Abstract
Summary
Background and objectives
Peginesatide is a synthetic, PEGylated, investigational, peptide-based erythropoiesis-stimulating agent. We report the first assessment of its efficacy and safety in correcting renal anemia in a population of 139 nondialysis chronic kidney disease patients.
Design, setting, participants, & measurements
Chronic kidney disease patients who were not on dialysis and not receiving treatment with erythropoiesis-stimulating agents in the 12 weeks before study drug administration were sequentially assigned to one of 10 cohorts; cohorts differed in starting peginesatide dose (different body weight-based or absolute doses), route of administration (intravenous or subcutaneous), and frequency of administration (every 4 or 2 weeks).
Results
Across all cohorts, 96% of patients achieved a hemoglobin response. A dose-response relationship was evident for hemoglobin increase. Comparable subcutaneous and intravenous peginesatide doses produced similar hemoglobin responses. Rapid rates of hemoglobin rise and hemoglobin excursions >13 g/dl tended to occur more frequently with every-2-weeks dosing than they did with every-4-weeks dosing. The range of final median doses in the every-4-weeks dosing groups was 0.019 to 0.043 mg/kg. Across all cohorts, 20% of patients reported serious adverse events (one patient had a possibly drug-related serious event) and 81% reported adverse events (11.5% reported possibly drug-related events); these events were consistent with those routinely observed in this patient population.
Conclusions
This study suggests that peginesatide administered every 4 weeks can increase and maintain hemoglobin in nondialysis chronic kidney disease patients. Additional long-term data in larger groups of patients are required to further elucidate the efficacy and safety of this peptide-based erythropoiesis-stimulating agent.
Introduction
Anemia in patients with chronic kidney disease (CKD) is often accompanied by a decreased quality of life and an increased risk of red blood cell (RBC) transfusions (1,2). The mainstay of treatment for this condition is erythropoietin-replacement therapy with an erythropoiesis-stimulating agent (ESA), which has been associated with an improved quality of life (3) and fewer RBC transfusions (4). Several ESAs are currently available in the United States and Europe for the correction of anemia in CKD, but these agents are approved for dosing three times per week (such as epoetin alfa) or dosing every 2 weeks (such as darbepoetin alfa) (5–7). Another ESA (methoxy polyethylene glycol-epoetin beta) is approved and marketed for dosing every 2 weeks to once monthly in Europe. These large protein-based molecules require complex manufacturing processes using recombinant DNA technology and mammalian cell lines.
Peginesatide is a synthetic, PEGylated, investigational, peptide-based ESA that acts via stimulation of the erythropoietin receptor (8). In a study of healthy volunteers, peginesatide was well tolerated and associated with a clinically and statistically significant increase in hemoglobin (Hb) that was sustained for >1 month (9). Because the amino acid sequence of peginesatide is unrelated to that of erythropoietin, peginesatide is unlikely to induce a cross-reactive immune response against either endogenous or recombinant erythropoietin. Indeed, a clinical study of peginesatide showed that it increased the Hb in most patients with antierythropoietin antibody-mediated pure red cell aplasia (PRCA) from protein-based ESAs (10).
These preliminary findings supported further study in a broader population of nondialysis CKD patients who were anemic. Most patients in this study received every-4-weeks dosing with peginesatide from the outset. The primary objective of the study was to determine the range of peginesatide doses that increase and maintain Hb between 11 and 13 g/dl, an acceptable Hb range when the study was conducted. The safety and tolerability of peginesatide were also evaluated.
Materials and Methods
Study Population
The study included adult patients with CKD stage 3 or 4 (estimated GFR of ≤60 ml/min per 1.73 m2). Before study drug administration, patients were required to have two Hb values from 9.0 to <11.0 g/dl and adequate iron stores (serum ferritin concentration ≥100 ng/ml and transferrin saturation [TSAT] ≥20%). The main exclusion criteria included ESA treatment in the 12 weeks before study drug administration and previous hemodialysis or peritoneal dialysis. A complete list of eligibility criteria is provided in Supplementary Table 1.
Study Design
This was a phase 2, multicenter, open-label, sequential, dose-finding study conducted at six sites in the United Kingdom and seven sites in Poland. All of the sites received approval from their ethics committee/institutional review board, all of the patients provided written informed consent, and the study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The study was registered with ClinicalTrials.gov (NCT00228436).
The study design allowed for multiple cohorts of patients, with ≤15 patients sequentially enrolled in each. Patients received peginesatide treatment for ≤24 weeks. Cohorts differed in starting peginesatide dose (body weight–based or absolute doses), route of administration (intravenous [IV] or subcutaneous [SC]), and frequency of administration (every 4 weeks [Q4W] or every 2 weeks [Q2W]). In the first cohort, SC peginesatide was administered Q4W (0.05 mg/kg starting dose). The decision to add the next cohort in the sequence was made on the basis of predetermined criteria including the observed safety and pharmacologic response for this cohort. After week 5, each patient's peginesatide dose was adjusted on the basis of prespecified dosing guidelines (Supplementary Table 2). Enrollment of new patients and dosing of patients in a cohort was stopped if ≥3 patients had a grade 3 or 4 treatment-related adverse event (AE). A safety monitor, not a data and safety monitoring board, reviewed the clinical, laboratory, and safety data throughout the study.
Hemoglobin, reticulocytes, and reticulocyte hemoglobin content were monitored weekly through week 13 and every other week thereafter. AEs were monitored throughout the study. Vital signs, clinical laboratory parameters, and 12-lead electrocardiograms were assessed periodically during the study. Blood samples for antibody evaluations were collected periodically during the study and tested after study completion. Iron status, monitored by regular measurements of serum ferritin levels and TSAT, was recommended to be maintained according to European Best Practice Guidelines (11,12) with administration of supplemental iron as needed to prevent iron deficiency and maintain adequate iron stores. Patients not enrolling in a long-term extension study were followed for 28 days after their last peginesatide dose, until the stabilization of AEs, or until Hb concentration was <13.0 g/dl, whichever occurred later.
Study Drug
Peginesatide (HematideTM; Affymax, Inc., Palo Alto, CA) was supplied as a preservative-free, aseptically manufactured, sterile parenteral isotonic phosphate-buffered solution in a single-use vial.
Assessments
Pharmacodynamic response was assessed by the percentage of patients in each cohort who achieved a Hb response, defined as a Hb increase of ≥1.0 g/dl from baseline (mean of the three Hb values [two screening and one predose] collected most recently before the first peginesatide dose) and a Hb value of ≥11.0 g/dl at any time during the study. Other pharmacodynamic parameters were the mean changes from baseline in Hb and reticulocyte counts, and the percentages of patients during the first 8 weeks of the study who experienced rapid rates of Hb increase (>1 g/dl increase in any 2-week period or >2 g/dl increase in any 4-week period) and elevated Hb excursions (Hb >13 g/dl). Hemoglobin data collected subsequent to a phlebotomy, or within 4 weeks after a RBC transfusion, were excluded.
Safety was assessed from AE reports. AE severity was graded according to the World Health Organization Toxicity Criteria (grades 1 to 4; grades 1 and 2 were considered mild and moderate, respectively). The main serious AEs (SAEs) were fatal, life-threatening, or required hospitalization (>24 hours). Each AE and SAE was assessed by the investigators as being either related or unrelated to the study drug. Other safety end points included reasons for study withdrawal, number of phlebotomies and RBC transfusions, changes in clinical laboratory results and vital signs, and antibody evaluations.
Statistical Analyses
This was an exploratory study intended to characterize the preliminary efficacy and safety of peginesatide and inform dose selection for future studies. Therefore, sample sizes were not determined by power calculations to evaluate specific hypotheses. The number of patients per cohort was considered sufficient to generate meaningful pharmacodynamic and safety data to guide the design of larger investigational studies. The results for cohorts with identical starting doses and dosing strategies were combined for purposes of summarization. A cross-cohort multivariate regression analysis was performed to assess the effect of administration of the first peginesatide dose and selected demographic and baseline characteristic variables on maximum Hb change from baseline before administration of the second peginesatide dose.
Results
Patient Disposition and Baseline Characteristics
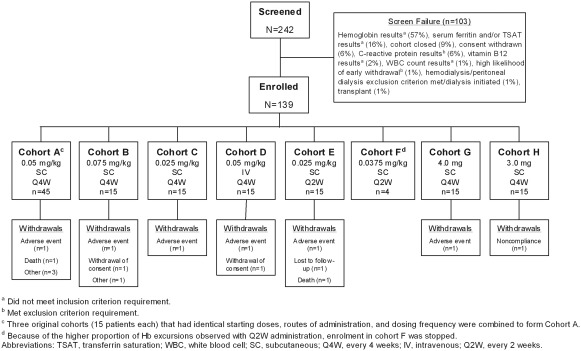
A total of 139 patients were enrolled in the study, which was conducted between September 2005 and November 2007 (Figure 1). Patients were assigned to one of 10 cohorts: three cohorts with the identical starting dose, dose frequency, and route of administration were combined (Cohort A), for a total of eight cohorts for analysis (Table 1). Body weight–based starting doses were investigated in Cohorts A through F, whereas absolute starting doses were investigated in Cohorts G and H. Q4W dosing was investigated in Cohorts A, B, C, D, G, and H, whereas Q2W dosing was investigated in Cohorts E and F. SC dosing was investigated in all cohorts except Cohort D (IV dosing). Two patients in Cohort E received all of their doses intravenously, in error, rather than subcutaneously. All of the enrolled patients received at least one peginesatide dose and were included in the analyses. Sixteen patients were withdrawn from the study (Figure 1).
Figure 1.
Patient disposition.
Table 1.
Dosing cohorts
| Cohort | n | Peginesatide Starting Dose | Dosing Frequency | Route of Administration |
|---|---|---|---|---|
| A | 45 | 0.05 mg/kg | Q4W | SC |
| B | 15 | 0.075 mg/kg | Q4W | SC |
| C | 15 | 0.025 mg/kg | Q4W | SC |
| D | 15 | 0.05 mg/kg | Q4W | IV |
| E | 15 | 0.025 mg/kg | Q2W | SCa |
| F | 4 | 0.0375 mg/kg | Q2W | SC |
| G | 15 | 4.0 mg | Q4W | SC |
| H | 15 | 3.0 mg | Q4W | SC |
Q4W, every 4 weeks; SC, subcutaneous; IV, intravenous; Q2W, every 2 weeks.
Because of dosing errors, two patients received their peginesatide doses via the intravenous route rather than the subcutaneous route.
Demographic and baseline characteristics are detailed in Table 2. Patients had a mean (SD) age of 65 (14) years and were predominantly male (54%) and white (73%). The primary causes of CKD were diabetes (40%) or other (29%; mainly included interstitial nephritis or renovascular disease). The mean (SD) estimated GFR was 25 (10) ml/min per 1.73 m2.
Table 2.
Demographic and baseline characteristics
| Characteristic | Cohort A (0.05 mg/kg SC Q4W n = 45) | Cohort B (0.075 mg/kg SC Q4W n = 15) | Cohort C (0.025 mg/kg SC Q4W n = 15) | Cohort D (0.05 mg/kg IV Q4W n = 15) | Cohort E (0.025 mg/kg SC Q2W n = 15) | Cohort F (0.0375 mg/kg SC Q2W n = 4) | Cohort G (4.0 mg SC Q4W n = 15) | Cohort H (3.0 mg SC Q4W n = 15) | Total (n = 139) |
|---|---|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 64.8 (13.6) | 64.7 (14.3) | 63.9 (12.3) | 59.9 (16.0) | 62.5 (14.6) | 68.3 (12.6) | 68.7 (13.0) | 65.7 (15.0) | 64.5 (13.8) |
| Men, n (%) | 26 (57.8) | 11 (73.3) | 8 (53.3) | 5 (33.3) | 6 (40.0) | 0 | 12 (80.0) | 7 (46.7) | 75 (54.0) |
| Race, n (%) | |||||||||
| white | 32 (71.1) | 7 (46.7) | 10 (66.7) | 10 (66.7) | 13 (86.7) | 4 (100.0) | 11 (73.3) | 14 (93.3) | 101 (72.7) |
| black | 7 (15.6) | 3 (20.0) | 2 (13.3) | 1 (6.7) | 1 (6.7) | 0 | 1 (6.7) | 0 | 15 (10.8) |
| other | 6 (13.3) | 5 (33.3) | 3 (20.0) | 4 (26.7) | 1 (6.7) | 0 | 3 (20.0) | 1 (6.7) | 23 (16.5) |
| Weight, mean (SD), kg | 77.9 (16.3) | 81.3 (13.8) | 84.7 (26.0) | 71.5 (17.4) | 81.4 (23.9) | 67.3 (21.0) | 82.0 (19.7) | 76.2 (16.7) | 78.6 (18.8) |
| Primary cause of CKD, n (%) | |||||||||
| diabetes | 14 (31.1) | 7 (46.7) | 8 (53.3) | 6 (40.0) | 6 (40.0) | 1 (25.0) | 8 (53.3) | 5 (33.3) | 55 (39.6) |
| hypertension | 7 (15.6) | 0 | 1 (6.7) | 0 | 2 (13.3) | 1 (25.0) | 1 (6.7) | 0 | 12 (8.6) |
| polycystic kidney disease | 5 (11.1) | 0 | 1 (6.7) | 0 | 0 | 0 | 1 (6.7) | 1 (6.7) | 8 (5.8) |
| urologic | 3 (6.7) | 0 | 0 | 1 (6.7) | 0 | 0 | 0 | 0 | 4 (2.9) |
| autoimmune disease | 1 (2.2) | 0 | 0 | 0 | 1 (6.7) | 0 | 0 | 1 (6.7) | 3 (2.2) |
| unknown | 6 (13.3) | 0 | 0 | 2 (13.3) | 3 (20.0) | 1 (25.0) | 3 (20.0) | 2 (13.3) | 17 (12.2) |
| othera | 9 (20.0) | 8 (53.3) | 5 (33.3) | 6 (40.0) | 3 (20.0) | 1 (25.0) | 2 (13.3) | 6 (40.0) | 40 (28.8) |
| eGFR, mean (SD), ml/min per 1.73 m2 | 24.4 (8.3) | 26.9 (9.8) | 25.7 (14.2) | 25.7 (8.9) | 24.9 (10.7) | 17.8 (5.6) | 25.5 (6.8) | 25.3 (12.9) | 25.0 (9.8) |
| Hemoglobin, mean (SD), g/dl | 10.3 (0.5) | 10.2 (0.5) | 10.2 (0.5) | 10.0 (0.5) | 10.1 (0.5) | 10.3 (0.4) | 10.1 (0.7) | 10.1 (0.6) | 10.2 (0.5) |
SC, subcutaneous; Q4W, every 4 weeks; IV, intravenous; Q2W, every 2 weeks; CKD, chronic kidney disease; eGFR, estimated GFR.
Mainly included interstitial nephritis or renovascular disease.
Pharmacodynamics
Across all cohorts, 96% of patients achieved a Hb response; a comparable proportion of patients in each cohort achieved a Hb response (Table 3). Across all cohorts, the mean (SD) Hb concentration increased by 1.4 (1.3) g/dl from baseline to study end.
Table 3.
Hemoglobin parameters, by cohort: hemoglobin response, rapid rates of increased hemoglobin, and hemoglobin excursions
| Hemoglobin Parameter, n (%) | Cohort A (0.05 mg/kg SC Q4W n = 45) | Cohort B (0.075 mg/kg SC Q4W n = 15) | Cohort C (0.025 mg/kg SC Q4W n = 15) | Cohort D (0.05 mg/kg IV Q4W n = 15) | Cohort E (0.025 mg/kg SC Q2W n = 15) | Cohort F (0.0375 mg/kg SC Q2W n = 4) | Cohort G 4.0 mg (SC Q4W n = 15) | Cohort H (3.0 mg SC Q4W n = 15) | Total (n = 139) |
|---|---|---|---|---|---|---|---|---|---|
| Patients who achieved a Hb response | 44 (97.8) | 15 (100.0) | 15 (100.0) | 14 (93.3) | 14 (93.3) | 4 (100.0) | 13 (86.7) | 14 (93.3) | 133 (95.7) |
| Patients with >1 g/dl Hb increase during any 2-week perioda | 31 (68.9) | 13 (86.7) | 9 (60.0) | 11 (73.3) | 13 (86.7) | 3 (75.0) | 12 (80.0) | 11 (73.3) | 103 (74.1) |
| Patients with >2 g/dl Hb increase during any 4-week perioda | 12 (26.7) | 3 (20.0) | 0 (0) | 5 (33.3) | 12 (80.0) | 1 (25.0) | 4 (26.7) | 3 (20.0) | 40 (28.8) |
| Patients with Hb excursionsa,b | 6 (13.3) | 1 (6.7) | 0 (0) | 2 (13.3) | 5 (33.3) | 1 (25.0) | 1 (6.7) | 1 (6.7) | 17 (12.2) |
SC, subcutaneous; Q4W, every 4 weeks; IV, intravenous; Q2W, every 2 weeks; Hb, hemoglobin.
During the first 8 weeks of the study.
Hemoglobin concentrations >13 g/dl on at least two consecutive measurements.
A lower starting dose (0.025 mg/kg) of SC Q4W peginesatide (Cohort C) increased Hb more slowly than did a higher starting dose (0.05 mg/kg, Cohort A), suggesting a relationship between starting doses and Hb response rate (Figure 2A). Similar initial increases in mean Hb levels from baseline were observed in Cohorts A (0.05 mg/kg SC Q4W starting dose) and B (0.075 mg/kg SC Q4W starting dose; Figure 2A). Similar starting doses of body weight-based and absolute-dose SC Q4W regimens (median starting doses of 3.8 and 4.0 mg for Cohorts A and G, respectively) resulted in comparable Hb responses (Figure 2A). A multivariate analysis was performed to evaluate the maximum Hb change from baseline before administration of the second dose, using the first dose and baseline demographics and characteristics as covariates; among all possible linear combinations of the baseline variables, the best model for predicting maximum Hb change included only the first dose (P = 0.02). Each cohort confidence interval (CI) did not include zero; Cohort G had the largest change (1.59 g/dl, 95% CI 1.21 to 1.95), and Cohort C had the smallest change (0.91 g/dl, 95% CI 0.55 to 1.28).
Figure 2.
Mean hemoglobin change from baseline. (A) In SC Q4W Cohorts. (B) With matched peginesatide starting doses delivered subcutaneously (Cohort A) or intravenously (Cohort D). (C) With equivalent (i.e., the same dose over a 4-week time period) peginesatide starting doses delivered Q4W (Cohort A) or Q2W (Cohort E). Q2W, every 2 weeks; Q4w, every 4 weeks.
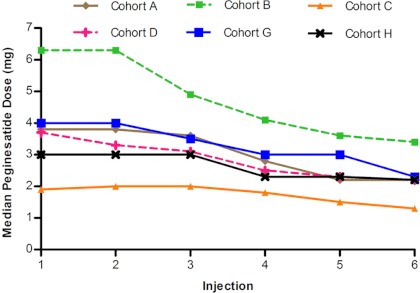
Identical starting doses of SC (Cohort A) and IV (Cohort D) Q4W peginesatide resulted in comparable mean Hb levels (Figure 2B). Notably, the median doses in Cohorts A and D were similar throughout the study (Figure 3), suggesting that a given dose may produce a similar Hb response irrespective of the route of administration.
Figure 3.
Median peginesatide doses in the every-4-weeks cohorts, by injection.
Hemoglobin concentrations of patients who received Q2W peginesatide (Cohort E) tended to increase more rapidly, and to higher concentrations, than did those of patients who received an equivalent (same total dose over a 4-week period) starting Q4W peginesatide dose (Cohort A; Figure 2C). Rapid rates of Hb increase, particularly increases >2 g/dl during any 4-week period (e.g., 80.0% versus 26.7%, Cohorts E versus A, P < 0.001), and Hb excursions (e.g., 33.3% versus 13.3%, Cohorts E versus A, P = 0.12) generally occurred in more patients in the Q2W than the Q4W cohorts (Table 3). Because of the higher proportion of Hb excursions observed with Q2W administration, enrollment in Cohort F was stopped on the basis of sponsor judgment to prevent rapid rates of Hb increase, and not because of AEs. These Cohort F patients had their next dose reduced, and thereafter their dosing was per the protocol-specified dose-adjustment guidelines.
Peginesatide Dosing
The median dose for the Q4W cohorts generally decreased during the study (Figure 3), and the median final dose ranged from 0.019 to 0.044 mg/kg. This effect was particularly pronounced in the highest starting dose cohort (Cohort B; 0.075 mg/kg) and was also evident in the Q2W cohorts.
Safety
AEs were reported by 112 of 139 patients (81%); most of these patients (90 [65%]) had only AEs that were considered mild to moderate in severity. The most frequently reported AEs (occurring in ≥5% of patients) are detailed in Table 4. AEs considered possibly related to peginesatide by the investigators were reported in 16 (11.5%) patients; the most common (occurring in ≥2 patients) were hypertension (n = 9, 6.5%), arthritis (n = 2, 1.4%), and headache (n = 2, 1.4%).
Table 4.
Most frequently (occurring in ≥5% of patients) reported adverse events, by cohort
| Adverse Event, n (%) | Cohort A (0.05 mg/kg SC Q4W n = 45) | Cohort B (0.075 mg/kg SC Q4W n = 15) | Cohort C (0.025 mg/kg SC Q4W n = 15) | Cohort D (0.05 mg/kg IV Q4W n = 15) | Cohort E (0.025 mg/kg SC Q2W n = 15) | Cohort F (0.0375 mg/kg SC Q2W n = 4) | Cohort G (4.0 mg SC Q4W n = 15) | Cohort H (3.0 mg SC Q4W n = 15) | Total (n = 139) |
|---|---|---|---|---|---|---|---|---|---|
| Nasopharyngitis | 3 (6.7) | 2 (13.3) | 1 (6.7) | 2 (13.3) | 5 (33.3) | 1 (25.0) | 3 (20.0) | 1 (6.7) | 18 (12.9) |
| Hypertension | 3 (6.7) | 3 (20.0) | 1 (6.7) | 1 (6.7) | 3 (20.0) | 1 (25.0) | 0 (0) | 2 (13.3) | 14 (10.1) |
| Diarrhea | 4 (8.9) | 0 | 1 (6.7) | 0 | 0 | 2 (50.0) | 0 | 3 (20.0) | 10 (7.2) |
| Urinary tract infection | 6 (13.3) | 1 (6.7) | 0 | 0 | 0 | 1 (25.0) | 1 (6.7) | 1 (6.7) | 10 (7.2) |
| Nausea | 1 (2.2) | 2 (13.3) | 1 (6.7) | 1 (6.7) | 1 (6.7) | 1 (25.0) | 0 | 2 (13.3) | 9 (6.5) |
| Back pain | 3 (6.7) | 2 (13.3) | 0 | 0 | 0 | 1 (25.0) | 1 (6.7) | 1 (6.7) | 8 (5.8) |
| Peripheral edema | 2 (4.4) | 0 | 0 | 0 | 4 (26.7) | 0 | 1 (6.7) | 1 (6.7) | 8 (5.8) |
| Chronic renal failure | 1 (2.2) | 0 | 1 (6.7) | 1 (6.7) | 1 (6.7) | 0 | 1 (6.7) | 3 (20.0) | 8 (5.8) |
| Increased blood pressure | 2 (4.4) | 0 | 1 (6.7) | 0 | 3 (20.0) | 1 (25.0) | 0 | 0 | 7 (5.0) |
SC, subcutaneous; Q4W, every 4 weeks; IV, intravenous; Q2W, every 2 weeks.
A total of 36 SAEs were reported by 27 of 139 patients (20%). The most frequently reported SAEs (occurring in ≥2 patients) were chronic renal failure (i.e., worsening of renal function; n = 6, 4.3%), bronchitis (n = 2, 1.4%), and diabetic ketoacidosis (n = 2, 1.4%). A single SAE (embolic cerebral infarction) was considered possibly related to peginesatide by the investigator; see patient narrative in Supplementary Table 3.
Two deaths occurred during the study, one caused by chronic renal failure and one caused by acute myocardial infarction and bronchitis. Neither death was reported as being related to peginesatide; see Supplementary Table 3 for patient narratives.
Six patients were withdrawn from the study because of AEs. Two patients withdrew because of nonserious AEs (dizziness and nausea in one patient and skin necrosis in the other). Four patients withdrew because of SAEs (chronic renal failure, renal failure, multiple myeloma, and catheter site hematoma and peritonitis [after peritoneal dialysis]; none were considered related to peginesatide).
A single patient in Cohort B underwent a phlebotomy (500 ml), 27 days after the second peginesatide dose, because of a Hb concentration of 15.2 g/dl. The patient's Hb progressively decreased to 12.2 g/dl within 30 days after the phlebotomy. The patient's third peginesatide dose was administered 30 days after the phlebotomy. Subsequent peginesatide doses were delayed and/or decreased because of Hb concentrations >12.5 g/dl. The patient did not have any AE considered possibly related to peginesatide by the investigator. Five patients received RBC transfusions during the study (Cohort A, n = 1; Cohort C, n = 1; Cohort G, n = 2; and Cohort H, n = 1), two patients because of anemia/low Hb concentrations and three patients because of posthemorrhage anemia.
Iron status was maintained throughout the study with the use of IV iron. For the cohorts that included ≥15 patients, 20 to 60% of patients per cohort received IV iron at some point during the study. Mean initial and end-of-study values were 269 and 260 ng/ml, respectively, for ferritin; 25.9 and 30.1%, respectively, for TSAT; and 32.8 and 33.4 pg, respectively, for reticulocyte hemoglobin content.
No patient had an electrocardiogram abnormality interpreted by the investigator as clinically significant that was not present at screening. Alanine aminotransferase elevations at least three times the upper limit of normal were observed once each in four patients; no SAEs were associated with these elevations (see Supplementary Table 3 for patient narratives). Other clinical laboratory tests and vital sign assessments generally remained within normal limits and were similar to baseline values throughout the study.
Peginesatide-specific antibodies were detected in two patients. The first patient was from Cohort B, and antibodies were first detected in the sample collected before the patient's fifth Q4W peginesatide dose (week 17). At week 25, the binding antibody titer reached 1:160, and a low, in vitro neutralizing antibody titer (1:20) was recorded. The neutralizing antibodies did not appear to affect the patient's Hb response (Hb at baseline, 10.7 g/dl; Hb at weeks 19 to 25, 11.7 to 11.9 g/dl) or safety profile. The second patient was from Cohort E, and antibodies were first detected in the sample collected before the patient's twelfth Q2W peginesatide dose (week 25). At week 29, the binding antibody titer reached 1:20, and a low, in vitro neutralizing antibody titer (1:10) was recorded. The neutralizing antibodies did not appear to affect the patient's Hb response (Hb at baseline, 9.9 g/dl; Hb at weeks 25 to 29, 11.4 to 12.0 g/dl) or safety profile. This patient had a slight decrease in Hb after discontinuation of peginesatide, but the Hb always remained above 10 g/dl. The patient initiated darbepoetin alfa treatment after discontinuation of peginesatide. In both patients, the peginesatide-specific antibodies did not cross-react with erythropoietin.
Discussion
This phase 2 dose-finding study found that 96% of patients achieved an Hb response by study end. Moreover, mean Hb values across all cohorts increased by 1.4 g/dl from baseline to study end. These results indicate that different peginesatide starting doses and dosing strategies (e.g., IV or SC) can elicit a robust pharmacodynamic response in nondialysis CKD patients who were recently naive to ESA treatment.
Across all cohorts, the median peginesatide dose decreased during the study. This effect was particularly pronounced in cohorts with the highest starting doses. The doses required to maintain Hb in the target range (final median doses of 0.019 to 0.044 mg/kg in the Q4W cohorts) guided the selection of peginesatide starting doses for this patient population in phase 3 studies (0.025 and 0.04 mg/kg).
Studies of epoetin alfa have demonstrated that lower doses can be used with SC than with IV administration (13). The results from this study suggest that Hb responses to comparable doses of IV and SC peginesatide may be similar, which has also been shown with the long-acting ESAs, darbepoetin alfa (14–17) and methoxy polyethylene glycol-epoetin beta (18,19). Future studies are needed to evaluate the similarity of IV and SC dose requirements for peginesatide.
The rate of Hb increase was higher, and there tended to be more Hb excursions >13 g/dl with Q2W peginesatide compared with an equivalent Q4W dose. This information helped inform the selection of a Q4W dosing interval in the phase 3 trials.
Most patients in this study (81%) experienced at least one AE with 90 (65%) patients experiencing only AEs considered mild to moderate in severity. The most common AE considered possibly related to peginesatide by the investigators was hypertension. Of the 36 reported SAEs, a single SAE (embolic cerebral infarction) was considered possibly related to peginesatide by the investigator. The AEs and SAEs reported in this study were consistent with those observed in this patient population; however, this study was not designed to definitively determine the safety of peginesatide relative to an active control. Two patients developed peginesatide-neutralizing antibodies during this study. The antibody titers were low and did not appear to affect the patients' Hb concentrations or safety profiles; however, this study did not have long-term follow-up of these patients. Importantly, given the lack of immunological cross-reactivity of peginesatide with erythropoietin (20), the potential risk of inducing antibody-mediated PRCA with peginesatide is extremely low. No patients with peginesatide-induced PRCA have been reported during clinical trials. Cases of antibody-mediated PRCA have been described with protein-based ESAs, albeit rarely (5,6).
This study had several limitations: the sample size was relatively small, follow-up was limited to the 24-week study period, subjects were not randomized to the different cohorts because the cohorts were enrolled sequentially, and there was no control group. Hence, these results may not be fully generalizable to the larger CKD population.
In conclusion, the results of this study show that Q4W peginesatide can increase and maintain Hb in nondialysis CKD patients. These results guided the dosing strategies used in the phase 3 studies in nondialysis patients.
Disclosures
Iain C. Macdougall has received consultancy fees, research support, and lecture honoraria from Amgen, Ortho Biotech, Roche, Affymax, Takeda, and Vifor Pharma. Andrzej Wiecek received speakers fees from Amgen, Roche, and Hospira; was an Advisory Board member of Affymax/Takeda, Bayer, Jannesen-Cilag, Roche, and Hospira; and was an investigator for studies sponsored by Jannsen-Cilag, Roche, Affymax/Takeda, and Hospira. Ashraf Mikhail was an investigator for studies sponsored by Amgen, Roche, Affymax/Takeda; received sponsorship to attend scientific meetings from Amgen, Roche, and Johnson & Johnson; and received consultancy fees from Amgen, Roche, Astellas, Takeda, and Lipoxen. Olgierd Smolenski was an investigator for a clinical study sponsored by Amgen. Beatriz Tucker, Michal Nowicki, Iain MacPhee, Michal Mysliwiec, Władysław Sułowicz, and Magdi Yaqoob have nothing to disclose. Martha Mayo, Carol Francisco, Krishna Polu, Peter J. Schatz, and Anne-Marie Duliege are employees of Affymax, Inc.
Supplementary Material
Acknowledgments
This study was supported by Affymax, Inc. Palo Alto, CA. We would like to thank Alex Yang, Steve Brunell, and Richard Rowell, who are employees or consultants of Affymax, Inc., for their medical writing and statistical support. Dr. Brunell, an Affymax, Inc. consultant, prepared the preliminary draft of the manuscript under the direction of the lead author, Dr. Iain C. Macdougall. Dr. Macdougall maintained full editorial control of the manuscript and its conclusions throughout its development. Some data contained in this manuscript were presented in abstract form at the 2007 and 2009 World Congress of Nephrology conferences, the 2006 and 2007 American Society of Nephrology annual meetings, the 2006 and 2007 Congresses of the European Renal Association-European Dialysis and Transplant Association, and the 2006 International Lübeck Conference on the Pathophysiology and Pharmacology of Erythropoietin and other Hemopoietic Growth Factors. The following investigators participated in this study: Vipula DeSilva, Mayday University Hospital, Croydon, United Kingdom; Simon Fletcher, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, United Kingdom; Kevin P. G. Harris, Leicester General Hospital, Leicester, United Kingdom; Iain C. Macdougall, King's College Hospital, London, United Kingdom (lead investigator for the United Kingdom); Iain MacPhee, St. George's Hospital, London, United Kingdom; Ashraf I. Mikhail, Morriston Hospital, Swansea, United Kingdom; Muhammad Magdi Yaqoob, The Royal London and St. Bartholomew's Hospitals, London, United Kingdom; Michal Mysliwiec, Wojewodzki Szpital, Bialystok, Poland; Michal Nowicki, Samodzielny Publiczny Szpital Kliniczny, Lodz, Poland; Olgierd Smolenski, Centrum Dializ Fresenius NephroCare, Krakow, Poland; Wladyslaw Sulowicz, Oddział Kliniczny Kliniki Nefrologii Szpitala Uniwersyteckiego, Krakow, Poland; Andrzej Wiecek, Medical University of Silesia, Katowice, Poland (lead investigator for Poland); Richard J. Fluck, Derby City General Hospital, Derby, United Kingdom; Boleslaw Rutkowski, Klinika Nefrologii Transplantologii, Gdansk, Poland; Ryszard Gellert, IV Oddzial Chorob Wewnetrznych z Pododdzialem Nefrologicznym, Szpital, Warszawa, Poland; and Philip A. Kalra, Hope Hospital, Salford, United Kingdom.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org
References
- 1. Evans RW, Rader B, Manninen DL: The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. Cooperative Multicenter EPO Clinical Trial Group. JAMA 263: 825–830, 1990 [PubMed] [Google Scholar]
- 2. Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S: Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J: The effect of anemia treatment on selected health-related quality-of-life domains: A systematic review. Clin Ther 25: 1786–1805, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, Korbet S, Krantz S, Lundin P, Nissenson A, Ogden D, Paganini E, Rader B, Rutsky E, Stivelman J, Stone WJ, Teschan P, Van Stone JC, Van Wyck DB, Zuckerman K, Adamson JW: Recombinant human erythropoietin in anemic patients with end-stage renal disease: Results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Procrit® (epoetin alfa) for injection [prescribing information]. Thousand Oaks, CA: Amgen Inc.; October, 2009 [Google Scholar]
- 6. Aranesp® (darbepoetin alfa) for injection [prescribing information]. Thousand Oaks, CA: Amgen Inc.; April, 2009 [Google Scholar]
- 7. European Medicines Agency Website Aranesp: darbepoetin alfa. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000332/human_med_000651.jsp&murl=menus/medicines/medicines.jsp&jsenabled=true. Revised April 20, 2010 Accessed September 3, 2010
- 8. Fan Q, Leuther KK, Holmes CP, Fong KL, Zhang J, Velkovska S, Chen MJ, Mortensen RB, Leu K, Green JM, Schatz PJ, Woodburn KW: Preclinical evaluation of Hematide, a novel erythropoiesis stimulating agent, for the treatment of anemia. Exp Hematol 34: 1303–1311, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Stead RB, Lambert J, Wessels D, Iwashita JS, Leuther KK, Woodburn KW, Schatz PJ, Okamoto DM, Naso R, Duliege AM: Evaluation of the safety and pharmacodynamics of HematideTM, a novel erythropoietic agent, in a phase 1, double-blind, placebo-controlled, dose-escalation study in healthy volunteers. Blood 108: 1830–1834, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Macdougall IC, Rossert J, Casadevall N, Stead RB, Duliege AM, Froissart M, Eckardt KU: A peptide-based erythropoietin-receptor agonist for pure red-cell aplasia. N Engl J Med 361: 1848–1855, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Macdougall IC, Hörl WH, Jacobs C, Valderrábano F, Parrondo I, Thompson K, Cremers S: European best practice guidelines 6–8: Assessing and optimizing iron stores. Nephrol Dial Transplant 15[Suppl 4]: 20–32, 2000 [PubMed] [Google Scholar]
- 12. Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougall IC, Macleod A, Wiecek A, Cameron S: Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19[Suppl 2]: ii1–ii47, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Besarab A, Reyes CM, Hornberger J: Meta-analysis of subcutaneous versus intravenous epoetin in maintenance treatment of anemia in hemodialysis patients. Am J Kidney Dis 40: 439–446, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Macdougall IC: Optimizing the use of erythropoietic agents: Pharmacokinetic and pharmacodynamic considerations. Nephrol Dial Transplant 17[Suppl 5]: 66–70, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Vanrenterghem Y, Barany P, Mann JF, Kerr PG, Wilson J, Baker NF, Gray SJ: Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int 62: 2167–2175, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Locatelli F, Canaud B, Giacardy F, Martin-Malo A, Baker N, Wilson J: Treatment of anaemia in dialysis patients with unit dosing of darbepoetin alfa at a reduced dose frequency relative to recombinant human erythropoietin (rHuEpo). Nephrol Dial Transplant 18: 362–369, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Brunkhorst R, Bommer J, Braun J, Haag-Weber M, Gill C, Wagner J, Wagener T: Darbepoetin alfa effectively maintains haemoglobin concentrations at extended dose intervals relative to intravenous or subcutaneous recombinant human erythropoietin in dialysis patients. Nephrol Dial Transplant 19: 1224–1230, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, Dougherty FC, Reigner B: Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol 1: 1211–1215, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, Villa G, Rosansky S, Adamis H, Beyer U: C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol 28: 280–289, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Woodburn KW, Fan Q, Winslow S, Chen MJ, Mortensen RB, Casadevall N, Stead RB, Schatz PJ: Hematide is immunologically distinct from erythropoietin and corrects anemia induced by antierythropoietin antibodies in a rat pure red cell aplasia model. Exp Hematol 35: 1201–1208, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.