Abstract
Summary
Background and objectives
Endotoxin (ET) is recognized to cause adverse effects on cardiovascular (CV) structure. Circulatory translocation of gut bacterial ET is described in heart failure. Chronic kidney disease (CKD) is common in older people and aggressive BP control is the cornerstone of management. We therefore studied ET after improvement of the overall CV milieu with introduction of optimized antihypertensive therapy (AHT).
Design, setting, participants, & measurements
We recruited 40 hypertensive nondiabetic patients (≥70 years) with CKD stages 3 and 4 and hypertensive non-CKD matched controls. Assessment was performed after complete AHT washout and repeated after AHT reintroduction to target BP 130/80 mmHg. Pulse wave velocity (PWV) and analysis were assessed by applanation tonometry, central hemodynamics by continuous digital pulse wave analysis, vascular calcification (VC) by superficial femoral artery CT, and serum ET by Limulus Amebocyte assay.
Results
Mean age was 76 ± 5 years, estimated GFR (eGFR) (CKD group) was 40 ± 14 ml/min per 1.73 m2, and achieved BP was 128/69 mmHg. Washout ET was 0.042 ± 0.011 EU/ml and was independent of renal function, gender, age, BP, VC, arterial stiffness, and high-sensitivity C-reactive protein. ET significantly decreased with AHT (to 0.020 ± 0.028 EU/ml; P < 0.001) and was associated with eGFR (R = −0.38; P = 0.02), arterial wave reflection (Augmentation Index R = −0.42; P = 0.01), and degree of tonic vasodilatation (total peripheral resistance R = −0.37; P = 0.03), but not VC, PWV, gender, age, BP, or high-sensitivity C-reactive protein.
Conclusions
Elderly patients with hypertension have elevated serum ET. Improvement of their CV status with optimized AHT is associated with a significant reduction in endotoxemia. Further investigation of the potential pathophysiological mechanisms linking CV disease and CKD with this previously unappreciated effect of AHT appears warranted.
Introduction
Translocation of endotoxin across the gut wall is well described in severe heart failure (1), and occurs both in the setting of shock and in severe decompensated hepatic impairment with portal hypertension (2). Endotoxemia is associated with a wide range of well recognized pathophysiological processes; it is a profoundly proinflammatory stimulus (3) and is seen in pathologic conditions associated with malnutrition and wasting (1,2).
Endotoxin (without sepsis) was initially proposed as a stimulus for immune activation in the proinflammatory state in congestive heart failure (CHF) (4). Endotoxin enters the circulation by translocation from the gut, with bowel edema and hypoperfusion being the two main factors thought to influence bowel wall permeability in CHF (5). A study of clinically stable CHF patients has shown structurally and functionally altered gut, with increased bowel wall thickness suggestive of edema, and increased intestinal mucosal permeability suggestive of inadequate bowel mucosal perfusion (6). Endotoxin and cytokine levels are elevated in CHF patients with recent-onset edema compared with stable CHF patients, with a significant reduction in endotoxin levels following diuretic treatment (7). In decompensated CHF, endotoxin levels are significantly higher in the hepatic veins compared with the left ventricle, suggesting endotoxin translocation from the gut is the probable source (8).
Endotoxemia has been previously recognized in patients on dialysis and with nondialyzed chronic kidney disease (CKD) (9–12). We have recently reported significant incremental endotoxemia across the full range of CKD patients. Although patients receiving dialysis were particularly affected, levels of serum endotoxin were markedly higher in CKD stage 3 and 4 patients when compared with hypertensive non-CKD patient controls (13). Acutely, haemodialysis (HD) with ultrafiltration causes a reduction in splanchnic blood volume. This associated reduction may occur with or without hypotension (14,15), with the resulting mesenteric ischemia leading to disrupted gut mucosal structure and function (with increased gut permeability) (16). Circulating endotoxin levels are around 500 to 1000 times greater than in non-CKD patients, levels roughly triple after initiation of HD (as compared with patients with stable cardiac troponin T CKD stage 5). These elevated levels in HD patients correlate with intradialytic instability, systemic inflammation, cardiac troponin T levels, ultrafiltration volumes, dialysis-induced myocardial stunning, and risk of subsequent mortality (13). The biologic fate of endotoxin is well known and is not related to renal clearance (17–20).
Such recurrent acute circulatory stress, however, does not account for endotoxemia in either peritoneal dialysis (PD) patients or in nondialyzed CKD patients. In common with CHF, CKD is characterized by fluid overload, with management limited to drug therapy in those not yet dialyzed. We hypothesize that factors relating to ischemic potential or predisposing to intestinal vascular congestion (in the absence of overt CHF) may be associated with endotoxemia, and that addressing these factors with antihypertensive medication is capable of modulating systemic endotoxin exposure by optimization of fluid status and/or reduction in circulatory stress with concomitant improvement in endothelial dysfunction.
Materials and Methods
We recruited a subset of BP-controlled hypertensive patients with CKD stages 3 and 4 from a previous observational study (13) to participate in a study of the effect of antihypertensive treatment (AHT) on endotoxemia. The effects of AHT in these patients were compared with a control group of hypertensive non-CKD control patients (characteristics summarized in Table 1). Diabetics and patients with heart failure, ischemic heart disease, or malignant-phase hypertension likely to be destabilized by current therapy withdrawal were excluded. This study received additional approval by Local Regional Ethics Committee and was issued with a full clinical trials certificate by the Medicines and Healthcare products Regulatory Agency. Informed consent was obtained from all patients, who were recruited after being identified on primary care CKD chronic disease registers.
Table 1.
Description of baseline patient characteristics
| Non-CKD (15) | CKD (21) | Overall | P value | |
|---|---|---|---|---|
| Age (yrs) | 77 ± 5 | 76 ± 5 | 76 ± 5 | 0.53 |
| eGFR (ml/min per 1.73 m2) | – | 40 ± 14 | 40 ± 14 | – |
| Proteinuria (%) | 0 | 33 | 19 | 0.03 |
| Optimal BP (mmHg) | 130/71 | 126/68 | 128/69 | 0.42 |
| Male | 67% | 57% | 61% | 0.73 |
| Smoker | 27% | 10% | 17% | 0.21 |
| Alcohol (U/wk) | 3 ± 12 | 3 ± 8 | 3 ± 7 | 0.52 |
| Albumin (g/L) | 39 ± 5 | 39 ± 4 | 39 ± 4 | 0.90 |
| Corrected calcium (mmol/L) | 2.39 ± 0.09 | 2.34 ± 0.09 | 2.36 ± 0.09 | 0.13 |
| Phosphate (mmol/L) | 1.05 ± 0.16 | 1.10 ± 0.15 | 1.08 ± 0.15 | 0.36 |
| Hemoglobin (g/dl) | 14.2 ± 1.8 | 12.8 ± 1.8 | 13.4 ± 1.9 | 0.04 |
| AIx (%) | 29 ± 8 | 26 ± 6 | 27 ± 7 | 0.29 |
| PWVcr (m/s) | 10 ± 1 | 10 ± 1 | 10 ± 1 | 0.13 |
| PWVcf (m/s) | 14 ± 3 | 13 ± 3 | 13 ± 3 | 0.17 |
| SFA VC (units) | 4 ± 30 | 4 ± 116 | 4 ± 75 | 0.89 |
All continuous data are presented as mean ± SD except alcohol and VC, which are median ± interquartile range. CKD, chronic kidney disease; eGFR, estimated GFR (modification of diet in renal disease 4-variable); AIx, augmentation index; PWVcr, carotid-radial pulse wave velocity; PWVcf, carotid-femoral pulse wave velocity; SFA VC, superficial femoral artery vascular calcification.
All subjects underwent a full initial assessment after having had all current antihypertensive agents withdrawn with a minimum washout period of 2 weeks. Circulating endotoxin levels were measured at this point. AHT was then reintroduced and escalated to achieve current guidelines (130/80 mmHg). All BP measurements were performed with an appropriately calibrated, serviced, and approved oscillometric device (as above). BP was measured in both arms, with the higher arm used for all subsequent measurements. At all visits, three measurements were performed, at least 2 minutes apart. The mean was used for further analysis. Postural BP was recorded at all visits. A single set of measurements was taken at sitting (0), 1, and 3 minutes. If there was a postural drop of >20/10 mmHg, AHT was reduced.
In the washout period, BP was checked after 2 weeks. If systolic BP was >180 mm Hg or diastolic BP >110 mm Hg during the washout period, AHT was restarted and the patient was withdrawn from the study. Reintroduction of AHT was based on renin-angiotensin-aldosterone system (RAAS) inhibition but was guided by knowledge of previously tolerated and effective agents within the individuals. If BP was <110/60 mmHg after an increase in treatment, that increase was reversed. Once a patient's BP was stable for 4 weeks at this target, he or she attended for a further visit to allow evaluation of the CV system and repeat measurement of circulating endotoxin levels. A maximum period of 3 months was allowed to achieve target BP.
Body Composition Assessment
Bioelectrical impedance (BIA) was measured using InBody S20® body composition analyzer (Biospace, Korea) to detect changes in total body water and soft tissue composition.
Circulating Endotoxin Level Measurement
The method of plasma lipopolysaccharide (LPS) quantification has been described previously (21). Briefly, serum samples were diluted to 20% with endotoxin-free water and then heated to 70°C for 10 minutes to inactivate plasma proteins. We then quantified serum LPS with a commercially available Limulus Amebocyte assay (Cambrex, Verviers, Belgium), according to the manufacturer's protocol. The detection limit of this assay was 0.01 EU/ml. Samples with LPS level below the detection limit were taken as 0.01 EU/ml. All samples were run in duplicate and background subtracted.
Blood and Urine Samples Collected
Blood samples were collected at baseline after AHT washout and after retitration. Serum sodium, potassium, urea, creatinine, 25-OH vitamin D, phosphate (PO4), albumin corrected calcium (CCa), albumin, and intact parathyroid hormone (PTH) were analyzed using standard autoanalyzer techniques (Roche diagnostics modular IIP®). Serum PTH was measured using the immunometric Immulite® 2000 assay (normal range 7 to 53 pg/ml). Commercially available enzyme-linked immunosorbent assay (ELISA) kits (DRG instruments, Germany) were used to assess high-sensitivity C-reactive protein (hsCRP). The detection limit was 0.1 mg/L with the coefficient of variation (CoV was 7.2%). Patients were staged on four-variable modification of diet in renal disease estimated GFR (eGFR). Proteinuria was quantified by the average of three sequential early morning urine samples for protein-creatinine ratio. All blood samples were taken in the hospital at an investigationary visit to allow for rapid separation of serum and storage at −85°C before endotoxin measurement.
Assessment of Vascular Calcification and Arterial Stiffness
Multislice computed tomography (MSCT) and measurement of hemodynamic variables were performed by a single observer. Briefly, MSCT was used to quantify calcification in a standardized section of the superficial femoral artery. Each slice was scored individually and a calcification score was generated. Calcification was considered to be present if an area ≥1 mm (2) displayed a density >130 Hounsfield units (22). Validation studies confirmed that the scoring technique is highly reproducible. Interobserver reproducibility between the investigator and a consultant radiologist was assessed in a 1-in-20 sample. The intraclass correlation was 1 (confidence interval [CI] 1 to 1) and the CoV was 3.9%. Repeatedly scored scans showed an intraobserver intraclass correlation of 1 (CI 1 to 1) and a CoV of 2.4%.
Applanation tonometry was performed at the radial artery using a SphygmoCor® (AtCor Medical Pty Ltd., Australia). Augmentation was assessed as a derived central pressure wave. Electrocardiogram gated PWV was assessed between the carotid and radial as well as the carotid and femoral arteries.
Measurement of Hemodynamic Variables
Noninvasive continuous pulse wave analysis was used to determine hemodynamic variables. The Finometer® (FMS, Amsterdam, The Netherlands) utilizes photoplethysmography to derive a pulse wave from a finger pressure cuff containing an infrared optical source and detection unit. The pulse wave is then subject to mathematical analysis based on the transport characteristics of peripheral blood vessels to reconstruct a central pressure waveform. This, in turn, is analyzed to calculate a wide variety of hemodynamic variables, including systolic BP, diastolic BP, mean arterial pressure, heart rate, cardiac output, stroke volume, and total peripheral resistance on a beat-to-beat basis. All data were subsequently downloaded to a personal computer–based analysis program (Beatscope™), allowing averaging of results over the 10-minute assessment period. Patients were assessed while supine after at least 15 minutes of rest.
Statistical Analysis and Sample Size Justification
The principal primary end point was to detect a 50% difference in circulating endotoxin levels after reintroduction of AHT. A sample size of at least 36 patients was needed to detect this difference at 90% power.
Group data are presented as mean ± SD, unless otherwise stated. All data were tested for normality. Analysis was performed using SPSS v12.0.1 (SPSS Inc., Chicago, IL). Categorical data were compared using the chi-squared test, continuous data using the paired or the unpaired t test, the Mann–Whitney U test, or the Wilcoxon test, as appropriate. Correlation between continuous variables was examined by the Spearman rank correlation coefficient. A two-tailed P value of less than 0.05 was considered significant.
Results
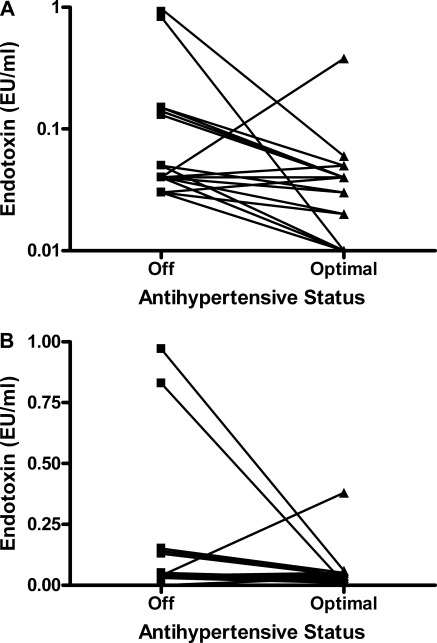
The patient characteristics and achieved BP after drug reintroduction, and agents utilized to achieve this, are summarized in Tables 1 and 2. The patient groups were well matched for all factors other than renal function. Achieved mean BP was 128/69 mmHg. Washout median (±interquartile range[IQR]) endotoxin level was 0.042 ± 0.010 EU/ml and was not associated with renal function, hemoglobin, gender, age, BP, vascular calcification, arterial stiffness, or hsCRP (non-CKD, 0.042 ± 0.010; CKD, 0.042 ± 0.011 EU/ml). Circulating endotoxin level significantly decreased with the reintroduction and optimization of AHT (to 0.020 ± 0.028 EU/ml, P < 0.001, Figure 1) in both CKD and non-CKD groups (non-CKD, 0.011 ± 0.006, P = 0.008; CKD, 0.032 ± 0.028 EU/ml, P = 0.002). This reduction was greater by 50% in the non-CKD patients (0.032 versus 0.010 EU/ml), despite similar drug usage and achieved BP.
Table 2.
Agents used during antihypertensive reintroduction in the study
| % Use | Non-CKD (15) | CKD (21) | P Value |
|---|---|---|---|
| ACEi/ARB | 40 | 57 | 0.31 |
| Calcium blocker | 47 | 48 | 0.96 |
| Beta blocker | 27 | 24 | 0.85 |
| Diuretic | 33 | 33 | 1.00 |
| Alpha blocker | 33 | 24 | 0.53 |
CKD, chronic kidney disease; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 1.
Effect of optimization of BP with antihypertensive therapy on circulating endotoxemia in the entire cohort.
Antihypertensive reintroduction resulted in a reduction in mean carotid-femoral PWV (−1.1 m/s, P < 0.001) and rate-corrected augmentation index (−3.2%, P < 0.001). Mean BIA measured intracellular and extracellular water tended to be lower (400 ml, P = 0.05; 223 ml, P = 0.07, respectively), lean mass decreased (837 g, P < 0.05), but body fat mass was unchanged with AHT reintroduction. Significant VC was present in 17 of 36 patients, median (± IQR) VC score 4 ± 75 units.
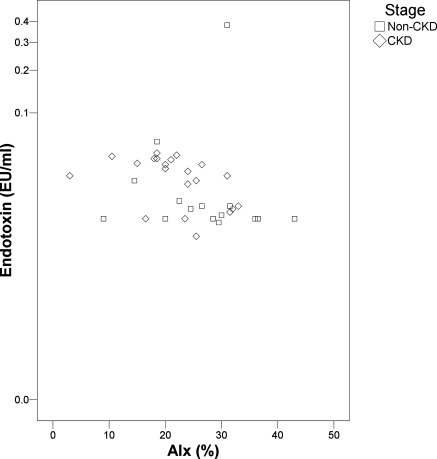
After reintroduction of antihypertensive medications, circulating endotoxin was associated with eGFR (R = −0.38; P = 0.02) as well as arterial wave reflection (Augmentation Index R = −0.42; P = 0.01; Figure 2) and degree of tonic vasodilatation (total peripheral resistance, R = −0.37; P = 0.03), but not VC, PWV (carotid-radial or carotid-femoral), gender, age, BP, or hsCRP. There were no associations between the degree of ET reduction and change in hemoglobin, arterial stiffness, central hemodynamics, renal function, or hsCRP. There was, however, little evidence of significant systemic inflammation within this group with median (± IQR) hsCRP being only 2.3 ± 3.2 mg/ml, which was similar in non-CKD and CKD groups.
Figure 2.
Relationship of endotoxemia with arterial stiffness (augmentation index; AIx) after reintroduction of antihypertensive therapy in the entire cohort. CKD, chronic kidney disease.
Discussion
This is the first study to recognize that serum endotoxin levels in hypertensive patients with CKD can be modified by AHT therapy use. This reduction in endotoxemia is associated with an improvement of factors previously implicated in CV health.
The biologic fate of plasma-free endotoxin is not dependent on the kidney. Clearance of endotoxin is affected both by humoral inactivation and through uptake into liver and mononuclear phagocyte cells (17–20), and is influenced by both host and LPS-specific factors (23,24). There is a complex relationship among binding of endotoxin to antibodies, to platelets, and to lipoproteins in the modulation of its biologic effects and clearance (23,24). Endotoxemia has been initially recognized in severe decompensated heart failure (4). Significant heart failure (New York Heart Association 3 to 4) was an exclusion criterion in this study, as was any recent decompensating acute illness. The degree of venous congestion has been reported as being increased with reducing GFR, but only in the setting of previously diagnosed significant CHF (25).
AHT appears to be able to reduce endotoxin levels. This is the first report that a pharmacologic intervention targeted at the cardiovascular system is capable of modulating such a potentially important factor as the intestinal translocation of preformed endotoxin in the pathophysiology of CV disease. Almost all of the patients studied had a reduction in endotoxemia after reintroduction of AHT. This resulted in around a halving of the levels of circulating endotoxin as compared with values obtained after full washout of AHT. Most patients were treated with RAAS inhibition, but a variety of agents were utilized. There were a variety of effects resulting from introduction of these agents, which might have influenced the exposure to intestinal endotoxin translocation. Reduction in BP itself is capable of modifying endothelial vascular function (26) and altering potential for relative gut ischemia; a variety of the agents utilized (particularly angiotensin-converting enzyme inhibitors) also directly influence vascular biology, potentially reducing demand ischemia (27,28). There was a modest reduction in extracellular water with drug introduction, and a potential reduction in venous congestion may have played a role, but the patients with established CHF were specifically excluded from study, and the absolute change in extracellular water was small. PWV and augmentation index both improved with reintroduction of antihypertensive medication; reduction in arterial stiffness is known to reduce demand ischemia (29). There was a correlation between augmentation index (as a composite measure of cardiac performance and peripheral arterial structure and function) and the degree of endotoxemia, but not with PWV as measure of arterial compliance itself. A reduction in endotoxemia was seen in all of the hypertensive patients, but the reduction was reduced in percentage terms in those with the added factor of CKD. Given the potential biologic effects and influence of endotoxin at many levels of vascular biology (NO production, direct vasodilatation, Pro-inflammatory cytokine production, etc.) further investigation of potential pathophysiological mechanisms appears warranted.
Limitations
This study is limited by a variety of factors. Although the sample size is sufficient to meet the primary end point of detecting the reduction in circulating endotoxin level, the sample size is inadequate to fully resolve factors relating to the degree of endotoxemia or associated outcomes. The study would have been enhanced if further assessment of endothelial function had been performed. Although the groups were well matched, hemoglobin was lower in the CKD group. However, Hb levels were not associated with endotoxin in this study or previously across the spectrum of CKD (13). Endotoxin is also recognized to be a driver of atherosclerosis and is associated with increased intimal medial thickness measurements in peritoneal dialysis patients (11). This study did not include a direct measure of atherosclerotic burden. Plaque disease and classic thrombotic complications appear, however, to be significantly less important in the genesis of CV outcomes in this patient group compared with nonuremic patients.
Conclusions
Endotoxemia in CKD stage 3 and 4 patients appears to be modifiable by the use of pharmacologic intervention primarily directed at reducing BP. The observed reduction is associated with an improvement in factors associated with cardiovascular health and a subclinical reduction in overall hydration status. This previously unappreciated effect of AHT may be of significant importance in the pathophysiology of CV disease and progressive loss of renal function in patients with CKD.
Disclosures
Dr. C.W. McIntyre was previously chief medical officer of INEOS healthcare.
Acknowledgments
We gratefully acknowledge the assistance of Mrs. Margaret Baker, Mrs. Amanda Gates. and Mr. Apostolos Fakis. This work was presented at ASN Renal week 2009. This work was supported by a research project grant from the British Renal Society.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Charalambous BM, Stephens RC, Feavers IM, Montgomery HE: Role of bacterial endotoxin in chronic heart failure: The gut of the matter. Shock 28: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Lumsden AB, Henderson JM, Kutner MH: Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 8: 232–236, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ: Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395: 284–288, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ: Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 79: 1426–1430, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Krack A, Sharma R, Figulla HR, Anker SD: The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J 26: 2368–2374, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD: Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD: Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 353: 1838–1842, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J: Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail 5: 609–614, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Markum HM, Suhardjono, Pohan HT, Suhendro, Lydia A, Inada K: Endotoxin in patients with terminal renal failure undergoing dialysis with re-processing dialyser. Acta Med Indones 36: 93–96, 2004 [PubMed] [Google Scholar]
- 10. Nisbeth U, Hallgren R, Eriksson O, Danielson BG: Endotoxemia in chronic renal failure. Nephron 45: 93–97, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK: Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3: 431–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goncalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, Fuerbringer R, Sauthier SM, Riella MC: Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant 21: 2788–2794, 2006 [DOI] [PubMed] [Google Scholar]
- 13. McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK: Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu AW, Nawab ZM, Barnes WE, Lai KN, Ing TS, Daugirdas JT: Splanchnic erythrocyte content decreases during hemodialysis: A new compensatory mechanism for hypovolemia. Kidney Int 51: 1986–1990, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J: Splanchnic perfusion during hemodialysis: Evidence for marginal tissue perfusion. Crit Care Med 29: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Khanna A, Rossman JE, Fung HL, Caty MG: Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J Surg Res 99: 114–119, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Caridis DT, Reinhold RB, Woodruff PW, Fine J: Endotoxaemia in man. Lancet 1: 1381–1385, 1972 [DOI] [PubMed] [Google Scholar]
- 18. Casey WF, Hauser GJ, Hannallah RS, Midgley FM, Khan WN: Circulating endotoxin and tumor necrosis factor during pediatric cardiac surgery. Crit Care Med 20: 1090–1096, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Freudenberg MA, Galanos C: Metabolism of LPS in vivo. In: Bacterial endotoxic lipopolysaccharides, edited by Morrison DC, Ryan JL, Boca Raton, FL, CRC Press, 1992, pp 275–294 [Google Scholar]
- 20. Skarnes RC: In vivo distribution and detoxification of endotoxins. In: Handbook of endotoxins, edited by Berry LJ. Amsterdam, The Netherlands, Elsevier, 1984, pp 56–81 [Google Scholar]
- 21. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12: 1365–1371, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Sigrist M, Bungay P, Taal MW, McIntyre CW: Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 21: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Harris HW, Grunfeld C, Feingold KR, Rapp JH: Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest 86: 696–702, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liao W, Floren CH: Hyperlipidemic response to endotoxin–a part of the host-defence mechanism. Scand J Infect Dis 25: 675–682, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL: Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9: 872–878, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Siragy HM: Improving vascular function in hypertension: Potential benefits of combination therapy with amlodipine and renin-angiotensin-aldosterone system blockers. J Hypertens 28: 2–8, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Morishita T, Tsutsui M, Shimokawa H, Tasaki H, Suda O, Kobayashi K, Horiuchi M, Okuda H, Tsuda Y, Yanagihara N, Nakashima Y: Long-term treatment with perindopril ameliorates dobutamine-induced myocardial ischemia in patients with coronary artery disease. Jpn J Pharmacol 88: 100–107, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Schmermund A, Lerman LO, Ritman EL, Rumberger JA: Cardiac production of angiotensin II and its pharmacologic inhibition: Effects on the coronary circulation. Mayo Clin Proc 74: 503–513, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y: Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol 21: 1497–1506, 1993 [DOI] [PubMed] [Google Scholar]