Abstract
Summary
Background and objectives
To date there is no reliable marker for the differentiation of prerenal and intrinsic acute kidney injury (AKI). We investigated whether urinary calprotectin, a mediator protein of the innate immune system, may serve as a diagnostic marker in AKI.
Design, setting, participants, & measurements
This was a cross-sectional study with 101 subjects including 86 patients with AKI (34 prerenal, 52 intrinsic including 23 patients with urinary tract infection) and 15 healthy controls. Assessment of urinary calprotectin concentration was by ELISA and immunohistochemistry of kidney biopsy specimens using a calprotectin antibody. Inclusion criteria were: admission to hospital for AKI stage 1 to 3 (Acute Kidney Injury Network); exclusion criteria were: prior renal transplantation and obstructive uropathy.
Results
Median urinary calprotectin was 60.7 times higher in intrinsic AKI (1692 ng/ml) than in prerenal AKI (28 ng/ml, p <0.01). Urinary calprotectin in prerenal disease was not significantly different from healthy controls (45 ng/ml, p = 0.25). Receiver operating curve curve analysis revealed a high accuracy of calprotectin (area under the curve, 0.97) in predicting intrinsic AKI. A cutoff level of 300 ng/ml provided a sensitivity of 92.3% and a specificity of 97.1%. Calculating urinary calprotectin/creatinine ratios did not lead to a further increase of accuracy. Immunostainings of kidney biopsies were positive for calprotectin in intrinsic AKI and negative in prerenal AKI.
Conclusions
Accuracy of urinary calprotectin in the differential diagnosis of AKI is high. Whereas calprotectin levels in prerenal disease are comparable with healthy controls, intrinsic AKI leads to highly increased calprotectin concentrations.
Introduction
Acute kidney injury (AKI) is a serious problem occurring in 1% to 5% of all hospitalized patients. Currently, the incidence of AKI in hospitalized patients increases by 11% per year (1). The prognosis of AKI depends crucially on an early diagnosis and an immediate onset of therapy. The earlier the cause of AKI is identified, the more successful is the specific therapy (2). A multitude of causes may lead to AKI, and they are commonly classified according to their origin as prerenal, intrinsic (intrarenal), and postrenal. Prerenal AKI means a loss of renal function despite intact nephrons, e.g., because of volume depletion and/or hypotension. There is a broad spectrum of intrinsic causes of AKI including acute tubular necrosis (ATN), interstitial nephritis, glomerulonephritis, and vasculitis.
The diagnosis of postrenal AKI may be established easily by ultrasound. The differentiation of prerenal and intrinsic AKI, however, may be difficult. To date, there is no reliable marker for the detection of prerenal AKI. Response to fluid repletion is still regarded as the gold standard in the differentiation between prerenal and intrinsic AKI. Return of renal function to baseline within 24 to 72 hours is considered to indicate prerenal AKI, whereas persistent renal failure indicates intrinsic disease. This criterion is not valid, however, in cases of short-lived ATN. Furthermore, intrarenal causes such as crescentic glomerulonephritis require immediate biopsy to avoid delay in the initiation of therapy. Therefore, waiting 72 hours before initiation of therapy is unacceptable. Finally, rapid fluid application is contraindicated in a substantial number of patients, such as those with congestive heart failure.
In addition to the fluid challenge, there are laboratory parameters that are propagated for the differentiation of prerenal and intrinsic AKI.
The most commonly used parameter is the fractional excretion of sodium (FENa) (3). There are several conditions, however, in which this parameter is not accurate: FENa may not be used in the presence of diuretics, because diuretics increase sodium excretion independent of the underlying cause of AKI (4). Furthermore, in the setting of reduced effective blood volume, such as congestive heart failure or liver cirrhosis, FENa is reduced, making its interpretation in AKI impossible. Hence, FENa cannot be used in the majority of elderly patients with pre-existing diseases. The fractional excretion of urea (FEUrea) is an alternative marker for the differentiation of prerenal and intrinsic AKI. In contrast to FENa, FEUrea is rather independent of diuretic therapy. Pépin et al. (4) reported sensitivity and specificity of 79 and 33%, respectively, in patients administered diuretics, which, however, is still too low to guide therapy.
A reliable noninvasive test for the differentiation of prerenal and intrinsic AKI would be desirable, because it would shorten the time to initiation of therapy, and it would prevent unnecessary biopsies in prerenal disease. In this study, urinary calprotectin, a mediator protein of the innate immune system, is evaluated as a urinary biomarker in the differential diagnosis of AKI. In gastroenterology, fecal calprotectin is a well established parameter for the differentiation between inflammatory bowel disease and irritable bowel syndrome. Because there is no relevant mucosal inflammation activity in irritable bowel syndrome, fecal calprotectin concentrations are low in this condition. They are highly increased, however, in inflammatory bowel disease. In analogy to irritable bowel syndrome, epithelial structures are completely intact in prerenal AKI. Therefore, the basic hypothesis of this study was that urinary calprotectin concentrations are low in prerenal AKI, whereas they are increased in intrinsic AKI.
Materials and Methods
Study Design and Protocol
This work constitutes a cross-sectional study at the Charité University Hospital Berlin. Patients referred to the Department of Nephrology for AKI of unknown origin were consecutively enrolled in the study from May 2009 to September 2010. The presence of AKI, defined according to the Acute Kidney Injury Network (AKIN) criteria, was an inclusion criterion (5). Patients were excluded for prior renal transplantation and obstructive uropathy. Diagnoses of prerenal and intrinsic AKI were established as follows: a histologic diagnosis was regarded as the gold standard. Hepatorenal syndrome, cardiorenal syndrome, and bilateral renal artery stenosis were regarded as prerenal AKI. In those subjects who did not undergo renal biopsy and had none of the above mentioned entities, the diagnosis was established by predefined clinical criteria (Table 1) (4): rapid response of renal function to volume repletion was defined as an obligatory criterion (category A) for the diagnosis of prerenal AKI. Compatibility of history, physical findings, and urinary findings were regarded as category B criteria. Diagnosis of prerenal AKI required two criteria of category B and the obligatory criterion of category A. Eight patients with intrinsic AKI and one patient with prerenal AKI underwent a kidney biopsy. Written informed consent was obtained by the participants. The study was approved by the local ethics committee of the Charité-Universitätsmedizin Berlin.
Table 1.
Diagnostic criteria of prerenal and intrinsic AKI in the absence of a histological diagnosis
| Clinical Criteria | Prerenal AKI | Intrinsic AKI |
|---|---|---|
| Category A | ||
| response to volume repletion | Rapid decrease of creatine with convergence to baseline levels | No reconstitution of renal function |
| Category B | ||
| history compatible | Dehydration, loss of fluid, e.g., by gastroenteritis, heart failure, liver failure, inadequate use of diuretics, etc. | Prolonged shock, exposition to nephrotoxins, extrarenal suggestive symptoms like pulmorenal syndrome, etc. |
| physical findings compatible | Low blood pressure, low jugular pulse, tachycardia, orthostatic changes, poor skin turgor | Absence of signs of dehydration, cardiac monitoring shows adequate volemia |
| urine examinations | Absence of proteinuria, hematuria, and leukocyturia | Proteinuria and/or hematuria and/or leukocyturia |
The criteria of category A are obligatory and criteria of category B are optional for the diagnosis of prerenal or intrarenal AKI. Diagnosis of prerenal AKI required two criteria of category B and the obligatory criterion of category A. Hepatorenal syndrome, cardiorenal syndrome, and bilateral renal artery stenosis were regarded as prerenal AKI independent of these criteria. AKI, acute kidney injury.
Study Population
Eighty-six patients with AKI were enrolled in the study. Fifteen healthy individuals (medical staff, no history of hypertension, diabetes, or renal disease) served as control. The characteristics of the study population are presented in Table 2. Baseline creatinine values were available in 60 of 86 AKI patients.
Table 2.
Characteristics of study population
| Prerenal (n = 34) | Intrinsic (n = 52) | P | |
|---|---|---|---|
| Female | 6 (17.6%) | 27 (51.9%) | <0.01 |
| Male | 28 (82.4%) | 25 (48.1%) | |
| Age (years) | 68.4 ± 14.1 | 70.5 ± 16.5 | 0.53 |
| Body mass index (kg/m2) | 26.4 ± 4.9 | 26.1 ± 4.5 | 0.76 |
| Preexisting CKD | 14 (41.2%) | 26 (50%) | 0.51 |
| Biopsy | 1 (2.9%) | 8 (15.4%) | 0.08 |
| Origin of AKI | |||
| dehydration by gastroenteritis | 12 (35.3%) | ||
| hemodynamic, drug-induced (diuretics, ACE-I, ARB, NSAID) | 11 (32.4%) | ||
| other causes of dehydration | 5 (14.7%) | ||
| cardiorenal syndrome | 2 (5.9)% | ||
| hepatorenal syndrome | 1 (2.9%) | ||
| bilateral renal artery stenosis | 1 (2.9%) | ||
| short-term hypotension | 2 (5.9%) | ||
| hypotension-induced acute tubular necrosis | 4 (7.7%) | ||
| toxic acute tubular necrosis | 2 (3.8%) | ||
| crescentic glomerulonephritis | 3 (5.8%) | ||
| lupus nephritis | 2 (3.8%) | ||
| other forms of glomerulonephritis | 4 (7.7%) | ||
| thrombotic thrombocytopenic purpura | 1 (1.9%) | ||
| drug-induced acute interstitial nephritis | 3 (5.8%) | ||
| renal infarction | 1 (1.9%) | ||
| rhabdomyolysis | 1 (1.9%) | ||
| urosepsis/severe urinary tract infection | 22 (42.3%) | ||
| contrast-media induced | 3 (5.8%) | ||
| multiple myeloma | 1 (1.9%) | ||
| unknown | 5 (9.6%) | ||
| Concomitant diseases | |||
| diabetes mellitus | 10 (29.4%) | 15 (28.8%) | 1.0 |
| hypertension | 29 (85.3%) | 36 (69.2%) | 0.12 |
| urinary tract infection | 0 (0%) | 23 (44.2%) | <0.01 |
| Medication on admission | |||
| ACE-I/ARB | 19 (55.9%) | 17 (32.7%) | 0.07 |
| diuretics | 23 (67.6%) | 22 (42.3%) | 0.04 |
| eGFR on admission (ml/min per 1.73 m2) | 18.2 ± 9.9 | 19.9 ± 13.4 | 0.52 |
| creatinine on admission (mg/dl) | 4.4 ± 2.7 | 4.1 ± 2.2 | 0.55 |
| creatinine prior to admission (mg/dl, if available) | 1.4 ± 0.7 | 1.4 ± 0.8 | 0.88 |
| creatinine at discharge (mg/dl) | 1.7 ± 0.9 | 2.2 ± 1.6 | 0.047 |
| proteinuria (mg/L) | 98 (66 to 178) | 393 (167 to 1023) | <0.01 |
| proteinuria (mg/L)/urinary creatinine (g/L) | 165 (75 to 309) | 987 (295 to 2071) | <0.01 |
| C-reactive protein (mg/dl) | 5.2 ± 4.6 | 6.7 ± 9.7 | 0.40 |
| urinary creatinine (g/L) | 0.71 ± 0.32 | 0.61 ± 0.38 | 0.23 |
| urinary sodium (mmol/L) | 67.0 ± 32.4 | 67.5 ± 39.4 | 0.95 |
| plasma sodium (mmol/L) | 137.6 ± 4.5 | 137.4 ± 5.5 | 0.90 |
| fractional excretion of sodium (%) | 3.5 ± 2.5 | 5.2 ± 6.5 | 0.10 |
| urinary calprotectin (ng/ml) | 28 (13 to 73) | 1692 (765 to 4735) | <0.01 |
| urinary calprotectin (ng/ml)/urinary creatinine (g/L) ratio | 52 (23 to 123) | 3698 (1330 to 7812) | <0.01 |
The numeric data are presented as means ± standard deviation in case of normal distribution, otherwise as median and interquartile range (calprotectin, calprotectin/creatinine ratio, and proteinuria). Statistical tests used for the individual parameters are presented in the statistics section. P < 0.05 was regarded as statistically significant. CKD, chronic kidney disease; AKI, acute kidney injury; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; eGFR, estimated GFR.
Measurement of Calprotectin in the Urine
Urine samples (10 ml) were collected three times weekly at our nephrological ward. Thus, we tried to keep the time between diagnosis of AKI and collection of urine sample ≤3 days. The samples were stored frozen (−20°C, no centrifugation) until assessment of calprotectin concentration took place. Urine concentrations of calprotectin were quantified using an ELISA kit (PhiCal® Calprotectin, catalog number K 6935; Immundiagnostik AG, Bensheim, Germany) according to the manufacturer's protocol. The coefficient of variation for duplicate measurements using this ELISA kit was <6%. To take the current concentration status of the urine into account, creatinine was assessed in the urine, and calprotectin/creatinine relations were calculated. The person, who performed the ELISA measurements, was blinded for clinical data.
Immunohistochemistry Studies in Biopsy Specimens
Kidney tissue was obtained by needle biopsy. After embedding in paraffin, 5-μm-thick serial sections were cut, and periodic acid Schiff reaction staining was performed. Subsequently immunohistochemistry was performed using the alkaline phosphatase anti-alkaline phosphatase technique (6). Local distribution of calprotectin was analyzed by immunostaining with a monoclonal antibody recognizing an epitope on the S100A8/A9 heterocomplex (calprotectin) that is not exposed on the individual subunits S100A8 (MRP8) or S100A9 (MRP14) (Product number T-1023; BMA Biomedicals, Augst, Switzerland). We investigated the sample of a patient that fulfilled the clinical criteria of prerenal AKI (urinary calprotectin, 3 ng/ml), a sample of a patient with lupus nephritis (urinary calprotectin, 1422 ng/ml), and, as a positive control, a sample of a patient with pyelonephritis (urinary calprotectin, 8232 ng/ml). Because there was no patient in our study population who underwent a biopsy because of pyelonephritis, we made use of the biopsy specimen of a renal transplant recipient. The urinary data of this patient were not included in the study.
Statistical Methods
Distribution of numeric data were analyzed by the Kolmogorov-Smirnov Test. In case of normal distribution, the data are presented as the means ± SD (age, body mass index, estimated GFR [eGFR], C-reactive protein, creatinine, sodium, and FENa), otherwise the data are presented as median and interquartile range (calprotectin, calprotectin/creatinine ratio, and proteinuria). Receiver operating characteristic (ROC) curves were formed in an attempt to determine the accuracy of calprotectin, calprotectin/creatinine ratio, FENa, and proteinuria in predicting intrinsic AKI. ROC curves were used to establish the best threshold for urinary calprotectin and urinary calprotectin/creatinine ratio in predicting intrinsic AKI. Sensitivity, specificity, and positive and negative predictive values were calculated for these thresholds. Comparison of normally distributed numeric parameters was performed by two-sided two-sample t tests. Comparison of not normally distributed numeric data were performed by using the Mann–Whitney U-test. Comparison of categorical parameters was performed by Fisher exact test in case of dichotomy (presence/absence of concomitant diseases, medication, biopsy, and gender) and by Pearson chi-squared test in case of polychotomy (stage of AKI). All of the statistical analyses were done using PASW Statistics 18.0 (SPSS Inc., Chicago, IL).
Results
Study Population
One hundred one subjects were enrolled in the study including 86 patients with AKI and 15 healthy controls. Classification according to biopsy results or, if not available, to the criteria presented in Table 1 revealed 34 patients with prerenal AKI and 52 patients with intrinsic AKI. Healthy controls (nine men and six women) had a mean age of 33.8 ± 10.0 years and a body mass index of 23.4 ± 3.6 kg/m2. Serum creatinine was 0.86 ± 0.12 mg/dl corresponding to an eGFR of 96.4 ± 22.2 ml/min per 1.73 m2. The epidemiologic data, stage, and origin of AKI, concomitant diseases, premedication, renal parameters, and findings of calprotectin measurements in the patients with AKI are presented in Table 2. In both prerenal and intrinsic AKI, the majority of cases corresponded to stage 3 according to AKIN criteria, followed by stage 2 and stage 1 (Table 3). Severity of AKI as measured by AKIN criteria was not significantly different between the prerenal and intrinsic AKI group (P = 0.82). The period between maximum serum creatinine and retrieval of urine sample was 3.2 ± 3.7 days. Creatinine and eGFR on admission were not significantly different (P = 0.55, P = 0.52). Creatinine at discharge from hospital, however, was significantly lower in prerenal AKI (P = 0.047), and values tended to baseline levels measured before admission. Creatinine before admission was available in 60 of 86 patients. The two groups differed significantly in the amount of proteinuria, being six times higher in intrinsic AKI (P < 0.01). C-reactive protein, urinary creatinine, urinary sodium, and plasma sodium were not significantly different in the two groups (P > 0.05 each). FENa tended to be higher in intrinsic AKI, but the difference did not reach any significance (P = 0.10). There were no urinary tract infections in the group of prerenal AKI and 23 cases in the intrinsic group (P < 0.001).
Table 3.
Classification of prerenal and intrinsic acute kidney injury according to the criteria of the Acute Kidney Injury Network (5)
| Prerenal (n = 34) |
Intrinsic (n = 52) |
|||||
|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 1 | Stage 2 | Stage 3 | |
| Number of probands | 7 (20.6%) | 9 (26.5%) | 18 (52.9%) | 13 (25.0%) | 15 (28.8%) | 24 (46.2%) |
| eGFR on admission (ml/min per 1.73 m2) | 29 (16 to 35) | 21 (19 to 31) | 10 (8 to 14) | 28 (18 to 48) | 19 (15 to 29) | 10 (7 to 17) |
| Creatinine on admission (mg/dl) | 2.3 (1.6 to 3.7) | 3.0 (2.5 to 3.1) | 5.2 (4.4 to 7.2) | 2.2 (1.4 to 3.1) | 3.2 (2.1 to 4.0) | 5.8 (3.7 to 6.9) |
| Creatinine prior to admission (mg/dl) | 1.6 (1.0 to 2.3) | 1.1 (1.0 to 1.3) | 1.1 (1.0 to 2.3) | 1.1 (0.8 to 1.5) | 1.3 (0.9 to 1.8) | 1.1 (1.0 to 1.7) |
| Creatinine at discharge (mg/dl) | 1.8 (1.4 to 2.1) | 1.1 (1.0 to 1.7) | 1.3 (1.1 to 1.8) | 1.3 (1.0 to 1.8) | 1.5 (1.1 to 2.3) | 1.9 (1.3 to 3.8) |
| Criteria stage 1 | Increase in creatinine of ≥0.3 mg/dl or increase to ≥150 to 200% from baseline OR diuresis <0.5 ml/kg per hour for >6 hours | |||||
| Criteria stage 2 | Increase in creatinine to >200 to 300% from baseline OR diuresis <0.5 ml/kg per hour for >12 hours | |||||
| Criteria stage 3 | Increase in creatinine to >300% from baseline OR creatinine ≥4.0 mg/dl with an acute increase of at least 0.5 mg/dl OR diuresis <0.3 ml/kg per hour for 24 hours or anuria for 12 hours | |||||
The data are presented as medians and interquartile ranges. Creatinine prior to admission was available in 60 of 86 patients. eGFR, estimated GFR.
Urinary Calprotectin and Calprotectin/Creatinine Ratios in AKI
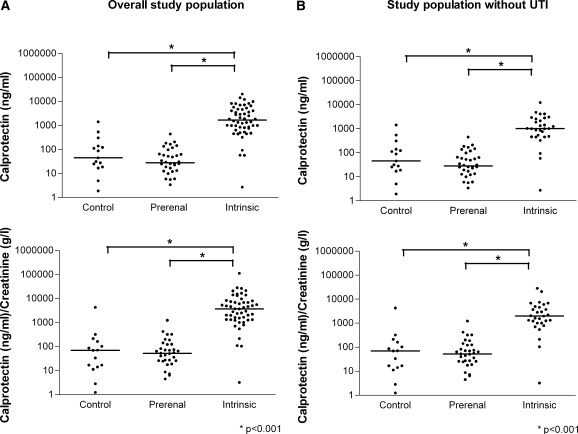
Assessment of urinary calprotectin concentration was successful in the whole study population. Figure 1A presents the individual results of calprotectin measurements in each group. The values ranged from 0 to 20,400 ng/ml. To take the current concentration status of the urine into account, urinary creatinine was assessed, and calprotectin/creatinine ratios were calculated ranging from 0 to 112,710 (ng/ml)/(g/L). The data are presented as medians and interquartile ranges. Calprotectin concentrations were not significantly different in healthy controls (45 ng/ml, 19 to 139) and prerenal AKI (28 ng/ml, 13 to 73; P = 0.25). Median urinary calprotectin was 60.7 times higher, however, in intrinsic AKI (1692 ng/ml, 765 to 4735) than in prerenal AKI (P < 0.01). Calprotectin/creatinine ratios did not differ significantly between healthy controls (70 (ng/ml)/(g/L), 14 to 162) and prerenal AKI (52 (ng/ml)/(g/L), 23 to 123; P = 0.97). The calprotectin/creatinine ratio in intrinsic AKI, however, was 71.1 times higher than in prerenal AKI (P < 0.01). The calprotectin and calprotectin/creatinine ratios in intrinsic AKI were significantly higher than those of healthy controls (P < 0.01 each). Because urinary tract infections go along with leukocyturia, potentially leading to high urinary calprotectin concentrations, we excluded all cases of urinary tract infection in a second analysis. Figure 1B illustrates the results of this analysis, showing that exclusion of urinary tract infection did not lead to a qualitative change of the findings in the overall study population. There were no cases of urinary tract infection in the prerenal AKI group; healthy controls were not tested for urinary tract infection. Accordingly, calprotectin and calprotectin/creatinine ratios did not change in these groups. Urinary calprotectin concentration was 36.1 times higher in intrinsic AKI (1007 ng/ml, 465 to 2,675) than in prerenal AKI (P < 0.01). The calprotectin/creatinine ratio was 38.2 times higher in intrinsic AKI (1987 (ng/ml)/(g/L), 1009 to 4744) when compared with prerenal AKI (P < 0.01).
Figure 1.
Individual measurement results of urinary calprotectin and calprotectin/creatinine ratio of healthy controls, prerenal acute kidney injury (AKI), and intrinsic AKI of the complete study population (A) and all subjects without urinary tract infection (UTI) (B). The data are presented as scatter plots (logarithmic y axis; medians are indicated by horizontal lines). Calprotectin concentrations and calprotectin/creatinine ratios did not significantly differ between healthy controls and prerenal AKI but were significantly higher in intrinsic AKI than in prerenal AKI and controls (P < 0.001 each). The difference remains highly significant after exclusion of subjects with urinary tract infection.
Diagnostic Accuracy of Urinary Calprotectin in Predicting Intrinsic AKI
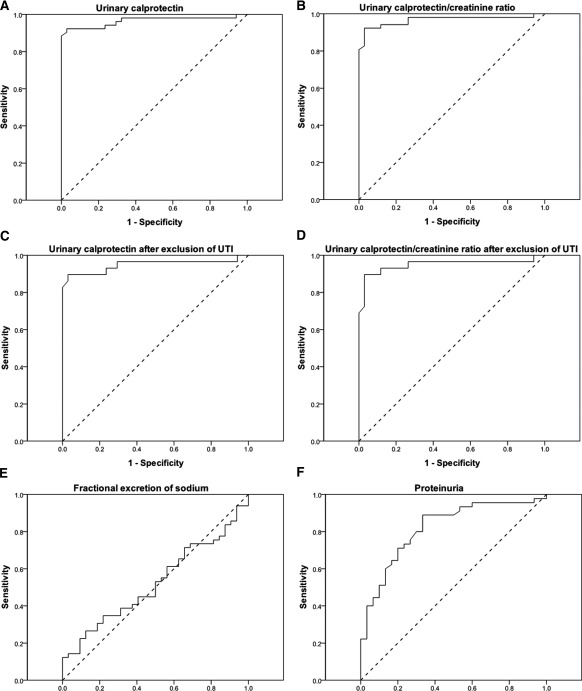
The accuracy of urinary calprotectin in the detection of intrinsic AKI was assessed by ROC curve analysis as presented in Figure 2. The ROC analysis of calprotectin revealed an area under the curve (AUC) of 0.97, and the calprotectin/creatinine ratio had an AUC of 0.97 as well. FENa performed worse with an AUC of 0.52. Proteinuria (AUC, 0.82) was more accurate than FENa but worse than calprotectin and the calprotectin/creatinine ratio. ROC analysis was used to determine the optimal cut off value for the differentiation of prerenal and intrinsic AKI. As presented in Table 4, calprotectin achieved a sensitivity of 92.3% and a specificity of 97.1% for a threshold value of 300 ng/ml. The resulting positive and negative predictive values were 98.0% and 89.2%, respectively. Figure 3 describes the accuracy of participants' assignment to prerenal and intrinsic AKI on the basis of the threshold of 300 ng/ml. Calprotectin/creatinine ratio achieved the same accuracy parameters with a cutoff value of 500. After exclusion of all cases with urinary tract infection, the ROC curve's AUC was 0.95 for calprotectin and 0.95 for the calprotectin/creatinine ratio. Specificity remained 97.1%, and sensitivity decreased slightly to 89.7% for both parameters. Accordingly, the positive predictive value decreased slightly to 96.3%, whereas the negative predictive value increased to 91.7%.
Figure 2.
(A and B) Diagnostic accuracy of urinary calprotectin (area under the curve [AUC], 0.97) (A) and urinary calprotectin/creatinine ratio (AUC, 0.97) (B) for the detection of intrinsic acute renal failure. (C and D) Accuracy of urinary calprotectin (C) and of calprotectin/creatinine ratio (D) after exclusion of subjects with urinary tract infection (UTI) (AUC, 0.95 and 0.95). (E and F) Accuracy of the fractional excretion of sodium (FENa; AUC, 0.52) (E) and of proteinuria (AUC, 0.82) (F). Shown are receiver operating characteristic curves. Diagonal scattered lines indicate differentiation of prerenal and intrinsic acute kidney injury by chance.
Table 4.
Performance of urinary calprotectin and calprotectin/creatinine ratio for the detection of intrarenal acute kidney injury in the overall study population and after exclusion of urinary tract infection
| Overall Study Population |
Subjects without Urinary Tract Infection |
|||
|---|---|---|---|---|
| Calprotectin (ng/ml) (Cutoff, 300) | Calprotectin (ng/ml)/Creatinine (g/L) (Cutoff, 500) | Calprotectin (ng/ml) (Cutoff, 300) | Calprotectin (ng/ml)/Creatinine (g/L) (Cutoff, 500) | |
| Sensitivity (%) | 92.3% | 92.3% | 89.7% | 89.7% |
| Specificity (%) | 97.1% | 97.1% | 97.1% | 97.1% |
| Positive predictive value (%) | 98.0% | 98.0% | 96.3% | 96.3% |
| Negative predictive value (%) | 89.2% | 89.2% | 91.7% | 91.7% |
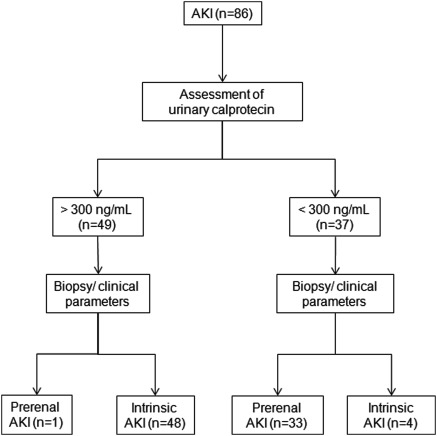
Figure 3.
Study flow diagram. Diagnostic performance of urinary calprotectin using a cut off level of 300 ng/ml for the assignment to prerenal and intrinsic acute kidney injury (AKI).
Immunohistological Examinations with the S100A8/A9 Antibody
Immunohistological examinations with the S100A8/A9 antibody were found to be positive in pyelonephritis and lupus nephritis. It was negative, however, in the patient who fulfilled the clinical criteria of prerenal AKI. A detailed description of the histologic findings is provided in the legend of Figure 4.
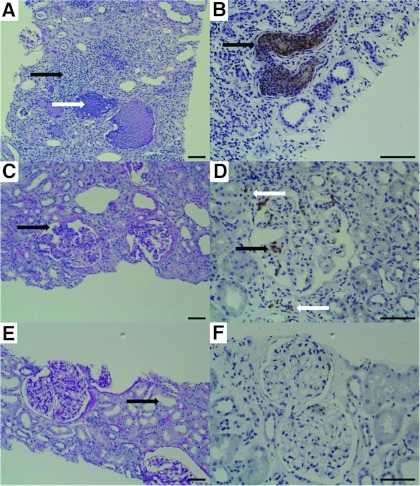
Figure 4.
Histologic findings in periodic acid Schiff reaction (PAS) staining (×100; scale bars, 100 μm) and immunohistochemistry with an antibody to the S100A8/A9 heterocomplex (×200, brown; scale bars, 100 μm) in pyelonephritis (A and B; urinary calprotectin, 8232 ng/ml), lupus nephritis (C and D; urinary calprotectin, 1422 ng/ml), and a patient fulfilling the clinical criteria of prerenal disease (E and F; urinary calprotectin 3 ng/ml). (A) PAS staining in pyelonephritis reveals marked tubulointerstitial nephritis, patchy interstitial inflammation by polymorphonuclear leukocytes (black arrow), and acute tubular injury with a dense inflammatory infiltrate (white arrow). (B) Neutrophils occurring in tubular microabscesses are strongly positive in the calprotectin staining (black arrow). (C) PAS staining in lupus nephritis shows two glomeruli with mild to moderate mesangial hypercellularity segmentally involving the glomerular tuft and increasing the mesangial matrix. Moreover, there is a small segmental lesion with endocapillary proliferation (black arrow). Tubular injury is mild with focal atrophy of tubular epithelial cells. (D) Calprotectin staining evoked positive signals in both the intraglomerular space (black arrow) and, to a lesser extent, in the interstitium (white arrows). (E) PAS staining in the kidney fulfilling the clinical criteria of prerenal acute kidney injury displays glomeruli without any histologic abnormalities. The glomerular tuft is normocellular, the glomerular capillaries are fully patent. Discrete tubular findings with mild widening of the tubular lumina (black arrow). (F) Calprotectin staining is negative.
Discussion
These findings show that urinary calprotectin may be a helpful tool for the differentiation of prerenal and intrinsic AKI. Whereas calprotectin levels in prerenal AKI are comparable with those of healthy subjects, high calprotectin levels indicate intrinsic disease.
Calprotectin is a calcium-binding complex of two proteins of the so-called S100 group (S100A8/S100A9). It is derived predominantly from neutrophils and, to a lesser extent, from monocytes (7). Moreover, the monomers S100A8 and S100A9 have been detected in urine and renal epithelial cells as well (8,9). In neutrophil cytoplasm it adds up to 60% of the cytosolic proteins (10). Calprotectin is an activator of the innate immune system. If a neutrophil granulocyte is stimulated by an invading microorganism, it releases preformed so-called “damage-associated molecular pattern proteins.” Calprotectin is one of these damage-associated molecular pattern proteins. It activates Toll-like receptor 4 (TLR4) and thereby amplifies inflammatory activity (11). In gastroenterology, fecal calprotectin concentration is used to differentiate between inflammatory bowel diseases (high calprotectin concentration) and merely functional disorders, such as irritable bowel syndrome (low calprotectin concentration) (12,13). Our findings show that prerenal AKI constitutes the renal analogue to irritable bowel syndrome. In both conditions there is a functional deficit despite intact epithelial structures leading to very low calprotectin concentrations. TLR4 is involved in the pathogenesis of several inflammatory renal diseases including glomerulonephritis, vasculitis, and interstitial nephritis (14–16). In ATN, the tubular injury leads to a secondary activation of the innate immune system: TLR4 is constitutively expressed in both proximal and distal tubules, the thin limb of the loop of Henle, and the collecting ducts. Expression is upregulated in these areas in ischemia reperfusion injury (17). Thus, calprotectin may be able to reflect all of the leading causes of intrinsic AKI. The immunohistological findings in Figure 4 provide insight in the local distribution of calprotectin in the kidney. The intensity of calprotectin signaling corresponded very well to the urinary calprotectin concentrations of >8000 ng/ml (pyelonephritis), 1400 ng/ml (lupus nephritis), and 3 ng/ml (prerenal disease).
Calprotectin has several characteristics that make it very attractive as a clinical marker. First, it is a measure of local inflammatory activity that seems to be unaffected by a variety of conditions that result in an elevation of systemic inflammation (13,18). Second, calprotectin shows an excellent stability at room temperature for up to as long as 1 week (10,19,20). The quantification is done using the inexpensive and easy ELISA technique. Hence, calprotectin is sensitive, specific, stable, and easily detectable.
The accuracy of urinary calprotectin in diagnosing intrinsic AKI was higher than that of current diagnostic markers like FENa or proteinuria. The relatively low accuracy of FENa may be explained primarily by the high percentage of patients with diuretics (43.7%), leading to high natriuresis even in prerenal AKI. The high percentage of patients with diuretic therapy in this study population, however, adequately reflects the situation in today's clinical practice, because the incidence of AKI increases with age and the presence of congestive heart failure. Interestingly, the accuracy of calprotectin was better than FENa, even if FENa was selectively regarded in patients without diuretic therapy. Because proteinuria is not affected by diuretic therapy, this marker was more accurate in diagnosing intrinsic AKI in our study population. Future studies on the use of calprotectin in AKI should include a comparison with the diagnostic performance of FEUrea. For logistical reasons, urine samples were not collected daily but only thrice weekly in this study. Thus, the temporal association between urine sample and maximal AKIN stage is not completely accurate. Moreover, we performed a single time assessment of urinary calprotectin. We will need longitudinal studies on the time-dependent development of calprotectin in the phases of initiation, maintenance, and recovery of AKI.
We regarded the large variability of urine concentration as a potential bias to the accuracy of urinary calprotectin in the differential diagnosis of AKI. Therefore, we calculated the urinary calprotectin/creatinine ratio. The accuracy of this ratio, however, was not superior to that of urinary calprotectin alone. ROC analysis revealed that a calprotectin concentration of 300 ng/ml was the optimal cut off value. In the prerenal group, only one out of 34 subjects had a higher urinary calprotectin. In the intrinsic group, four of the 52 patients had calprotectin levels lower than 300 ng/ml. Interestingly, two of these patients were classified as intrinsic AKI because of marginal histologic findings, although they fulfilled the clinical criteria of prerenal disease.
There are two potential limitations of urinary calprotectin that have to be discussed. First, the effect of different entities of chronic kidney disease on calprotectin needs further investigation. In this study population, calprotectin was able to differentiate prerenal and intrinsic AKI even in case of “acute on chronic” renal failure. This is evident in case of, for example, nephrosclerosis as the underlying chronic disease, but it appears probable that chronic glomerulonephritis, for example, leads to increased levels of urinary calprotectin independent of a potential acute prerenal deterioration of renal function. Second, urinary tract infection may increase urinary calprotectin, because it goes along with leukocyturia. Thus, urinary tract infections with or without coincidental signs of dehydration, being a cause of deteriorating renal function, will be classified as intrarenal AKI. Nevertheless, exclusion of subjects with urinary tract infection did not reduce specificity and evoked only a marginal decrease of sensitivity (Table 3). Hence, urinary tract infections were no relevant bias in this study population. In the healthy control group, however, three subjects with no history of renal disease had calprotectin concentrations of >300 ng/ml, including one person with a concentration of even 1413 ng/ml. All of these persons were female. All of the male probands had urinary calprotectin levels of <150 ng/ml. The two most obvious reasons are asymptomatic bacteriuria and potential (peri-) menstrual blood contaminations. To our mind, a low urinary calprotectin concentration largely excludes a severe intrarenal damage in both genders. In case of a high calprotectin concentration, menstrual bleeding and urinary tract infection should be ruled out.
Urinary calprotectin may be a helpful diagnostic tool in the clinical approach to AKI. The first step in daily clinical practice consists of exclusion of urinary tract obstruction by ultrasound. Afterwards, patients with low urinary calprotectin levels have a high probability of prerenal AKI and need immediate fluid repletion. In absence of urinary tract infection, high calprotectin levels indicate intrinsic renal failure. Patients might have to undergo renal biopsy and need specific treatment. Interestingly, there is a semiquantitative calprotectin test for stool, providing a result within minutes. From a technical point of view, adaptation of this test to the required cut off concentration may be performed easily. Thus, an assignment of AKI to a prerenal or intrinsic cause would be possible at the initial examination in the emergency room by dipstick test. In summary, these findings show that urinary calprotectin may be a very promising marker in the differential diagnosis of AKI.
Disclosures
None.
Acknowledgments
The study was funded by the German Research Foundation (Research Unit FOR1368).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
See related editorial, “Is It Time to Evolve Past the Prenatal Azotemia versus Acute Tubular Necrosis Classification,” on pages 2332–2334.
References
- 1. Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Schrier RW: Need to intervene in established acute renal failure. J Am Soc Nephrol 15: 2756–2758, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Steiner RW: Interpreting the fractional excretion of sodium. Am J Med 77: 699–702, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Pepin MN, Bouchard J, Legault L, Ethier J: Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 50: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY: Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 32: 219–229, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P: Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer's patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut 34: 1357–1363, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillay SN, Asplin JR, Coe FL: Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol 275: F255–F261, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Thurgood LA, Ryall RL: Proteomic analysis of proteins selectively associated with hydroxyapatite, brushite, and uric acid crystals precipitated from human urine. J Proteome Res 9: 5402–5412 [DOI] [PubMed] [Google Scholar]
- 10. Poullis A, Foster R, Northfield TC, Mendall MA: Review article: Faecal markers in the assessment of activity in inflammatory bowel disease. Aliment Pharmacol Ther 16: 675–681, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J: Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13: 1042–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Foell D, Wittkowski H, Roth J: Monitoring disease activity by stool analyses: From occult blood to molecular markers of intestinal inflammation and damage. Gut 58: 859–868, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Gisbert JP, McNicholl AG: Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis 41: 56–66, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Giorgini A, Brown HJ, Sacks SH, Robson MG: Toll-like receptor 4 stimulation triggers crescentic glomerulonephritis by multiple mechanisms including a direct effect on renal cells. Am J Pathol 177: 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soylu A, Kizildag S, Kavukcu S, Cingoz S, Turkmen M, Demir BK, Sakizli M: TLR-2 Arg753Gln, TLR-4 Asp299Gly, and TLR-4 Thr399Ile polymorphisms in Henoch-Schonlein purpura with and without renal involvement. Rheumatol Int 30: 667–670 [DOI] [PubMed] [Google Scholar]
- 16. Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J: The role of Toll-like receptors in renal diseases. Nat Rev Nephrol 6: 224–235 [DOI] [PubMed] [Google Scholar]
- 17. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C: In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I: Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 123: 450–460, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Lundberg JO, Hellstrom PM, Fagerhol MK, Weitzberg E, Roseth AG: Technology insight: Calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2: 96–102, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Angriman I, Scarpa M, D'Inca R, Basso D, Ruffolo C, Polese L, Sturniolo GC, D'Amico DF, Plebani M: Enzymes in feces: Useful markers of chronic inflammatory bowel disease. Clin Chim Acta 381: 63–68, 2007 [DOI] [PubMed] [Google Scholar]