Abstract
Summary
Background and objectives
Hypoalbuminemia and hyperphosphatemia have been shown to be strong predictors of mortality in dialysis patients that might not be independent from each other. We prospectively investigated the relationship and interaction between serum albumin and phosphorus with all-cause mortality in an inception cohort of incident dialysis patients.
Design, setting, participants, & measurements
We followed 235 incident dialysis patients in a prospective single-center cohort study (INVOR study) applying a time-dependent Cox proportional hazards model using all measured laboratory values (2887 albumin and 10306 phosphorus values).
Results
Eighty-two patients (35%) died during a median follow-up of 35.1 months. Albumin was inversely associated with mortality (hazard ratio [95% confidence interval]: 0.23 [0.14 to 0.36]; P < 0.001), whereas higher phosphorus concentrations showed a trend to an increasing risk for mortality (hazard ratio 1.57 [95% confidence interval 0.97 to 2.54]; P = 0.07). Importantly, we observed a significant interaction between albumin and phosphorus (P = 0.01). The lowest risk was found with concurrent low phosphorus and high albumin values, whereas risk was increased with either concurrent low phosphorus and low albumin values or high phosphorus and high albumin values.
Conclusions
In incident dialysis patients the associations of serum phosphorus and albumin concentrations with mortality are modified by each other over time. Phosphorus-lowering interventions that concomitantly can cause a fall in serum albumin level may be harmful and warrant additional studies. If confirmed, epidemiologic studies and therapeutic guidelines aiming for target values should consider this interplay.
Introduction
Patients with ESRD are at significantly increased mortality risk compared with the age-adjusted general population (1,2). A variety of risk factors have been found to be associated with this increased risk. Although the traditional cardiovascular risk factors do not exclusively explain the excessively increased mortality (3,4), markers of mineral and bone disorders and measures of protein-energy wasting are associated with an increased risk (5–9). Among these, hypoalbuminemia (10,11) and hyperphosphatemia (12,13) are both well established predictors of all-cause mortality in dialysis patients.
Prevention and reduction of overt hyperphosphatemia by the restriction of dietary phosphorus intake and by administering oral phosphate binders are cornerstones in the management of ESRD patients (14,15). The recently published Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that elevated serum phosphorus concentrations be lowered toward the normal range (16). Restriction of dietary phosphorus intake, however, requires a reduction in oral protein intake, as protein-rich foods are the main source of dietary phosphorus (17). On the other hand, lowering protein intake can lead to malnutrition and protein-energy wasting and thereby increasing mortality in ESRD patients (6,9). Hypoalbuminemia is the most commonly used surrogate parameter for protein-energy wasting in daily clinical practice, although it has also been shown to be a marker for inflammation (18,19).
There are only limited prospective data concurrently evaluating the relationship between the two biomarkers albumin and phosphorus as time-varying parameters and survival outcomes over a long-term observation period in incident dialysis patients (20–22). Therefore, in this study we aimed to investigate the association of albumin and serum phosphorus as single parameters as well as their interaction with all-cause mortality in a well characterized inception cohort of incident dialysis patients who were followed for up to more than 7 years. We hypothesized that albumin and phosphorus interact in their association with high mortality and thereby attenuate the single effects due to their seemingly opposing associations with survival. Most importantly, this investigation is not based on a single measurement of these parameters at baseline but makes use of all measured laboratory values during the entire observation period, providing a deep granularity of the data.
Materials and Methods
Patient Population
The INVOR Study (Study of Incident Dialysis Patients in Vorarlberg) is a single-center, prospective, observational cohort study of incident hemodialysis and peritoneal dialysis patients in Vorarlberg, the westernmost state of Austria with approximately 400,000 inhabitants. As described recently (23,24), all incident dialysis patients starting chronic dialysis treatment between May 1, 2000, and April 30, 2006, were enrolled. Patients having a malignant tumor at initiation of dialysis were excluded. A total of 235 patients were included in the study and followed for a maximum of almost 7.5 years until December 31, 2007, or until the patient died. Because mineral metabolism, cardiovascular risk, and overall survival considerably change after transplantation, the observations have been censored at transplantation. Four patients were lost to follow-up, three of them regained renal function and the other one moved away. The study was approved by the local ethics committees and all patients enrolled in the study provided written informed consent.
Data Description
As described recently (23,24), clinical, laboratory, and medication data were collected prospectively starting at the time of initiation of dialysis. Type of and change in dialysis therapy (hemodialysis [HD] or peritoneal dialysis [PD]) were recorded and considered as time-dependent treatment status for data analysis. All-cause mortality data including death causes (autopsy-proven in 33%) were evaluated. Laboratory parameters were measured in a central laboratory and recorded continuously during the study period. Blood for laboratory parameters was collected immediately before dialysis at different time intervals, most of them once to twice monthly (phosphorus, hemoglobin, creatinine, and calcium) and a few of them every 3 months (albumin, C-reactive protein [CRP], and immunoreactive parathyroid hormone [iPTH]). Patients had up to 52 measurements for albumin (median 11) and up to 188 phosphorus measurements (median 33) during the follow-up period. Overall, this resulted in 2887 albumin and 10,306 phosphorus measurements during the entire observation period, which were used in the time-dependent Cox regression model described below.
Statistical Analyses
The primary outcome of interest was all-cause mortality. To determine the effect of phosphorus and albumin concentrations and their interaction on mortality, a time-dependent Cox proportional hazards model (25) was used, allowing all variables to vary over different measurements during the entire observation period for each patient. That is, each time span between two successive measurements enters the model independently. Each covariate that entered the model was updated at the time it was measured and modeled in a time-dependent fashion. If not all variables were measured at a particular date, the values measured at the last observation of this variable were substituted for the respective missing values (“last observation carried forward”). The proportional hazards assumption was tested for each model by testing for zero slopes of scaled Schoenfeld residuals. The assumption of linear effects was tested in models excluding the interaction terms via nonlinear P splines with three degrees of freedom (26), which is a linear combination of cubic functions.
The final Cox proportional hazards models included linearly modeled effects of albumin, phosphorus, and albumin-phosphorus interaction, adjusted for age, gender, and type of dialysis therapy (simple adjustment model) as well as extended adjustment models, which additionally included adjustment for CRP, hemoglobin, calcium, and iPTH. For this extended adjustment model, a significance region was calculated (27) to determine for which values of albumin the effect of phosphorus on mortality was significantly different from zero and vice versa. This time-dependent modeling of interaction effects reflects the assumed effect modification of albumin on phosphorus and vice versa during the complete observation period.
Because low albumin values apart from malnutrition might also reflect inflammation, additional interaction models were calculated to improve insight into the nature of effect modification (phosphorus*CRP and phosphorus*creatinine). Further subgroup analysis was performed stratifying for the type of dialysis treatment (HD, PD). The correlation of albumin and phosphorus was evaluated using Spearman's correlation coefficient as well as linear mixed effect models accounting for the repeated measures.
All analyses were conducted in R (28) using the package “survival.”
Results
Patient Characteristics
Table 1 presents the baseline demographic, clinical, and laboratory characteristics of all 235 incident dialysis patients at the time dialysis treatment was started. Patients were followed for a median of 35.1 months ranging from 24 days to approximately 7.5 years. During this observation period 82 patients died (35%). Mortality rate was independent of gender (35% for both men and women). About 92% of all patients were treated with phosphate binders at least once during the observation period; 89% received calcium-based binders and 66% received calcitriol. The repeated measurements of albumin and phosphorus per patient were significantly but only weakly correlated (Spearman r = 0.15, P < 0.001).
Table 1.
Clinical characteristics of patients at baseline and during follow-up
| All Patients (n = 235) | |
|---|---|
| Characteristics at baseline | |
| age (years) | 61.7 ± 14.0 |
| gender (male/female), n (%) | 146 (62.1)/89 (37.9) |
| body mass indexa | 26.1 ± 4.5 |
| Start of dialysis with | |
| hemodialysis | 197 (83.8) |
| peritoneal dialysis | 38 (16.2) |
| Diabetes mellitus | 82 (34.9) |
| Systolic BP (mmHg) | 154.0 ± 22.7 |
| Diastolic BP (mmHg) | 83.0 ± 12.3 |
| Mean ± SD | 25th; 50th; 75th Percentile | |
|---|---|---|
| Laboratory parameters at baseline | ||
| albumin (g/dl) | 3.71 ± 0.65 | 3.30; 3.70; 4.20 |
| phosphorus (mmol/L) | 1.98 ± 0.61 | 1.57; 1.94; 2.30 |
| calcium (mmol/L) | 2.12 ± 0.27 | 1.98; 2.14; 2.30 |
| iPTH (pg/ml) | 350.5 ± 264.9 | 156.5; 287.2; 468.7 |
| hemoglobin (g/dl) | 11.17 ± 1.72 | 10.10; 11.30; 12.30 |
| creatinine (mg/dl) | 7.28 ± 2.64 | 5.50; 6.80; 8.60 |
| C-reactive protein (mg/dl) | 3.24 ± 5.34 | 0.30; 0.98; 3.00 |
| bicarbonate (mmol/L) | 21.0 ± 3.6 | 18.7; 21.0; 23.4 |
| ferritin (ng/ml) | 174.3 ± 206.5 | 44.0; 111.0; 234.0 |
| total cholesterol (mg/dl) | 189.8 ± 51.0 | 152.0; 184.0; 219.5 |
| leukocytes (g/L) | 8.18 ± 3.29 | 6.10; 7.40; 9.90 |
| Kt/V | 1.29 ± 0.41 | 1.10; 1.20; 1.43 |
| Comorbidities at baseline | |
| CADb | 40 (17.0) |
| CVDc | 70 (29.8) |
| PADd | 40 (17.0) |
| Follow-up | |
| follow-up time (months)e | 38.9 ± 23.2 |
| transplantation | 58 (24.7) |
| all-cause mortality | 82 (34.9) |
| Medications during follow-up | |
| phosphate binder | 217 (92.3) |
| calcium-based | 209 (88.9) |
| aluminum-based | 22 (9.4) |
| sevelamer-HCl | 125 (53.2) |
| erythropoietin | 234 (99.6) |
| iron supplements | 204 (86.8) |
| calcitriol | 155 (66) |
Values are mean ± SD or number with percentage in parentheses. iPTH, immunoreactive parathyroid hormone.
Calculated as weight in kilograms divided by height in meters squared (kg/m2).
Coronary artery disease (CAD): myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), aortocoronary bypass (ACBP).
Cardiovascular disease (CVD): myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), aortocoronary bypass (ACBP), coronary artery stenosis ≥50%, ischemic cerebral infarction, transient ischemic attack (TIA)/prolonged reversible ischemic neurological deficit (PRIND).
PAD (peripheral arterial disease): arterial stenosis, percutaneous transluminal angioplasty (PTA), peripheral bypass, amputation.
Follow-up time was calculated as the time from the start of dialysis until the patient died or the end of the observation period (December 31, 2007) was reached.
Associations between Albumin, Phosphorus, and All-Cause Mortality
Since linearity and proportional hazards assumptions held in all models, linear effects were estimated in Cox proportional hazards models. The corresponding results for the simple model (adjusted for age, gender, type of dialysis therapy) as well as the extended model (additionally adjusted for CRP, hemoglobin, calcium and iPTH) are shown in Table 2. Without the interaction term for albumin and phosphorus, phosphorus showed a positive trend toward increased overall mortality with higher values in the simple model (hazard ratio [HR] = 1.47, P = 0.08) and borderline significance in the extended model (HR 1.57; P = 0.07). In contrast, higher albumin concentrations were associated with a lower mortality risk in a highly significant manner in the simple adjustment model (HR = 0.19, P < 0.001). Adjusting for additional confounders in the extended model did not change the effect of albumin (HR = 0.23, P < 0.001).
Table 2.
Cox regression analysis
| Univariate |
Simple Adjustmenta |
Extended Adjustmentb |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Including only main effects of phosphorus and albumin | ||||||
| phosphorus (mmol/L) | 1.09 (0.75 to 1.58) | 0.67 | 1.47 (0.96 to 2.25) | 0.08 | 1.57 (0.97 to 2.54) | 0.07 |
| albumin (g/dl) | 0.21 (0.15 to 0.29) | <0.001 | 0.19 (0.13 to 0.28) | <0.001 | 0.23 (0.14 to 0.36) | <0.001 |
| Including main effects of phosphorus and albumin + interaction effect | ||||||
| phosphorus-albumin | 0.004 | 0.01 | ||||
Hazard ratios (HRs), 95% confidence intervals (CIs), and P-values of continuous and time-varying phosphorus and albumin concentrations as well as of their interaction term each in a univariate Cox model, in a simple adjustment model (adjusted for age, gender, and type of dialysis therapy) and an extended adjustment model (adjusted for age, gender, type of dialysis therapy, C-reactive protein, hemoglobin, calcium, and immunoreactive parathyroid hormone [iPTH]) on all-cause mortality risk.
Adjusted for age, gender, type of dialysis therapy.
Adjusted for age, gender, type of dialysis therapy, C-reactive protein, hemoglobin, calcium, and iPTH.
Interaction between Albumin and Phosphorus and Their Association with All-Cause Mortality
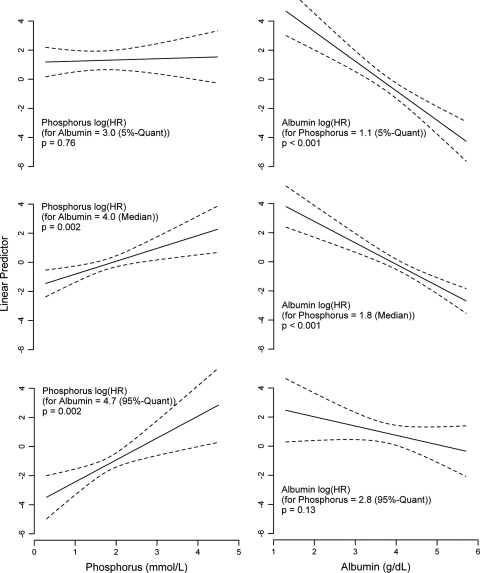
Albumin and phosphorus showed a clear and significant interaction, in both the simple (P = 0.004) and the extended adjustment (P = 0.01) models. Because the effect estimates for the albumin and phosphorus main effects are not interpretable without taking into account the interaction effect, phosphorus effects are shown for specific fixed quantiles of albumin and vice versa (Figure 1). For albumin values higher than 3.5 g/dl (significance region), increasing phosphorus concentrations were significantly associated with an increased mortality risk, while this association was not found at concurrently lower albumin values. The analysis of varying albumin values at simultaneously fixed phosphorus quantiles yielded a linear inverse association between albumin and mortality risk with attenuation of this effect at very high phosphorus concentrations. For phosphorus concentrations higher than 2.7 mmol/L, greater albumin values were not associated with an additional decrease in mortality risk.
Figure 1.
Cox regression results for the extended adjustment model. The y-axis shows the log hazard ratios for varying values of phosphorus with their corresponding P-values, keeping the albumin values fixed at specific values (5%, 50%, and 95% quantiles [Quant]; left panels) as well as the log hazard ratios (HRs) with P-values for varying values of albumin, keeping the phosphorus values fixed at specific values (5%, 50%, and 95% quantiles; right panels).
We could not find a significant interaction of phosphorus with CRP (P = 0.36 with log-transformed CRP) or creatinine (P = 0.17). However, the additional analyses suggest that the albumin effect was rather triggered by malnutrition than inflammation (Supplementary Material). Additional subgroup analysis revealed that the albumin-phosphorus interaction was only present in hemodialysis patients but could not be found in peritoneal dialysis patients.
Discussion
In the study at hand, we observed a significant interaction between time-varying albumin and phosphorus concentrations in their association with all-cause mortality in incident dialysis patients using a time-dependent multiple Cox proportional hazards analysis. Our data confirm earlier observations (20–22) that found an association between time-varying serum albumin and phosphorus and all-cause mortality and extend these findings by a new and important issue: the significant effect modification of one predictive variable on the other with regard to mortality.
Lower serum albumin has been shown to be independently associated with higher mortality in prevalent dialysis patients (10,29–32). Our data corroborate these studies, but in contrast to earlier reports in prevalent dialysis patients with a single measurement of albumin levels, we determined the mortality risk associated with serum albumin in incident dialysis patients using all available time-varying values. Thus, not only a single baseline measurement is used but the full spectrum of all measurements during the entire follow-up. This enables a better appraisal of the nutritional situation of each patient over time. In a large U.S. 2-year observational registry study, Kalantar-Zadeh et al. found a significantly increased all-cause and cardiovascular mortality with trimonthly varying serum albumin values <3.8 g/dl and estimated that hypothetically some 10,000 deaths could be prevented in the United States if serum albumin would be corrected to concentrations >3.8 g/dl (10). Although analyses were adjusted for several confounding factors related to the malnutrition-inflammation complex, serum phosphorus and its effect modification on albumin and vice versa have not been considered. We are not aware of other studies that have examined the effects of time-varying serum albumin and phosphorus and their interaction on all-cause mortality with multivariate adjustment.
Increasing oral protein intake may help increase serum albumin concentrations. KDOQI guidelines recommend 1.2 g protein/kg body weight per day (33). Because dietary phosphorus and protein content are closely related, one reasonable concern is that an increase in protein intake would entail an increased phosphorus intake. Noori et al. recently found a strong correlation between dietary protein and phosphorus intake and a significantly increased 5-year mortality risk with increasing dietary phosphorus intake and increasing dietary phosphorus/protein ratio (34). In our analyses a greater albumin concentration with concurrently higher phosphorus concentration was also associated with increased all-cause mortality. Above a phosphorus value of 2.7 mmol/L the beneficial mortality-lowering effect of higher albumin values was no longer obvious. Consequently, a fortified or even additional protein intake in this constellation to avoid malnutrition might not be useful for dialysis patients, considering the significant and clinically relevant albumin-phosphorus interaction. This latter association might illustrate the countervailing risks and benefits of high protein intake, probably caused by worsening hyperphosphatemia, and once again underscores the important interaction between serum albumin and phosphorus.
On the other hand, the highest mortality risk in our study was found for concurrently low albumin and phosphorus concentrations. Therefore, in patients with moderate hyperphosphatemia and decreased serum albumin, dietary phosphorus restriction via reduced protein intake may even be rather harmful than helpful to the patient. In support of our findings, a 3-year observational study among 30,000 prevalent hemodialysis patients showed a significant correlation between serum phosphorus and dietary protein intake as estimated by the normalized protein nitrogen appearance (nPNA). It furthermore found a decreased multivariable-adjusted death rate with increasing nPNA and concomitantly decreasing serum phosphorus, whereas decreasing nPNA with a concordant rise in serum phosphorus or decrease in both parameters was associated with increased mortality (17). In the same study similar results were observed when serum albumin was used as a surrogate for the nutritional status in multiple regression analysis. Moreover, in a recent post hoc analysis from the Hemodialysis (HEMO) study a more liberal phosphate prescription was associated with greater survival in prevalent hemodialysis patients (35). A decline in serum phosphorus observed over time should prompt a nutritional evaluation, and, if serum albumin has concurrently fallen, the patient might face a greater mortality risk.
One possibility for overcoming the problem of concordant overall protein restriction and the risk of malnutrition with reduced dietary phosphorus intake would be to avoid phosphorus-rich ingredients that are added to processed foods and beverages (36,37). Reducing the consumption of such phosphorus additives might help decrease phosphorus intake without the risk of protein-energy wasting. Besides, grain-based vegetarian sources of protein have been recently shown to allow a better phosphorus homeostasis compared with a meat-based diet (38). The lowest mortality risk in our study was found for simultaneously low phosphorus and high albumin values. Therefore, careful use of phosphate binders might be appropriate in patients with normal serum albumin. Clearly, a potential benefit of a phosphorus-lowering strategy with phosphate binders and dietary phosphorus restriction based on the concomitant assessment of albumin and phosphorus has to be evaluated in a prospective trial to prove the hypothesis generated by our observational study.
Although interventional trial data demonstrating a survival benefit for lowering serum phosphorus are still missing, experimental data suggest possible causal mechanisms of adverse outcome. Elevated serum phosphorus has been shown to stimulate phenotypic transformation of vascular smooth muscle cells into osteoblast-like cells, promoting vascular calcification, causing endothelial cell dysfunction, increased arterial stiffness, and cardiac fibrosis (7,39–41). Moreover, in large observational studies hyperphosphatemia has consistently been found to be an independent risk factor for all-cause mortality with the highest association of all markers of mineral and bone disorder (7). In our multiple-adjusted analyses of time-varying serum phosphorus we determined a borderline significant association between increasing phosphorus concentrations and all-cause mortality. These findings are well in line with the recently published results of the ARO (Analyzing Data, Recognizing Excellence, and Optimizing Outcomes) CKD Research Initiative investigating the association between markers of mineral and bone disease and mortality in 7970 prevalent European hemodialysis patients, which also found a trend to an increased all-cause mortality for elevated phosphorus concentrations (>1.78 mmol/L) without reaching statistical significance when using an adjusted time-dependent analysis (42).
Hypoalbuminemia is not only caused by low protein intake and malnutrition, but also by chronic inflammation, which might explain part of the association between low serum albumin and increased all-cause mortality (19). Adjusting for CRP, we consistently found higher albumin values to have a risk-lowering effect. Investigation of albumin-phosphorus interaction and its association with all-cause mortality showed concordant low serum albumin and phosphorus to pose the highest risk in our incident dialysis cohort. This might be explained as follows: First, uremia-associated protein-energy malnutrition and inflammation, together also known as malnutrition-inflammation complex syndrome, are a strong indicator of increased mortality in dialysis patients (43). Therefore, low serum albumin might be the surrogate marker of malnutrition and inflammation, with concurrently low phosphorus as an additional marker of malnutrition due to low protein intake in this situation. Additional interaction models determining the effect modification of phosphorus by CRP and creatinine did not enable us to definitely specify whether the albumin effect was predominantly influenced by inflammation or nutritional status. However, these analyses rather suggest an alimentary effect, as the linear inverse association between creatinine values and mortality risk is attenuated at higher phosphorus concentrations and the mortality risk association with increasing phosphorus increases with higher creatinine values. Second, malnutrition and hypoalbuminemia have been shown to be associated with adynamic bone disease (44,45), and chronic inflammation has been found to decrease PTH (46,47) and bone turnover. Diminished release from bone may partially explain low phosphate levels in these situations.
Strengths and Limitations
There are limitations to this study. Because it is an observational study, no causal inference can be made from the study results. A further limitation is the relatively small sample size. Finally, no further nutritional parameters such as normalized PCR data or further inflammation markers were collected. Nevertheless, our study has several notable strengths. It is a single-center study with uniform laboratory measurements of high frequency and continuity collected over a long follow-up period. The prospective recruitment of all patients starting dialysis treatment over a period of 6 years in a clearly defined area allowed complete recruitment of all patients requiring renal replacement therapy with almost no loss to follow-up. We can therefore exclude the most important bias of cross-sectional studies with a mix of prevalent and incident cases and the resulting survival bias. All patients were treated by the same physicians during the whole observation period following current best practice guidelines. Moreover, our study characterizes the association between time-varying albumin and phosphorus concentrations and their interaction and all-cause mortality in an inception cohort of incident dialysis patients considering all measurements in this observation period in a time-dependent modeling.
Conclusions
Time-varying albumin and phosphorus values significantly interact in their association with all-cause mortality in incident dialysis patients. The lowest risk is found for simultaneously low phosphorus and high albumin values, whereas risk is increased for either concurrent low phosphorus and low albumin values or for high phosphorus and high albumin values. In patients with low serum albumin concentration therapeutic attempts should be aimed at hypoalbuminemia. The correction of serum phosphorus values seems to be less important in this situation. With albumin values within the normal range, reduction of hyperphosphatemia by the restriction of dietary phosphorus intake and by administering oral phosphate binders seems to be clinically meaningful. If confirmed in larger studies, decisions about phosphorus-lowering therapy should also take albumin values into account and epidemiologic studies and therapeutic guidelines aiming for target values should consider this interplay.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by a grant from Hans Drexel to the Vorarlberg Institute for Vascular Investigation and Treatment (VIVIT) and by a grant from the Austrian National Bank (Project 13662) to the Innsbruck Medical University.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Zoccali C: Cardiovascular risk in uraemic patients—Is it fully explained by classical risk factors? [Editorial]. Nephrol Dial Transplant 15: 454–457, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ: Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol 13: 1918–1927, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA: The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 19: 1092–1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD: Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis 42: 864–881, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, Hakim RM: Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 17: 363–371, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr, Kopple JD, Greenland S: Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 20: 1880–1888, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol 7: 728–736, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Wald R, Sarnak MJ, Tighiouart H, Cheung AK, Levey AS, Eknoyan G, Miskulin DC: Disordered mineral metabolism in hemodialysis patients: An analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis 52(3): 531–540, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Block G, Uribarri J, Coladonato JA, Fan SL, Cunningham J, Nolan CR, Qunibi WY, Lindberg JS: How should hyperphosphatemia be managed in dialysis patients? Semin Dial 15: 315–328, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Nolan CR, Qunibi WY: Treatment of hyperphosphatemia in patients with chronic kidney disease on maintenance hemodialysis. Kidney Int Suppl: S13–S20, 2005 [DOI] [PubMed] [Google Scholar]
- 16. KDIGO CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl S1–130, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Shinaberger CS, Greenland S, Kopple JD, Van WD, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 19. de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW: Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 19: 127–135, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT: The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: Association with mortality in dialysis patients. Am J Kidney Dis 46: 925–932, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez-Benot A, Martin-Malo A, Alvarez-Lara MA, Rodriguez M, Aljama P: Mild hyperphosphatemia and mortality in hemodialysis patients. Am J Kidney Dis 46: 68–77, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Sturm G, Lamina C, Zitt E, Lhotta K, Lins F, Freistätter O, Neyer U, Kronenberg F: Sex-specific association of time-varying hemoglobin values with mortality in incident dialysis patients. Nephrol Dial Transplant 25: 2715–2722, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Gouya G, Sturm G, Lamina C, Zitt E, Freistätter O, Struck J, Wolzt M, Knoll F, Lins F, Lhotta K, Neyer U, Kronenberg F: The association of mid-regional pro-adrenomedullin and mid-regional pro-atrial natriuretic peptide with mortality in an incident dialysis cohort. PLoS One 6: e17803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model, New York: Springer, 2008 [Google Scholar]
- 26. Marx BD, Eilers PHC: Generalized linear regression on sampled signals and curves: A P-spline approach. Technometrics 41: 1–13, 1999 [Google Scholar]
- 27. Aiken LS, West SG: Multiple Regression: Testing an Interpreting Interactions, Thousand Oaks, CA: SAGE Publications, 1991 [Google Scholar]
- 28. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2008 [Google Scholar]
- 29. Beddhu S, Kaysen GA, Yan G, Sarnak M, Agodoa L, Ornt D, Cheung AK: Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis 40: 721–727, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Iseki K, Kawazoe N, Fukiyama K: Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int 44: 115–119, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15: 458–482, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Owen WF, Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 35: S1–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD: Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol 5: 683–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lynch KE, Lynch R, Curhan GC, Brunelli SM: Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol 6: 620–629, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sherman RA, Mehta O: Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin J Am Soc Nephrol 4: 1370–1373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherman RA, Mehta O: Dietary phosphorus restriction in dialysis patients: Potential impact of processed meat, poultry, and fish products as protein sources. Am J Kidney Dis 54: 18–23, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H: Vascular calcification and inorganic phosphate. Am J Kidney Dis 38: S34–S37, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Amann K, Tornig J, Kugel B, Gross ML, Tyralla K, El-Shakmak A, Szabo A, Ritz E: Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int 63: 1296–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR: Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 20: 381–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC, on behalf of the ARO Investigators: Serum iPTH, calcium and phosphate, and the risk of mortality in a European hemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Andress DL: Adynamic bone in patients with chronic kidney disease. Kidney Int 73: 1345–1354, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Sanchez-Gonzalez MC, Lopez-Barea F, Bajo MA, Selgas R: Serum albumin levels, an additional factor implicated in hyperparathyroidism outcome in peritoneal dialysis: A prospective study with paired bone biopsies. Adv Perit Dial 22: 198–202, 2006 [PubMed] [Google Scholar]
- 46. Carlstedt E, Ridefelt P, Lind L, Rastad J: Interleukin-6 induced suppression of bovine parathyroid hormone secretion. Biosci Rep 19: 35–42, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Nielsen PK, Rasmussen AK, Butters R, Feldt-Rasmussen U, Bendtzen K, Diaz R, Brown EM, Olgaard K: Inhibition of PTH secretion by interleukin-1 beta in bovine parathyroid glands in vitro is associated with an up-regulation of the calcium-sensing receptor mRNA. Biochem Biophys Res Commun 238: 880–885, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.