 mg/d (n = 22) | 90/30 mg/d (n = 24) | |
| Nasopharyngitis | 13 (76) | 2 (9) | 1 (4) |
| Thirst | 9 (53) | 8 (36) | 16 (67) |
| Pollakiuria | 2 (12) | 10 (46) | 14 (58) |
| Renal pain | 0 (0) | 10 (46) | 9 (38) |
| Nocturia | 2 (12) | 9 (41) | 3 (13) |
| Upper respiratory tract infection | 0 (0) | 1 (5) | 9 (38) |
| Polyuria | 1 (6) | 8 (36) | 6 (25) |
| Dizziness | 2 (12) | 8 (36) | 4 (17) |
| Contusion | 6 (35) | 1 (5) | 0 (0) |
| Fatigue | 1 (6) | 6 (27) | 8 (33) |
| Hypertension | 5 (29) | 4 (18) | 4 (17) |
| Abdominal pain | 1 (6) | 3 (14) | 7 (29) |
| Diarrhea | 2 (12) | 2 (9) | 7 (29) |
| Blood antidiuretic hormone increase | 5 (29) | N/Ad | N/Ad |
| Urinary tract infection | 1 (6) | 3 (14) | 6 (25) |
| Sinusitis | 2 (12) | 3 (14) | 6 (25) |
| Headache | 4 (24) | 3 (14) | 6 (25) |
| Blood uric acid increased | 4 (24) | 0 (0) | 0 (0) |
| Anemia | 1 (6) | 5 (23) | 1 (4) |
| Back pain | 3 (18) | 5 (23) | 3 (13) |
| Dry skin | 2 (12) | 5 (23) | 2 (8) |
| Dyspnea | 0 (0) | 1 (5) | 5 (21) |
| Constipation | 1 (6) | 0 (0) | 5 (21) |
| Dehydration | 3 (18) | 0 (0) | 2 (8) |
| Gastritis | 3 (18) | 0 (0) | 0 (0) |
| Palpitations | 3 (18) | 3 (14) | 1 (4) |
| Gastritis erosive | 3 (18) | 0 (0) | 0 (0) |
| Vertigo | 3 (18) | 0 (0) | 0 (0) |
| Dental caries | 3 (18) | 0 (0) | 0 (0) |
| Bronchitis | 1 (6) | 4 (18) | 2 (8) |
| Abdominal distension | 1 (6) | 3 (14) | 4 (17) |
| Nausea | 1 (6) | 1 (5) | 4 (17) |
| Cough | 0 (0) | 0 (0) | 4 (17) |
| Edema, peripheral | 1 (6) | 3 (14) | 3 (13) |
| Hypotension | 0 (0) | 3 (14) | 3 (13) |
| Arthralgia | 1 (6) | 3 (14) | 3 (13) |
| Chest pain | 1 (6) | 3 (14) | 2 (8) |
| Dry eye | 0 (0) | 3 (14) | 2 (8) |
| Polydipsia | 0 (0) | 3 (14) | 1 (4) |
| Erythema | 0 (0) | 3 (14) | 0 (0) |
| Weight increase | 0 (0) | 3 (14) | 0 (0) |
| Blood creatinine increase | 2 (12) | 1 (5) | 3 (13) |
| Viral upper respiratory tract infection | 0 (0) | 1 (5) | 3 (13) |
| Dyspepsia | 0 (0) | 1 (5) | 3 (13) |
| Dry mouth | 0 (0) | 0 (0) | 3 (13) |
| Vomiting | 0 (0) | 1 (5) | 3 (13) |
| Insomnia | 1 (6) | 1 (5) | 3 (13) |
| Rash | 0 (0) | 0 (0) | 3 (13) |
| Muscle spasms | 0 (0) | 0 (0) | 3 (13) |
| Anxiety | 0 (0) | 0 (0) | 3 (13) |
| Pain in extremity | 2 (12) | 2 (9) | 0 (0) |
| Blood triglyceride increase | 2 (12) | 0 (0) | 2 (8) |
| Hyperuricemia | 2 (12) | 0 (0) | 2 (8) |
| Arthropod sting | 2 (12) | 1 (5) | 1 (4) |
| Tinea pedis | 2 (12) | 1 (5) | 0 (0) |
| Musculoskeletal pain | 2 (12) | 0 (0) | 1 (4) |
| Neck pain | 2 (12) | 0 (0) | 1 (4) |
| Keratitis | 2 (12) | 0 (0) | 0 (0) |
| Gastric polyps | 2 (12) | 0 (0) | 0 (0) |
| Malaise | 2 (12) | 0 (0) | 0 (0) |
| Muscle injury | 2 (12) | 0 (0) | 0 (0) |
| Alanine aminotransferase increased | 2 (12) | 0 (0) | 0 (0) |
| Blood cholesterol increase | 2 (12) | 0 (0) | 0 (0) |
| Blood glucose increase | 2 (12) | 0 (0) | 0 (0) |
| Hemoglobin decrease | 2 (12) | 0 (0) | 0 (0) |
| Blood phosphorus increase | 2 (12) | 0 (0) | 0 (0) |
| Spinal; osteoarthritis | 2 (12) | 0 (0) | 0 (0) |
| Intervertebral disc protrusion | 2 (12) | 0 (0) | 0 (0) |
| Intracranial aneurysm | 2 (12) | 0 (0) | 0 (0) |
| Eczema | 2 (12) | 0 (0) | 0 (0) |
| Pruritus | 2 (12) | 0 (0) | 0 (0) |
| Upper respiratory tract inflammation | 2 (12) | 0 (0) | 0 (0) |
An adverse event (AE) is a new or worsening untoward symptom or sign occurring after receiving at least one dose of tolvaptan. Medical Dictionary for Regulatory Activities.
Values in parentheses are percentage.
Subjects receiving at least one dose from Japanese 156-05-002 (n = 17) studies.
Subjects receiving at least one dose from U.S. 156-04-250 TEMPO42 (n = 46) studies who were randomized to separate dose groups.
Not applicable (n/A) because antidiuretic hormone (vasopressin) was not assessed in TEMPO.
Twelve (19%) patients withdrew from the study. Reasons for withdrawal are shown in Supplemental Table 2. AEs accounted for six (50%) of the withdrawals, including renal impairment, acute renal failure, benign pituitary tumor, transient ischemic attack, eye swelling, and subarachnoid hemorrhage with a fatal outcome.
Urine osmolality below 300 mOsm/kg·H2O was used in the TEMPO42 and 156-05-002 study as a target for tolvaptan inhibition of vasopressin activity. The TEMPO42 and 156-05-002 mean (median) Uosm premorning dose was 264 (228) and 343 (310) mOsm/kg per H2O. This was below mean (median) baseline levels of 472 (409) and 478 (461) mOsm/kg per H2O.
Tolvaptan subject cohorts were closely matched to controls for gender and hypertension status, age, height, race, and baseline parameter of interest. Mean TKV was 13% lower, and age was 5 years younger in the TKV controls (Table 2). Forty-five percent and 37% of tolvaptan-treated patients and 47% and 23% of CRISP matches were treated with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Forty-two percent of subjects in the MDRD study were treated with angiotensin-converting enzyme inhibitors (16).
Table 2.
Baseline demographic profile of North American and Japanese completing subjects and their matched controls
| Parameter | Tolvaptan (n = 51)a | TKV Control (n = 102)b | eGFR Control (n = 102)c |
|---|---|---|---|
| Maled | 17 (33.3) | 34 (33.3) | 34 (33.3) |
| Femaled | 34 (66.7) | 68 (66.7) | 68 (66.7) |
| Age (years)e | 42 (8.8) | 37 (6.5) | 42 (9.4) |
| Raced | |||
| Caucasian | 38 (74.5) | 89 (87.3) | 90 (88.2) |
| Black | 1 (2.0) | 9 (8.8) | 6 (5.9) |
| Asian | 12 (23.5) | 1 (1.0) | 1 (1.0) |
| Other | 0 (0) | 3 (2.9) | 5 (4.9) |
| Hypertensiond | 43 (84.3) | 86 (84.3) | 86 (84.3) |
| Height (cm)e | 170 (9.8) | 172 (11) | 171 (10.1) |
| Weight (kg)e | 77 (21.1) | 78 (18.2) | 78 (16.3) |
| TKV (ml)e | 1635 (978) | 1422 (725) | — |
| eGFR (ml/min per 1.73 m2)f | 62 (20.1) | 77 (18.4) | 62 (19.1) |
TKV, total kidney volume; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease Study; CRISP, Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease; —, not available.
Tolvaptan subjects for TKV and eGFR derived from those completing 3 years' treatment in the U.S. 156-04-250 TEMPO42 (n = 39) and 156-05-002 (n = 12) studies.
Control subjects for TKV derived from CRISP cohort.
Control subjects for eGFR derived from CRISP (n = 66) and MDRD (n = 36) cohorts.
Values are number of subjects with percentage in parentheses.
Values are mean with SD in parentheses.
eGFR by MDRD = 186 × (SCr) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.21 if African American or 0.808 if Japanese).
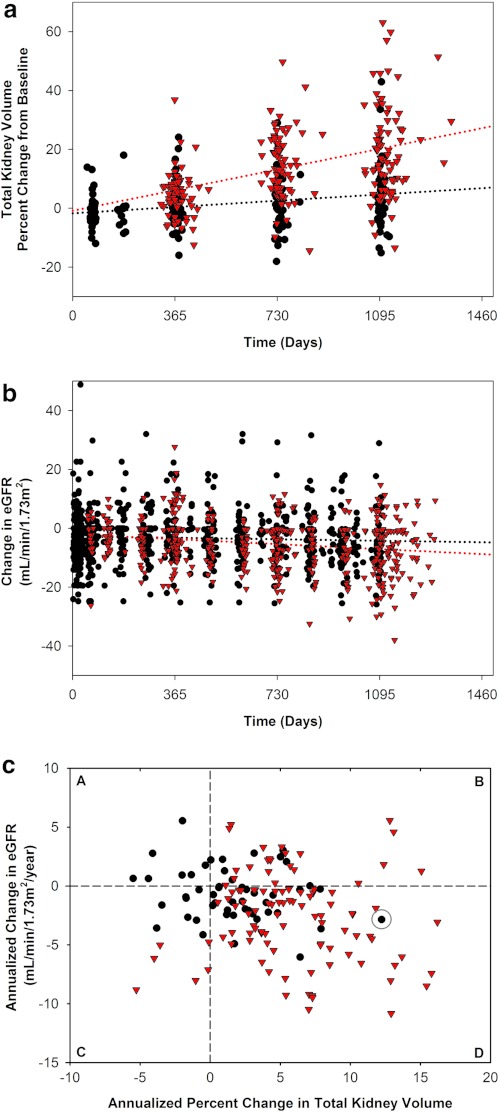
TKV growth in tolvaptan-treated subjects was 1.7%/yr compared with 5.8%/yr for control CRISP subjects (P < 0.001, ratio of geometric mean [RGM] 0.96, 95% confidence interval 0.95 to 0.97) (Table 3, Figure 3a). This represents a 70% slower growth rate per year in tolvaptan-treated patients. A similar result was obtained when all completers and withdrawn subjects were included in the analysis: 1.7%/yr and 5.8%/yr, respectively (P < 0.001, RGM 0.96, 95% confidence interval 0.95 to 0.97) (Supplemental Table 3). The corresponding slopes of eGFR were −0.71 in tolvaptan-treated patients and −2.1 ml/min per 1.73 m2/yr in CRISP (n = 66) and MDRD (n = 36) control subjects (P = 0.01, linear mixed model [LMM] group difference 1.1, 95% confidence interval 0.24 to 1.9) for a group difference of approximately 65% (Table 3, Figure 3b). Results were similar, but less marked (15% effect size), for the all completers and withdrawn group comparison; −1.7 versus −2.0 ml/min per 1.73 m2/yr (P = 0.02, LMM group difference 0.95, 95% confidence interval 0.13 to 1.8) (Supplemental Table 3).
Table 3.
Annualized progression rate of TKV and eGFR in completing subjects projected over 3 years
| Group | N | Annualized TKV Growth Ratea |
P-Value | |
|---|---|---|---|---|
| Annual Change (%/yr)b | RGMc | |||
| TKV control | 102 | 5.8 (4.3) | 0.96 (0.95 to 0.97) | <0.01 |
| Tolvaptan | 51 | 1.7 (3.5) | ||
| Group | N | Annualized eGFR Sloped |
P-Value | |
|---|---|---|---|---|
| Annual Change (ml/min per 1.73 m2 per year)b | LS Mean Difference (ml/min per 1.73 m2 per year)c | |||
| eGFR control | 102 | −2.1 (3.1) | 1.1 (0.24 to 1.92) | =0.01 |
| Tolvaptan | 51 | −0.7 (2.2) | ||
TKV, total kidney volume; eGFR, estimated GFR; N, number of subjects; RGM, ratio of geometric means; LS, least significant, MDRD, Modification of Diet in Renal Disease.
Summary statistics were derived by regressing logarithm-transformed TKV against time, and then exponential the regression slopes and P-value of ratio of geometric means were derived from testing the time-treatment interaction using linear mixed model in which both intercept and slope are fixed and random effects. The ratio of geometric means is an estimate of the ratio of the geometric mean of annualized growth rate of tolvaptan and control.
Values are mean with SD in parentheses.
Values in parentheses are 95% confidence interval.
Summary statistics were derived by slope of change by regressing eGFR data against time by subject. LS means difference derived from testing the time-treatment interaction using a linear mixed model in which both intercept and slope are fixed and random effects. eGFR by MDRD = 186 × (SCr) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.21 if African American or 0.808 if Japanese).
Figure 3.
Change in total kidney volume and renal function over 3 years of tolvaptan compared with matched control and their correlation for individual subjects. The primary analysis for slope of total kidney volume (TKV) and estimated GFR (eGFR) use each parameter's annualized change and a linear mixed model to test for group differences. Because these data are not easily displayed, subjects completing 3 years of tolvaptan (black circles) and their controls (red triangles) are displayed for TKV (a) and eGFR (b). Control subjects were matched 1:2 by gender, presence of hypertension, age, and either baseline TKV (a) or baseline eGFR (b). Values for change from baseline (as percent TKV or ml/min per 1.73 m2 per year) are plotted, and dotted lines representing a linear regression of group trend were drawn. (c) These subjects' annualized change in eGFR (by MDRD formula) and the annualized percent change in TKV were plotted, and the entire data set's correlation (r = −0.21, P < 0.01) was evaluated. Dashed lines marking a zero change in TKV and eGFR parameters are drawn, creating quadrants representing improvement in both parameters (A), improvement in eGFR but worsened TKV (B), worsened eGFR but improved TKV (C), and worsening in both parameters (D) are shown. The data point circled represents the subject in whom compliance with tolvaptan was not supported by tolvaptan metabolite data.
The effect of tolvaptan on kidney growth was confirmed by the MMRM sensitivity analysis. At each visit, average TKV trended upward or downward as expected (Table 4). Over 3 years, mean TKV increased by 98 ml (5.3%, from 1635 to 1734 ml) in the all completers tolvaptan-treated group, compared with 300 ml (19%, from 1422 to 1722 ml) in the matched control group. These TKV changes were all significantly different between the groups at each yearly assessment. In contrast, eGFR declined from a mean of 61.6 to 55.9 for all completers treated subjects and from 61.9 to 55.4 ml/min per 1.73 m2 for matched controls. Although the difference between groups in the mean change at year 3 in eGFR was only 1.1 ml/min per 1.73 m2, the maximum difference was 3.1 ml/min per 1.73 m2 at year 2, reflecting a high degree of variability of average eGFR over time and yielding nonsignificant differences between the groups at 1, 2, or 3 years. Results were similar when using data from all subjects (Supplemental Table 4).
Table 4.
Change in mean TKV and eGFR in all completing subjects by visit
| Control |
Tolvaptan |
Group Difference; P-valuea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Mean % Change | SD | N | Mean | SD | Mean % Change | SD | ||
| TKV (ml) | |||||||||||
| Baseline | 102 | 1422 | 724.8 | 51 | 1635 | 977.5 | |||||
| Month 12 | 100 | 1515 | 776.3 | 4.8 | 7.0 | 51 | 1644 | 983.7 | 0.8 | 7.3 | −4.41 (−0.71 to −8.11); P = 0.02 |
| Month 24 | 95 | 1660 | 896.7 | 13.8 | 11.0 | 51 | 1670 | 1015 | 1.6 | 8.5 | −12.5 (−8.79 to −16.2); P < 0.0001 |
| Month 36 | 102 | 1722 | 945.5 | 19.0 | 15.9 | 51 | 1733 | 1052 | 5.3 | 11.0 | −14.3 (−10.6 to −18.0); P < 0.0001 |
| N | Mean | SD | Mean Change | SD | N | Mean | SD | Mean Change | SD | Group Difference; P-Valuea | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR ml/min per 1.73 m2b | |||||||||||
| Baseline | 102 | 61.9 | 19.1 | 51 | 61.6 | 20.1 | |||||
| Month 12 | 100 | 59.4 | 22.1 | −2.6 | 8.5 | 49 | 60.0 | 22.2 | −1.5 | 12.8 | 1.0 (−2.3 to 4.3); P = 0.54 |
| Month 24 | 100 | 56.2 | 22.1 | −5.6 | 8.4 | 50 | 59.3 | 21.5 | −2.6 | 8.8 | 2.9 (−0.4 to 6.1); P = 0.09 |
| Month 36 | 101 | 55.4 | 22.3 | −6.8 | 10.5 | 51 | 55.9 | 21.1 | −5.7 | 9.2 | 1.1 (−2.2 to 4.4); P = 0.50 |
TKV, total kidney volume; eGFR, estimated GFR; MDRD, Modification of Diet in Renal Disease.
Values in parentheses are 95% confidence interval.
eGFR by MDRD = 186 × (SCr) − 1.154 × (age) − 0.203 × (0.742 if female) × (1.21 if African American or 0.808 if Japanese).
The slopes for TKV and eGFR were significantly and negatively correlated. Greater increases in TKV were correlated with greater declines in eGFR, with lesser changes for both occurring in the tolvaptan-treated patients (r = −0.21, P < 0.01) (Figure 3c).
Separate analyses of the TEMPO42 North American (Supplemental Tables 5 and 6) and 156-05-002 Japanese completers yielded similar results for trends in TKV (both groups showing significance; LMM group difference 0.97, 95% CI 0.95 to 0.98, P < 0.001, for TEMPO42; 0.935, 95% CI 0.92 to 0.95, P < 0.001, for 156-05-002) and eGFR (significant only for the larger TEMPO42 cohort).
Only eight subjects were not receiving antihypertensive therapy at baseline. Three became hypertensive during the 3 years of treatment. The small number of subjects in the nonhypertensive category at baseline prevented useful analyses of effects on hypertension progression.
Discussion
Cyst growth progressed more slowly in the tolvaptan-treated patients than in historical controls. Eighty-one percent of subjects completed 3 years of treatment. Although all of the participants experienced AEs and six participants withdrew from the study because of AEs, most were mild to moderate in severity. Of 39 TEMPO42 study completers, 92% were eligible and enrolled in an extension of open-label treatment.
Fifty-one subjects treated with tolvaptan for 3 years were compared with 102 untreated, matched ADPKD controls. Efficacy of tolvaptan in blocking the vasopressin V2 receptor was evident in that the group median trough Uosm before the premorning dose (the time point where the drug would have least affected urine osmolality) at the maximally tolerated dose for TEMPO42 and at 15/15 mg/d for 156-05-002 were 228 and 310 mOsm/kg per H2O, respectively; below or near serum osmolality (290 mOsm/kg per H2O). In comparison to recently published data on water-loaded ADPKD subjects, this spot-urine value reflects the maximum urine osmolality, rather than a 24-hour integration of osmolality, which would be a lower value (17).
Tolvaptan had a strong effect on TKV growth, consistent with a potent effect on cyst growth. Data from the 2-month time point in the TEMPO42 study and 6-month time point in the 156-05-002 study suggest an early decrease in TKV, likely attributable to changes in cyst fluid secretion (18). Beyond this 2- to 6-month period, TKV growth appears to resume, but at a substantially slower rate than in the untreated subjects.
The results of our analysis showed significant effect of tolvaptan on the rate (slope) of eGFR decline. MMRM analysis using data limited to annual visits, however, was not significant for change in eGFR at year 1 or year 3, with only a trend toward significance at year 2. The high physiologic variability of GFR compared with TKV and the lower sensitivity of the MMRM compared with the slope analysis may account for the different results.
The results also showed a significant negative correlation between annualized slope of TKV and slope of eGFR (r = −0.21, P < 0.01). In Figure 3c, subjects treated with tolvaptan cluster near zero change for both parameters. The only subjects without an increase in TKV or a decline in eGFR were treated with tolvaptan (quadrant A). More control subjects tended to worsen in both (TKV increase and eGFR decline) in number and extent (quadrant D). Likewise, the mean change in eGFR over 3 years of treatment with tolvaptan was marginally less than in the control even although the tolvaptan group had larger kidneys at baseline. The CRISP study suggested that both TKV and renal function worsen more quickly for those with larger kidney volumes (7). In the current study, TKV was 13% lower at baseline in the control compared with the tolvaptan group, a difference that should have favored slower growth and functional decline. On the other hand, the mean age of the control group was 5 years younger (37 versus 42 years), and younger subjects with larger kidneys grew most rapidly in the CRISP study (7). TKV growth rates in the whole CRISP cohort averaged 5.3% to 9.5% and 5.2% to 6.8% per year for <30- and ≥30-year-old subjects, respectively, more than three times that observed for the group receiving tolvaptan (1.7%/yr) (7) in this study. Thus, TKV and renal function appear to be linked, and treatment to slow the rate of TKV growth appears to be accompanied by a slower rate of decline in renal function.
This study has limitations. Twelve of 51 (approximately 25%) of the tolvaptan-treated patients were Japanese, whereas both of the matched-control patient cohorts were predominantly Caucasian (approximately 90%) with only approximately 1% Asian. It should be noted, however, that the tolvaptan effects on TKV and eGFR are also significant when matched control comparisons are restricted to the North American patients (Supplemental Tables 5 and 6). Another limitation is that controls were not studied concurrently. This is less likely to have influenced TKV comparisons (because controls derived exclusively from contemporaneous CRISP) than eGFR comparisons using controls from CRISP (65% of patients) and from the MDRD (35% of patients) conducted almost two decades earlier. A sensitivity analysis using only CRISP eGFR-matched controls for all subjects in both studies provides a poorer baseline match, but yields equally significant results for eGFR (−1.7 versus −1.0 ml/min/1.73 m2 per year [P = 0.01, LMM group difference 1.1, 95% CI 0.23 to 2.0] using only CRISP controls as compared with −1.7 versus −2.0 ml/min/1.73 m2 per year [P = 0.02, LMM group difference 0.95, 95% CI 0.13 to 1.8] using CRISP and MDRD controls). Given these limitations, the results of the current study, while promising, should be viewed cautiously.
To be clinically meaningful, the 15% to 65% or 0.3- to 1.4-ml/min per 1.73 m2 per year advantage would need to be sustained over a period of many more years. Confirming this degree of benefit in the ongoing 1445-subject, placebo-controlled ADPKD trial (NCT00428948) are needed to provide the evidence necessary to establish a causal link between this therapy's ability to slow kidney volume expansion with preservation of kidney function.
General Acknowledgments
The CRISP and MDRD studies were conducted by the CRISP and MDRD investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the CRISP and MDRD studies reported here were supplied by the NIDDK Central Repositories. The analyses presented in this publication were not prepared in collaboration with Investigators of the CRISP and MDRD studies and does not necessarily reflect the opinions or views of the CRISP and MDRD studies, the NIDDK Central Repositories, or the NIDDK. We acknowledge the contributions of the volunteer subjects who have and continue to patiently offer their time and cooperation during the several years of these studies; the study investigators, coordinators, Radiology Centers and other staff; the TEMPO scientific advisors: Akira Hishida (Hamamatsu University, Shizuoka, Japan); Fumitake Gejyo, M.D. (Nigata University, Niigata, Japan); Toshiaki Nitatori, M.D. (Kyorin University School of Medicine, Mitaka, Tokyo); the TEMPO Independent Data Monitoring Committee members: Sidney Goldstein, M.D. (Henry Ford Hospital, Detroit, MI); Benjamin Cowley, M.D. (University of Oklahoma, Oklahoma City, OK); Masafumi Fukagawa, M.D., Ph.D. (Tokai University School of Medicine, Isehara City, Japan); Roser Torra, M.D. (Fundacio Puigvert, Barcelona, Spain); Lee-Jen Wei, Ph.D. (Harvard School of Public Health, Boston, MA): the Otsuka and CRO project managers, Data Monitors, Data Managers, Programmers; Osamu Sato and Tadashi Okada (Otsuka Pharmaceutical Corporation, Tokyo, Japan); and the radiologists at Central Reading Facilities (Biovisiq, Osaka, Japan, and Perceptive, Billerica, MA).
Funding and Disclosures
The TEMPO42 trial was funded by Otsuka Pharmaceutical Development & Commercialization, Inc; the 002 trial was funded by Otsuka Pharmaceutical, Ltd.; the CRISP and MDRD trials were funded by the U.S. National Institutes of Health; Dr. Higashihara reports having served as a consultant to Otsuka. Dr. Torres reports having served as a consultant to Hoffman La-Roche, Primrose, and Amgen, receiving grants/research support from Otsuka and Novartis. Dr. Chapman reports having served as a consultant to Otsuka and Novartis. Dr. Grantham reports having served as a consultant to Otsuka and receiving grants/research support from Otsuka. Dr. Bae reports having served as a consultant to Otsuka. Dr. Watnik reports serving as an investigator for the Otsuka Tolvaptan Program, holding a patent in area of ADPKD genetics, licensed to Athena Laboratories through Johns Hopkins University (JHU) for which her and her spouse's royalties are managed by JHU and donated to the Polycystic Kidney Disease Research Foundation. Drs. Horie and Nutahara have served as consultants and investigators for Otsuka. Drs. Ouyang and Czerwiec and Ms. Krasa are employees of Otsuka. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Appendix
156-04-250 TEMPO42 Principle Investigators (PI), Subinvestigators (SI), and Radiologists (R): United States
William Bennett (PI), Michael Walczyk (SI), Northwest Renal Clinic, Inc., Portland, OR; Jon Blumenfeld* (PI), Stephanie Donahue (SI), Martin Prince* (R), Rogosin Institute,* New York, NY, and Weill Medical College; W. Kline Bolton (PI), Mitchell Rosner (SI), Clinical Research Center at the University of Virginia, Charlottesville, VA; Arlene Chapman (PI), Frederick Rahbari-Oskoui (SI), Diego Martin (R), Emory Midtown Hospital, Atlanta, GA; Charles Edelstein (PI), Tomas Berl (SI), Janis Cicerchi (SI), Brian L. Burke (R), University of Colorado Denver, Aurora, CO; Michael Koren (PI), Susan N. Greco (SI), Jeffry Jacqmein (SI), Darlene Bartilucci (SI), Mark Frisk (R), Jacksonville Center for Clinical Research, Jacksonville, FL; Gerald Schulman (PI), Julia Lewis (SI), Thomas Golper (SI), Ronald Arildsen (R), Vanderbilt University Medical Center, Nashville, TN; Suzanne Swan (PI), Courtney Cannon (SI), James Cunningham–R. Davita Clinical Research, Minneapolis, MN; Vicente Torres (PI), Marie Hogan (SI), Fernando Fervenza (SI), James Glockner (R), Mayo Clinic, Rochester, MN; Terry Watnick (PI), Sharon Turban (SI), Katarzyna J. Macura (R), John Hopkins School of Medicine, Baltimore, MD; Franz Winklhofer (PI), Judson Bertsch (SI), Connie Wang (SI), Louis Wetzel (R), University of Kansas Medical Center, Kansas City, KS.
156-05-002 Investigators: Japan)
Yasuhiko Iino (PI), Nippon Medical School Hospital, Tokyo; Kouju Kamata (PI), Hisato Sakamoto (SI), Kitasato University Hospital, Sagamihara, Kanagawa; Koichi Kamura (PI), National Hospital Organization Chiba-East Hospital, Chiba; Tatsuhiko Kanno (PI), Hidetomo Nakamoto (SI), Musashi Ranzan Hospital, Ranzan-machi, Saitama; Satoru Muto (PI), Mitsuko Yasuda (SI), Shigeo Horie (SI), Hisamitsu Ide (SI), Teikyo University Hospital, Tokyo; Yukio Naya (PI), Naoki Nihei (PI), Kazuhiro Araki (SI), Chiba University Hospital, Chiba; Kosaku Nitta (PI), Ken Tsuchiya (SI), Junko Arai (SI), Tokyo Women's Medical University Hospital, Tokyo; Takatsugu Okegawa (PI), Eiji Higashihara (SI), Kyorin University Hospital, Mitaka, Tokyo; Kenmei Takaichi (PI), Yoshifumi Ubara (SI), Toranomon Hospital, Tokyo; Yusuke Tsukamoto (PI), Tomokazu Okado (SI), Michio Kuwahara (SI), Mikiko Ayata (SI), Shigeru Takada (SI), Seiji Inoshita (SI), Tamaki Kuyama (SI), Tomoki Asai (SI), Shuwa General Hospital, Kasukabe, Saitama.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Gabow P: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 323–342, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Dalgaard OZ: Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med Scand 328: 1–255, 1957 [PubMed] [Google Scholar]
- 4. Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bajwa ZH, Gupta S, Warfield CA, Steinman TI: Pain management in polycystic kidney disease. Kidney Int 60: 1631–1644, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Torres VE: Vasopressin in chronic kidney disease: An elephant in the room? Kidney Int 76: 925–928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Grantham JJ, Chapman AB, Torres VE: Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin J Am Soc Nephrol 1: 148–157, 2006 [DOI] [PubMed] [Google Scholar]
- 9. USDA: Develop biomarkers to aid in the development of therapies for polycystic kidney disease (PKD). In: Key FDA critical path activities under way in 2007. Washington, D.C., U.S. Department of Health and Human Services Food and Drug Administration, June 2008, page 5 [Google Scholar]
- 10. Klahr S, Levey A, Beck G, Caggiula A, Hunsicke L, Kusek J, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Stevens L, Greene T, Levey A: Surrogate endpoints for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Klahr S, Breyer J, Beck G, Dennis V, Hartman J, Roth D, Steinman T, Wang S, Yamamoto M: Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease modification of diet in renal disease study group. J Am Soc Nephrol 5: 2037–2047, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S: Modification of the CKD epidemiology collaboration (CDK-EPI) equation for Japanese: Accuracy and use for population estimates. Am J Kidney Dis, 56: 32–38, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Wang CJ, Creed C, Winklhofer FT, Grantham JJ: Water prescription in autosomal dominant polycystic kidney disease: A pilot study. Clin J Am Soc Nephrol 6: 192–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TJ, Krasa HB, Ouyang J, Czerwiec FS: Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 80: 295–301, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.