Abstract
Summary
Background and objectives
The effect of increased fluid intake on kidney function is unclear. This study evaluates the relationship between urine volume and renal decline over 6 years in a large community-based cohort.
Design, setting, participants, & measurements
This prospective cohort study was undertaken in Canada from 2002 to 2008. We obtained 24-hour urine samples from adult participants with an estimated GFR (eGFR) ≥60ml/min per 1.73 m2 at study entry. Percentage annual change in eGFR from baseline was categorized as average decline <1% per year, between 1% and 4.9% (mild-to-moderate decline) or ≥5% (rapid decline).
Results
2148 participants provided valid 24-hour urine samples, grouped as <1 L/d (14.5%); 1 to 1.9 L/d (51.5%); 2 to 2.9 L/d (26.3%); and ≥3 L/d (7.7%). Baseline eGFR for each category of urine volume was 90, 88, 84, and 87 ml/min per 1.73 m2, respectively. Overall, eGFR declined by 1% per year, with 10% demonstrating rapid decline and 40% demonstrating mild-to-moderate decline. An inverse, graded relationship was evident between urine volume and eGFR decline: For each increasing category of 24-hour urine volume, percentage annual eGFR decline was progressively slower, from 1.3%, 1.0%, 0.8%, to 0.5%, respectively; P = 0.02. Compared with those with urine volume 1 to 1.9 L/d, those with urine volume ≥3 L/d were significantly less likely to demonstrate mild-to-moderate decline (adjusted odds ratio 0.66; 95% confidence interval 0.46 to 0.94) or rapid decline (adjusted odds ratio 0.46; 95% confidence interval 0.23 to 0.92); adjusted for age, gender, baseline eGFR, medication use for hypertension (including diuretics), proteinuria, diabetes, and cardiovascular disease.
Conclusions
In this community-based cohort, decline in kidney function was significantly slower in those with higher versus lower urine volume.
Introduction
The message to drink “at least 8 glasses of water a day” is widespread, despite a lack of evidence to support it (1–4). Two major medical journals, the British Medical Journal and The Lancet, rightly describe this “fluid craze” as a medical myth propagated by the popular press (1,5). Aside from preventing kidney stone formation (6,7), few studies have demonstrated a beneficial effect of increased fluid intake in adequately hydrated individuals (3,4). Previous research evaluating the relationship between fluid intake and kidney function has produced equivocal results; however, much of this research was conducted in animal models (8) or was restricted to patients with chronic kidney disease (CKD) (9). To date, no study has prospectively examined the effect of urine volume on kidney function in the general population. The Walkerton Health Study, a prospective community-based cohort study, provided a unique opportunity to evaluate the relationship between urine volume and renal decline over 7 years of follow-up.
Materials and Methods
Participants and Study Design
Participants were from the Walkerton Health Study (2002 to 2008, Canada), a prospective cohort study evaluating the long-term health sequelae from exposure to water contaminated with Escherichia coli O157 and Campylobacter. The design and methodology of the Walkerton Health Study are described elsewhere (10). Briefly, residents of the Walkerton area were invited to participate in the study and attend an annual clinic, irrespective of whether they had been exposed to contaminated water or developed an acute illness. The study sample has previously been shown to be representative of the target population (11). Written consent was obtained from all participants. Ethics approval was obtained from the University of Western Ontario's Research Ethics Board for Health Sciences. The present analysis was limited to adult participants ≥18 years who joined the study in 2002 or 2003 (n = 3154), provided a valid 24-hour urine samples at study entry, had an estimated GFR (eGFR) ≥60 ml/min per 1.73 m2 at baseline, and had at least two annual eGFR assessments.
Measures
Participants attended an annual clinic and completed a computer-assisted in-person interview that included questions on family history, past and current medication use, risk factors, and several physician-diagnosed health conditions, including diabetes and cardiovascular disease. Questionnaire development was guided by the U.S. Third National Health and Nutrition Survey (NHANES III) and Statistics Canada's National Population Health Survey. Height and weight were measured by trained study personnel.
Serum creatinine was assessed annually and eGFR was calculated using the abbreviated Modification of Diet in Renal Disease equation (12). Participants provided a 24-hour urine sample at study entry and again at years 5 and 7. Under- or overcollection of 24-hour urine samples were identified if 24-hour urine creatinine was less than or greater than the laboratory's reference range (7 to 25 mmol/d). Serum and urine creatinine were measured by the modified kinetic method of Jaffe using the Vitros 950 autoanalyzer (interassay coefficient of variation <4%) (Johnson and Johnson, Skillman, NJ). Based on evidence that 24-hour urine protein was systematically overestimated in samples with higher urine volumes (13), proteinuria was measured using a urine dipstick (Bayer 8SG Multistix) from a random spot urine sample obtained at study entry. In 2003, participants were offered an 8-hour fasting plasma glucose test and an oral glucose tolerance test (OGTT), if fasting glucose was 5.5 to 6.9 mmol/L, to increase the sensitivity of detecting diabetes.
Definitions
Participants were grouped into four categories of urine volume based on values at study entry (<1 L/d, 1 to 1.9 L/d, 2 to 2.9 L/d, and ≥3 L/d) (14). To calculate the rate of change in kidney function over time, we fitted an ordinary least-squares regression line to all eGFR measures for each participant. The slope of the regression line describes the rate of change in kidney function (eGFR) over time. While the distribution of the absolute annual change was strongly skewed to the right and susceptible to highly variable rates of annual decline in those with eGFR ≥90 ml/min per 1.73 m2, the distribution of percentage annual change ([slope/baseline eGFR]×100) was more symmetrically balanced around the median. Therefore, for our primary analysis, we defined change in renal function as percentage annual change in eGFR from baseline, categorized as average decline <1% per year (reference), between 1% and 4.9% per year (mild-to-moderate decline), or ≥5% (rapid decline) (15,16). Cardiovascular disease was defined as a self-reported, doctor-diagnosed heart attack, stroke, or congestive heart failure. Diabetes mellitus was defined using the current diagnostic criteria based on the presence of fasting plasma glucose ≥7.0 mmol/L, random or 2-hour post OGTT plasma glucose ≥11.1 mmol/L, a medical diagnosis of diabetes, and/or the use of oral hypoglycemic agents or insulin (17).
Statistical Analyses
Normally distributed data were summarized by the mean and standard deviation (SD), and skewed distributions by the median and interquartile range (IQR). Bivariable associations were assessed using the chi-squared test, ANOVA, or the Kruskal–Wallis test, as appropriate. We performed a multiple linear regression to compare renal decline across categories of urine volume while adjusting for confounders. We performed a multinomial logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for rapid decline and mild-to-moderate renal decline. The reference category for all analyses was urine volume 1 to 1.9 L/d, which contained the median value for the population. Age and gender were included in all models. Possible confounders considered for statistical adjustment included medication use for hypertension and risk factors for renal progression, including dipstick proteinuria (missing for 31), cardiovascular disease, diabetes, smoking, obese body mass index at study entry (≥30 kg/m2), and family history of hypertension (missing for 5), diabetes (missing for 2), or kidney failure (missing for 1). Information on risk factors was collected from multiple sources, and in the absence of any evidence, missing data on risk factors was set to zero (absent), which produced similar or more conservative results than a complete case analysis. Models were reduced using backward elimination at alpha = 0.15 (18,19) unless elimination changed the exposure-outcome association by >10% (19,20). Models were run with and without outliers, with no appreciable effect on results. To assess the potential impact of information bias from differential follow-up times, we examined the change in risk when (1) follow-up time was entered into the model, and (2) number of annual eGFR assessments was entered into the model. In addition, we examined whether the main results differed when renal decline was defined using a minimum of three versus two annual eGFR assessments. Additional sensitivity analyses were run to confirm that results were robust to differing variable definitions and modeling techniques.
Results
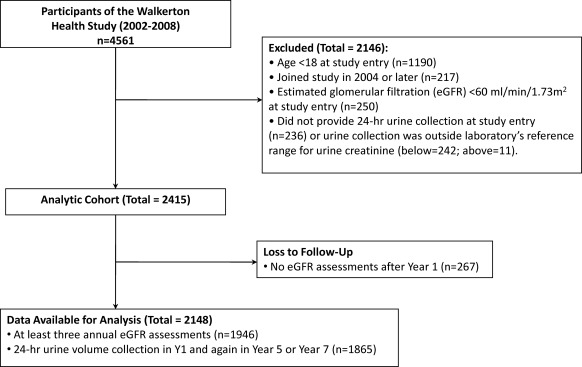
Of 3371 participants ≥18 years, 2148 provided valid 24-hour urine samples at study entry and had at least two annual eGFR assessments (Figure 1). Participants were 56% female, with an average age of 46 years. At baseline, median urine volume was 1.8 L/d (IQR 1.2 to 2.2) and mean eGFR was 87 ml/min per 1.73 m2. Participants received a median of 6 (IQR 4 to 7) annual eGFR assessments, and 82% received at least four annual assessments. Participant characteristics overall, and by 24-hour urine volume, are shown in Table 1. Those with higher urine volumes tended to be older and were more likely to be obese, smokers, or taking medication for hypertension, which included diuretics. Baseline eGFR for each category of urine volume (<1 L/d, 1 to 1.9 L/d, 2 to 2.9 L/d, and ≥3 L/d) was 90, 88, 84, and 87 ml/min per 1.73 m2, respectively. The median number of annual eGFR assessments (6) was similar across categories of urine volume.
Figure 1.
Flow of participants from recruitment to analysis.
Table 1.
Characteristics of participants, overall and by 24-hour urine volume at baseline
| Overall (n = 2148) | 24-hour Urine Volume |
Pa | ||||
|---|---|---|---|---|---|---|
| <1 L (n = 312) | 1 to 1.9 L (n = 1107) | 2 to 2.9 L (n = 564) | ≥3 L (n = 165) | |||
| Female gender | 1207 (56.2%) | 182 (58.3%) | 607 (54.8%) | 328 (58.2%) | 90 (54.5%) | 0.48 |
| Age at entry (years), mean (SD) | 46.3 (15.0) | 39.2 (14.5) | 46.0 (15.3) | 50.2 (13.6) | 48.4 (13.9) | <0.001 |
| Age at last follow-up (years), mean (SD) | 51.5 (15.3) | 44.3 (14.7) | 51.2 (15.6) | 55.6 (13.9) | 53.9 (14.0) | <0.001 |
| Years followed, median (IQR) | 5.7 (3.9, 6.0) | 5.6 (3.8, 6.0) | 5.7 (3.8, 6.0) | 5.8 (4.1, 6.0) | 5.8 (4.3, 6.1) | 0.001 |
| Number of eGFR assessments, median (IQR) | 6.0 (4, 7) | 6 (4, 7) | 6 (4, 7) | 6 (5, 7) | 7 (5, 7) | 0.001 |
| 24-hour urine volume (L), median (IQR) | 1.8 (1.2, 2.2) | 0.8 (0.65, 0.9) | 1.4 (1.2, 1.7) | 2.3 (2.2, 2.6) | 3.3 (3.1, 3.8) | <0.001 |
| eGFR ml/min per 1.73 m2, mean (SD) | 86.9 (15.2) | 90.3 (15.5) | 87.6 (15.6) | 83.8 (14.1) | 86.5 (13.9) | <0.001 |
| Serum creatinine (mg/dl), mean (SD) | 0.88 (0.15) | 0.88 (0.15) | 0.88 (0.15) | 0.89 (0.14) | 0.88 (0.14) | 0.61 |
| Dipstick protein ≥1 g/L | 41 (1.9%) | 9 (2.9%) | 18 (1.6%) | 11 (2.0%) | 3 (1.8%) | 0.02 |
| Obese (BMI ≥30 kg/m2) | 783 (36.5%) | 97 (31.1%) | 402 (36.3%) | 214 (37.9%) | 70 (42.4%) | 0.07 |
| Family history | ||||||
| hypertension | 955 (44.5%) | 128 (41.0%) | 492 (44.4%) | 255 (45.2%) | 80 (48.5%) | 0.44 |
| kidney failure | 52 (2.4%) | 10 (3.2%) | 21 (1.9%) | 13 (2.3%) | 8 (4.8%) | 0.10 |
| diabetes | 584 (27.2%) | 68 (21.8%) | 311 (28.1%) | 162 (28.7%) | 43 (26.1%) | 0.12 |
| Smoker | 970 (45.2%) | 113 (36.2%) | 502 (45.3%) | 271 (48.0%) | 84 (50.9%) | 0.003 |
| Medications for hypertension (including diuretics) | 601 (28.0%) | 50 (16.0%) | 306 (27.6%) | 189 (33.5%) | 56 (33.9%) | <0.001 |
| Diabetes | 270 (12.6%) | 31 (9.9%) | 132 (11.9%) | 83 (14.7%) | 24 (14.5%) | 0.15 |
| Cardiovascular disease | 175 (8.1%) | 15 (4.8%) | 92 (8.3%) | 57 (10.1%) | 11 (6.7%) | 0.05 |
Values are numbers (percentages) unless stated otherwise (n = 2148). IQR, interquartile range; eGFR, estimated GFR; BMI, body mass index.
Variables were compared using the chi-squared test, analysis of variance, or the Kruskal-Wallis test, as appropriate.
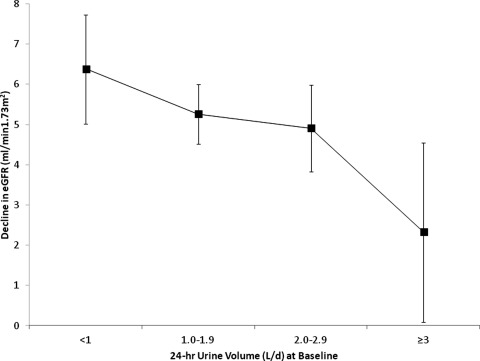
The annual change in eGFR was −0.83 ml/min per 1.73m2/yr (−0.81 ml/min per 1.73m2/yr for men and −0.84 ml/min per 1.73m2/yr for women). The annualized change in reciprocal serum creatinine was −0.005 (mg/dl)−1 per yr (−0.004 (mg/dl)−1 per yr for males and −0.006 (mg/dl)−1 per yr for females). Overall, eGFR declined by 1.0% per year, with 10% demonstrating rapid decline (eGFR decline ≥5%) and 40% demonstrating mild-to-moderate decline (eGFR decline between 1% and 4.9%). The difference in eGFR between the first and last assessment was 5.1 ml/min per 1.73 m2, and eGFR fell below 60 ml/min per 1.73 m2 for 9.6% of participants. Despite having similar levels of renal function at baseline (87 ml/min per 1.73 m2), eGFR fell below 60 ml/min per 1.73 m2 for twice as many participants with urine volume <3 L/d compared with those with urine volume ≥3 L/d (10.0% versus 5.5%; P = 0.07). An inverse, graded relationship was evident between urine volume and renal decline: For each increasing category of 24-hour urine volume (<1 L/d, 1 to 1.9 L/d, 2 to 2.9 L/d, and ≥3 L/d), percentage annual decline in eGFR was progressively slower (1.3%, 1.0%, 0.8%, and 0.5%, respectively; P = 0.02). A similar pattern was seen for reciprocal serum creatinine, where the annualized change was: −0.007, −0.006, −0.005, −0.002 (mg/dl)−1 per yr, respectively, P = 0.06. In terms of absolute eGFR decline, Figure 2 shows the difference in eGFR between the first and last assessment (median 5.7 years); the decrease in eGFR was nearly three times greater in those with the lowest versus highest urine volumes: 6.4 ml/min per 1.73 m2 versus 2.3 ml/min per 1.73 m2; P = 0.01. The increase in serum creatinine between the first and last assessment showed a similar pattern, with the increase becoming progressively smaller across increasing categories of urine volume: 0.03 mg/dl, 0.03 mg/dl, 0.02 mg/dl, and 0.01 mg/dl; respectively; P = 0.04. Overall, the age- and gender-adjusted average annual eGFR decline was 0.6 ml/min per 1.73 m2/yr slower in those with urine volume ≥3 L/d compared with those with smaller urine volumes (P = 0.01). As shown in Table 2, percentage annual decline in eGFR remained significantly slower in adults with urine volume ≥3 L/d compared with those in the reference category (1 to 1.9 L/d) after adjusting for age, gender, medication use for hypertension, dipstick protein, and cardiovascular disease (difference = 0.9%/yr; P = 0.02).
Figure 2.
Decline in kidney function between first and last assessment over 5.7 years (n = 2148). eGFR, estimated GFR.
Table 2.
Percentage annual change in kidney function in relation to 24-hour urine volume at baseline (n = 2145a)
| Age and Gender Adjusted |
Fully Adjusted |
|||
|---|---|---|---|---|
| Changeb | P | Changeb | P | |
| Intercept | −0.83 | 0.08 | −1.03 | 0.03 |
| Age | −0.01 | 0.01 | 0.01 | 0.50 |
| Gender (reference: male) | 0.06 | 0.76 | −0.08 | 0.71 |
| Urine volume (reference: 1 to 1.9 L/day) | ||||
| <1 L/day | −0.22 | 0.45 | −0.20 | 0.51 |
| 2 to 2.9 L/day | 0.11 | 0.64 | 0.12 | 0.62 |
| ≥3 L/day | 0.87 | 0.02 | 0.86 | 0.02 |
| Dipstick protein ≥1 g/L | −2.56 | <0.01 | ||
| Medications for hypertension (including diuretics) | −0.63 | 0.01 | ||
| Cardiovascular disease | −1.23 | 0.01 | ||
Excludes three outliers with strong positive skew in estimated GFR decline.
Multiple linear regression: models were reduced using backward elimination at alpha = 0.1518,19 unless elimination changed the association with urine volume by >10%;19,20 age and gender were forced into all models.
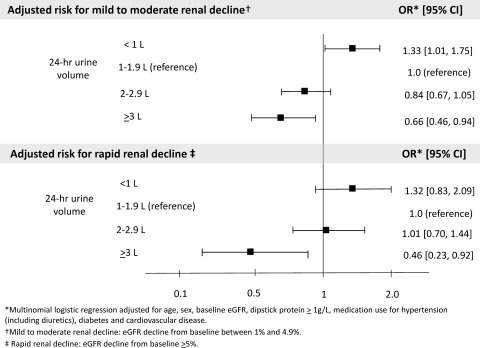
As shown in Table 3 and Figure 3, an inverse, graded relationship was evident between urine volume and the risk for mild-to-moderate and rapid renal decline. Compared with those with urine volume 1 to 1.9 L/d, those with urine volume ≥3 L/d were significantly less likely to demonstrate mild-to-moderate renal decline (adjusted OR = 0.66; 95% CI 0.46 to 0.94; P = 0.02) or rapid decline (adjusted OR = 0.46; 95% CI 0.23 to 0.92; P = 0.03).
Table 3.
Association between urine volume and renal decline in the general population (n = 2148)
| 24-hour Urine Volume | Mild to Moderate Renal Declinea |
Rapid Renal Declineb |
||||
|---|---|---|---|---|---|---|
| n = 861 | ORc (95% CI) |
n = 214 | ORc (95% CI) |
|||
| Age and Gender Adjusted | Multivariate Adjusted | Age and Gender Adjusted | Multivariate Adjusted | |||
| <1 L/day | 146 | 1.30 (1.00 to 1.70) | 1.33 (1.01 to 1.75) | 31 | 1.26 (0.81 to 1.97) | 1.32 (0.83 to 2.09) |
| 1 to 1.9 L/day | 454 | 1.0 | 1.0 | 111 | 1.0 | 1.0 |
| 2 to 2.9 L/day | 205 | 0.82 (0.66 to 1.02) | 0.84 (0.67 to 1.05) | 62 | 0.97 (0.68 to 1.36) | 1.01 (0.70 to 1.44) |
| ≥ 3 L/day | 56 | 0.67 (0.47 to 0.96) | 0.66 (0.46 to 0.94) | 10 | 0.48 (0.24 to 0.94) | 0.46 (0.23 to 0.92) |
OR, odds ratio; CI, confidence interval.
Mild to moderate renal decline: eGFR decline from baseline between 1% and 4.9%.
Rapid renal decline: estimated GFR decline from baseline ≥5%.
Odds ratios were estimated using multinomial regression.
Adjusted for age (in 1-year increments), gender, baseline estimated GFR, dipstick protein ≥1 g/L, medication use for hypertension (including diuretics), diabetes, and cardiovascular disease.
Figure 3.
Urine volume and risk for renal decline in the general population (n = 2148). eGFR, estimated GFR; OR, odds ratio; CI, confidence interval.
Sensitivity Analyses
Results of the regression analyses did not change after controlling for number of eGFR assessments, follow-up time, baseline eGFR, diabetes, and urine creatinine, or when renal decline was modeled as absolute versus percentage change or as the inverse of serum creatinine. Change in measured creatinine clearance between first and last assessment did not significantly associate with urine volume; however, measurement of serum creatinine was not timed to the 24-hour urine collection and therefore cannot be assumed to reflect the true clearance. When we restricted the analysis to those with a minimum of three annual eGFR assessments (n = 1865) and sustained polyuria (urine volume ≥3 L/d for at least two annual assessments; n = 95), percentage annual decline in eGFR was 0.6%/yr slower in those with sustained polyuria (P = 0.047). When 24-hour urine volume was averaged over the three follow-up assessments (intraclass correlation coefficient: 0.78; P < 0.001), the inverse relationship between averaged urine volume and renal decline remained the same; however, polyuria lost statistical significance (difference = 0.5%/yr; P = 0.28), and renal decline for those with the smallest urine volumes (<1 L/d) was significantly faster (difference = −0.7%/yr; P = 0.04) compared with the reference group (urine volume 1 to 1.9 L/d).
Discussion
In this prospective, community-based cohort study of adults with normal kidney function, decline in kidney function was significantly slower in those with higher versus lower urine volumes. The age- and gender-adjusted average annual decline in eGFR was 0.6 ml/min per 1.73 m2/yr slower for those with urine volume ≥3 L/d compared with those with smaller urine volumes. Over 10 years, this translates into a difference of 6 ml/min per 1.73 m2. The fastest rate of decline was observed for those with the smallest urine volumes (<1 L/d). Those with the largest urine volumes (≥3 L/d) were least likely to demonstrate mild-to-moderate renal decline or rapid decline. The inverse, graded relationship between urine volume and renal decline remained significant after controlling for age, gender, baseline eGFR, medication use for hypertension (including diuretics), dipstick proteinuria, diabetes, and cardiovascular disease.
Our findings are consistent with Strippoli et al., who recently reported a protective effect of higher self-reported fluid intake on kidney function in a large community-based cohort (21). These findings contrast with earlier studies showing no association or possible harm of greater fluid intake; however, much of this research was conducted in animal models (8) or CKD patients (9). In an observational study of CKD patients, higher urine volume and low urine osmolality were associated with faster decline in renal function (9). However, this association may be explained, in part, by greater diuretic use among those with higher urine volumes and the decreased ability of the kidneys to concentrate urine as function declines; and, thus, in CKD patients, high urine volume with low osmolality could be the result, not the cause, of faster decline (9,22). Evidence supporting the latter conclusion is provided by highly controlled studies in rats in which high hydration shows a persistent benefit in preserving renal function (8,22–25). Furthermore, an experimental study that randomized elderly men to increase fluid intake saw no change in eGFR over a 6-month follow-up (26), although a longer follow-up may be necessary to discern an appreciable effect on kidney function. In two small studies of acute water loading in healthy adults, a transient increase in albumin excretion was observed in one (27), and the other showed that the effect of fluid loading on GFR was dependent on whether participants had consumed a high-protein meal or were in a fasting state (28). Anastasio et al. underlined the acute nature of these experiments, which cannot be generalized to the effects of chronic high hydration (28). We previously noted an association between polyuria and 24-hour urine protein, but a subsequent investigation revealed that laboratory measurement error was the most likely explanation for these results (13,29).
The kidneys play a key role in regulating fluid balance, which is guided by tight homeostatic control of plasma osmolality. Whereas increased plasma osmolality stimulates the release of arginine vasopressin, causing the kidney to retain water and decrease urine production, decreased plasma osmolality inhibits the excretion of vasopressin, causing the kidney to increase urine output (30,31). In addition to regulating fluid balance, the kidneys filter waste from the blood and require a minimum obligate urine volume to remove the solute load (31,32). Kidneys may function more efficiently in the presence of an abundant supply of water (33). Higher fluid intake increases the clearance of sodium, urea, and osmoles (4,28,34), and high fluid intake is the most effective therapeutic measure to prevent kidney stones (6,7,35). If the kidneys are made to economize on water and produce more concentrated urine to maintain plasma osmolality, they may incur greater metabolic demand, as demonstrated in studies of rats (23,32,36,37).
Osmolar excretion and urine volume are affected by gender and race. Some argue that the greater food consumption among men compared with women, and their consequent higher daily osmolar loads and higher arginine vasopressin, might contribute to their increased susceptibility to kidney disease and salt-sensitive hypertension. Similarly, black individuals excrete similar daily osmolar loads as white individuals, but with less urine volume, which may, in part, explain their higher rate of kidney disease and salt-sensitive hypertension (38–40). Accelerated loss of kidney function can have a number of adverse consequences, including, but not limited to, poor BP control, various biochemical abnormalities, and higher levels of uraemic toxins (41).
We measured 24-hour urine volume in over 2000 individuals from the general community. To our knowledge, this is the largest study of its kind. We excluded participants with urine creatinine levels outside the specified laboratory reference ranges, minimizing over- or undercollection as a source of misclassification bias. We obtained accurate comorbidity and medication information through structured interviews, blood tests, and medical chart reviews; controlled for age, gender, presence of proteinuria, smoking, cardiovascular disease, and medication use for hypertension; and used a highly sensitive algorithm to detect the presence of diabetes. Estimated renal decline was calculated over a median follow-up time of 6 years. High variability in absolute change among those with eGFR ≥90 ml/min per 1.73 m2 made percentage change a better measure of renal decline in this general population sample (15). In addition, since percentage change adjusts for baseline eGFR, this method effectively adjusts for differences in baseline renal reserve, giving greater weight to small changes in the presence of reduced renal reserve and less weight to small changes at higher levels kidney function. However, results were similar when we analyzed the absolute annual change rather than percentage change. True decline in renal function may be underestimated when eGFR is used in place of measured GFR (42); however, this should not affect the difference between comparison groups. Although we did not measure fluid intake directly, urine volume is an excellent proxy since it is directly proportional to fluid intake (6). Moreover, we did not query participants on fluid consumption, and we do not know whether the type of fluid intake varied across categories of urine volume. For instance, a recent analysis of NHANES data showed a positive association between consumption of sugar-sweetened soft drinks and microalbuminuria (43). We did not measure use of lithium, which may be associated with polyuria; however, the prevalence of lithium use in the general population is extremely low and is unlikely to explain the inverse association between polyuria and renal decline. Finally, because the present study took place after the occurrence of an environmental disaster in which a substantial number of this study's participants were exposed to contaminated water containing E. coli O157 and Campylobacter, we considered the possibility that this exposure may have affected the results. However, the presence of acute illness at the time of the outbreak was not associated with polyuria or renal decline (data not shown).
In this prospectively followed community-based cohort, renal decline was significantly slower in adults with higher versus lower urine volume. Our results do not support aggressive fluid loading with its attendant risk for hyponatraemea (44,45) but are consistent with the popular belief in the benefits of a moderately increased fluid intake. This is the first large study of the general population to systematically study the relationship between urine volume and change in kidney function over time. Although this is an observational study and conclusions regarding causality must be cautious, these findings represent important initial evidence that higher fluid intake (2 to 3 L/d) may in fact benefit the kidney; however, it remains to be determined whether these results generalize to patients with CKD, the segment of the population where preservation of renal function is most crucial.
Disclosures
The funding source for this study was The Ontario Ministry of Health and Long-term Care. The funding source had no role in design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi disclosure.pdf (available, on request, from the corresponding author) and declare that (1) no authors have received support from any companies for the submitted work; (2) no authors have any relationships with any companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) no authors have nonfinancial interests that may be relevant to the submitted work.
Acknowledgments
An abstract of this work was presented at the North American Congress of Epidemiology, Montreal, Canada, June 21 to 24, 2011.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Fluid Intake for Kidney Disease Prevention: An Urban Myth,” on pages 2558–2560.
References
- 1. Vreeman RC, Carroll AE: Medical myths. BMJ 335: 1288–1289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wenzel UO, Hebert LA, Stahl RA, Krenz I: My doctor said I should drink a lot! Recommendations for fluid intake in patients with chronic kidney disease. Clin J Am Soc Nephrol 1: 344–346, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Valtin H: “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 x 8”? Am J Physiol Regul Integr Comp Physiol 283: R993–R1004, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Negoianu D, Goldfarb S: Just add water. J Am Soc Nephrol 19: 1041–1043, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Lette F, Dwyer JP: The fluid craze. Lancet 372: 782, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Siener R, Hesse A: Fluid intake and epidemiology of urolithiasis. Eur J Clin Nutr 57 Suppl 2: S47–S51, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Curhan GC, Willett WC, Speizer FE, Stampfer MJ: Beverage use and risk for kidney stones in women. Ann Intern Med 128: 534–540, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP: Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17: 2220–2227, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Hebert LA, Greene T, Levey A, Falkenhain ME, Klahr S: High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis 41: 962–971, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Clark WF, Sontrop JM, Macnab JJ, Salvadori M, Moist L, Suri R, Garg AX: Long term risk for hypertension, renal impairment, and cardiovascular disease after gastroenteritis from drinking water contaminated with Escherichia coli O157: H7: A prospective cohort study. BMJ 341: c6020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg AX, Macnab J, Clark W, Ray JG, Marshall JK, Suri RS, Devereaux PJ, Haynes B: Long-term health sequelae following E.coli and Campylobacter contamination of municipal water. Population sampling and assessing non-participation biases. Can J Public Health 96: 125–130, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 13. Ortho-Clinical Diagnostics Vitros UPRO Materials Sheet. No. J04089 EN Version 3.0. 2003. Rochester, New York, Ortho-Clinical Diagnostics, Inc [Google Scholar]
- 14. Sadjadi S: Genitourinary Disorders: Polyuria. In: The Merck Manuals: Online Medical Library, edited by Porter R, Kaplan J. Whitehouse Station, New Jersey, Merck Sharp & Dohme Corp., 2009 [Google Scholar]
- 15. Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark W, Macnab J, Sontrop J, Moist L, Jain A, Salvadori M, Suri R, Garg A: Dipstick proteinuria as a screening strategy to identify rapid renal decline. J Am Soc Nephrol, 2011, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26[Suppl 1]: S5–S20, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Mickey RM, Greenland S: The impact of confounder selection criteria on effect estimation. Am J Epidemiol 129: 125–137, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–936, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Greenland S, Rothman K: Introduction to stratified analysis. In: Modern Epidemiology, 2nd ed., edited by Rothman K, Greenland S. Philadelphia, PA, Lippincott-Raven, pp 253–279, 1998 [Google Scholar]
- 21. Strippoli GF, Craig JC, Rochtchina E, Flood VM, Wang JJ, Mitchell P: Fluid and nutrient intake and risk of chronic kidney disease. Nephrology (Carlton) 16: 326–334, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Torres VE: Water for ADPKD? Probably, Yes. J Am Soc Nephrol 17: 2089–2091, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Bouby N, Bachmann S, Bichet D, Bankir L: Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol- Renal Physiology 258: F973, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Grantham JJ: Therapy for polycystic kidney disease? It's water, stupid! J Am Soc Nephrol 19: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spigt MG, Knottnerus JA, Westerterp KR, Olde Rikkert MG, Schayck CP: The effects of 6 months of increased water intake on blood sodium, glomerular filtration rate, blood pressure, and quality of life in elderly (aged 55–75) men. J Am Geriatr Soc 54: 438–443, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Viberti GC, Mogensen CE, Keen H, Jacobsen FK, Jarrett RJ, Christensen CK: Urinary excretion of albumin in normal man: The effect of water loading. Scand J Clin Lab Invest 42: 147–157, 1982 [PubMed] [Google Scholar]
- 28. Anastasio P, Cirillo M, Spitali L, Frangiosa A, Pollastro RM, De Santo NG: Level of hydration and renal function in healthy humans. Kidney Int 60: 748–756, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Clark WF, Kortas C, Suri RS, Moist LM, Salvadori M, Weir MA, Garg AX: Excessive fluid intake as a novel cause of proteinuria. CMAJ 178: 173–175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramsay D: Homeostatic control of water balance. In: Hydration throughout Life, edited by Arnaud MJ. Montrouge, France, John Libbey Eurotext, pp 9–18, 1998 [Google Scholar]
- 31. Berl T: Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol 19: 1076–1078, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Popkin BM, D'Anci KE, Rosenberg IH: Water, hydration, and health. Nutrition Reviews 68: 439–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicolaidis S: Physiology of thirst. In: Hydration throughout Life, edited by Arnaud MJ. Montrouge, France, John Libbey Eurotext, pp 3–9, 1998 [Google Scholar]
- 34. Pitts RF: Physiology of the kidney and body fluids: An introductory text, Chicago, Illinois, Year Book Medical Publishers, 1974 [Google Scholar]
- 35. Consensus Conference Prevention and treatment of kidney stones. J Am Med Assoc 260: 977–981, 1988 [PubMed] [Google Scholar]
- 36. Bankir L, Bouby N, Trinh-Trang-Tan MM: Vasopressin-dependent kidney hypertrophy: role of urinary concentration in protein-induced hypertrophy and in the progression of chronic renal failure. Am J Kidney Dis 17: 661–665, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Sugiura T, Yamauchi A, Kitamura H, Matsuoka Y, Horio M, Imai E, Hori M: High water intake ameliorates tubulointerstitial injury in rats with subtotal nephrectomy: Possible role of TGF-[bgr]. Kidney Int 55: 1800–1810, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Adrogue HJ, Madias NE: Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356: 1966–1978, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Luft FC: Vasopressin, urine concentration, and hypertension: A new perspective on an old story. Clin J Am Soc Nephrol 2: 196–197, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Bankir L, Perucca J, Weinberger MH: Ethnic differences in urine concentration: Possible relationship to blood pressure. Clin J Am Soc Nephrol 2: 304–312, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Kovesdy CP: Rate of kidney function decline associates with increased risk of death. J Am Soc Nephrol 21: 1814–1816, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Xie D, Joffe MM, Brunelli SM, Beck G, Chertow GM, Fink JC, Greene T, Hsu CY, Kusek JW, Landis R, Lash J, Levey AS, O'Conner A, Ojo A, Rahman M, Townsend RR, Wang H, Feldman HI: A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol 3: 1332–1338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, Cooper RS: Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS One 3: e3431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gardner J: Death by water intoxication. Military Medicine 167: 432–434, 2002 [PubMed] [Google Scholar]
- 45. Noakes TD: Overconsumption of fluids by athletes. BMJ 327: 113, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]