Abstract
Summary
Background and objectives
Renal transplant recipients with pre-existing diabetes (PD) have reduced graft survival and increased risk of mortality and ischemic heart disease compared with nondiabetic transplant recipients. To assess the effect of belatacept in this high-risk group, we evaluated outcomes of the subpopulation with PD from previously published BENEFIT and BENEFIT-EXT trials.
Design, setting, participants, & measurements
A post hoc analysis evaluated pooled data from BENEFIT (living donors or standard criteria donors) and BENEFIT-EXT (extended criteria donors). Patients were randomized to receive cyclosporine or a more intensive (MI) or less intensive (LI) belatacept regimen.
Results
Of 1209 intent-to-treat patients, 336 had PD. At 12 months, the belatacept LI arm demonstrated a numerically higher rate of patients surviving with a functioning graft (90.4% MI [103 of 114], 92.8% LI [90 of 97], and 80.8% cyclosporine [101 of 125]), and fewer serious adverse events than cyclosporine or MI patients. Three cases of posttransplant lymphoproliferative disorder were reported in LI patients, one involving the central nervous system. Higher rates (% [95% confidence interval]: 22.8% MI [15.1 to 30.5]; 20.6% LI [12.6 to 28.7]; 14.4% cyclosporine (8.2 to 20.6]) and grades of acute rejection were observed with belatacept. Measured GFR (ml/min per 1.73 m2, 59.8 MI; 62.5 LI; 45.4 cyclosporine), and cardiovascular risk profile were better for belatacept versus cyclosporine.
Conclusions
In post hoc analysis of patients with PD, patient/graft survival and renal function at 12 months were numerically higher with belatacept versus cyclosporine, but not statistically significant. Further study is necessary to confirm the benefits belatacept may provide in these patients.
Introduction
The most common cause of death among renal transplant recipients (RTR) is cardiovascular disease (1). Diabetic transplant recipients are at increased risk for cardiovascular disease (2,3) and exhibit higher mortality (1,4–6) and reduced graft survival (7,8) compared with nondiabetic transplant recipients. Current therapies for maintenance immunosuppression have adverse effects on BP (9,10), lipids (9,10), and glycemic control (11–15) in RTR, thus contributing to increased cardiovascular morbidity and mortality. More selective immunosuppressive therapies without the cardiovascular and metabolic toxicities of current therapies may therefore improve outcomes in diabetic RTR.
Belatacept (LEA29Y) is a first-in-class costimulation blocker being developed for primary maintenance of immunosuppression. The pivotal Phase III trials BENEFIT (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial) (16) and BENEFIT-EXT (17) (Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression Trial—EXTended criteria donors), demonstrated similar patient/graft survival, better renal function, and an improved cardiovascular/metabolic risk profile in belatacept versus cyclosporine patients. Two belatacept dosing regimens, more intense (MI) and less intense (LI) regimens, were assessed in BENEFIT and BENEFIT-EXT. The belatacept MI and LI regimens had similar efficacy, and the LI regimen had fewer deaths and serious infections than the MI regimen (18), supporting a more favorable benefit/risk profile for the LI regimen.
Separate prespecified analyses of RTR with pre-existing diabetes (RTR-PD) were performed on BENEFIT (16) and BENEFIT-EXT (17) populations. To further understand the effect of belatacept treatment and increase the robustness of the analysis, a pooled post hoc analysis of the RTR-PD subpopulations from the two trials was performed. A pooled post hoc analysis of the patients without PD is also included for comparison.
Materials and Methods
Design
BENEFIT (16) and BENEFIT-EXT (17) are 3-year, randomized, partially blinded, active-controlled, parallel-group studies in adult de novo RTR. Analyses in the RTR-PD subpopulation of each trial were prespecified. Coprimary endpoints assessed at 12 months included composite patient and graft survival, composite renal impairment (defined as the percentage of patients exhibiting a measured glomerular filtration [mGFR] of <60 ml/min per 1.73 m2 at month 12 or a decrease in mGFR of ≥10 ml/min per 1.73 m2 from months 3 to 12), and acute rejection (AR) (BENEFIT only).
Patients
Patients were considered to have diabetes before transplantation (PD) if they had a medical history of diabetes or were taking insulin or oral antidiabetic medication at the time of transplantation.
Interventions
Patients were randomized 1:1:1 and stratified by study site to receive belatacept MI or LI or cyclosporine for maintenance immunosuppression. Details of the three treatment regimens were described previously (16,17).
Statistical Methods
Statistical methods for the BENEFIT and BENEFIT-EXT intent-to-treat (ITT) population were described previously (16,17) and have been similarly performed for the RTR-PD and non-RTR-PD subpopulations. To account for multiple comparisons, the nominal type I error (level of significance) was set at 2.7% (two-sided) for each belatacept arm versus cyclosporine arm and at 5% overall. Because these post hoc analyses were not powered by sample size, the resulting P values are not reported here. Any conclusions drawn from the analyses should be considered hypothesis generating and not hypothesis confirming.
The primary and key secondary efficacy endpoints, including composite patient/graft survival, composite renal impairment, AR, and chronic allograft nephropathy, were assessed using a DerSimonian-Laird (19) random-effects model to estimate the pooled risk difference (weighted difference) between the belatacept and cyclosporine arms and associated 97.3% confidence interval (CI). The endpoints, including mGFR, calculated GFR (cGFR, Modification of Diet in Renal Disease), systolic BP, and diastolic BP, were analyzed using an ANOVA with factor for randomization group (treatment). The change from baseline in non-HDL cholesterol and triglycerides were assessed using an analysis of covariance model with factor for treatment, study, and baseline measurement. The intensities of antihypertensive and antihyperlipidemic medications were calculated using a cumulative logit model with treatment and study as the covariates.
Imputation Rules
The composite renal impairment endpoint was assessed by mGFR at months 3 and 12. The imputation methods for missing data at months 3 and/or 12 were: (1) patients with graft loss or who died during first year posttransplant were considered as meeting the composite endpoint (note: missing GFR values were not imputed for these subjects) or (2) for other patients who had a missing mGFR assessment, the missing values were imputed on the basis of mGFR values at other time points or calculated values at the same time point. Patients without these alternate measurements could not be imputed and were excluded from the analysis.
The endpoint of mGFR at month 12 was supportive for the primary renal composite endpoint and was imputed within the frame of imputation methods defined for the composite endpoint. Therefore, mGFR values missing because of death or graft loss were not imputed.
Results
Patient Characteristics and Disposition
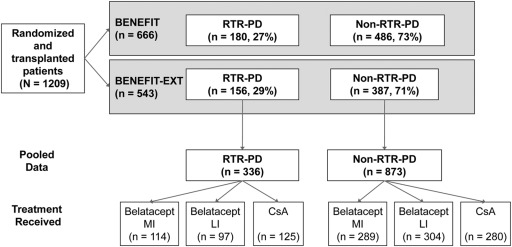
A total of 1209 patients across BENEFIT and BENEFIT-EXT were randomized and received a renal transplant (16,17). The proportion of patients who remained on treatment for the first 12 months was comparable across treatment arms (16,17). Patients who discontinued treatment and reasons for discontinuation have been described previously (16,17). The proportion of RTR-PD patients (27% and 29%, respectively) are shown in Figure 1. Table 1 lists pooled demographic and baseline characteristics of the RTR-PD subpopulation. Recipient characteristics were similar across all treatment groups. The proportion of RTR-PD patients across treatment arms was comparable in BENEFIT (29% MI, 26% LI, 27% cyclosporine). The proportion of RTR-PD cyclosporine patients in BENEFIT-EXT was higher than in the belatacept arms (28% MI, 22% LI, 36% cyclosporine).
Figure 1.
Patient disposition. CI, confidence interval; cGFR, calculated GFR; CsA, cyclosporine; LI, less intensive; MI, more intensive; RTR-PD, renal transplant recipient pretransplant diabetes.
Table 1.
Demographic and baseline characteristics of RTR-PD subpopulation
| Recipient Characteristic | Belatacept MI (n = 114) | Belatacept LI (n = 97) | CsA (n = 125) |
|---|---|---|---|
| Mean age, years (SD) | 53 (14.3) | 53 (11.9) | 53 (13.3) |
| Gender, n (%) | |||
| male | 82 (71.9) | 72 (74.2) | 88 (70.4) |
| female | 32 (28.1) | 25 (25.8) | 37 (29.6) |
| Race, n (%) | |||
| Caucasian | 70 (61.4) | 59 (60.8) | 80 (64.0) |
| African American | 15 (13.2) | 19 (19.6) | 22 (17.6) |
| Native American/Alaskan Native | 1 (0.9) | 1 (1.0) | 0 (0.0) |
| Asian | 15 (13.2) | 12 (12.4) | 9 (7.2) |
| other | 13 (11.4) | 6 (6.2) | 14 (11.2) |
| Geographic region, n (%) | |||
| North America | 63 (55.3) | 49 (50.5) | 57 (45.6) |
| South America | 14 (12.3) | 11 (11.3) | 22 (17.6) |
| Europe | 26 (22.8) | 25 (25.8) | 38 (30.4) |
| Asia/Pacific | 10 (8.8) | 10 (10.3) | 7 (5.6) |
| Africa | 1 (0.9) | 2 (2.1) | 1 (0.8) |
| Reported cause of ESRD, n (%) | |||
| glomerular disease | 9 (7.9) | 19 (19.6) | 18 (14.4) |
| diabetes | 57 (50.0) | 41 (42.3) | 62 (49.6) |
| polycystic kidneys | 9 (7.9) | 9 (9.3) | 8 (6.4) |
| hypertension (primary and secondary) | 19 (16.7) | 12 (12.4) | 17 (13.6) |
| congenital, familial, metabolic disorders | 2 (1.8) | 0 (0.0) | 3 (2.4) |
| tubular and interstitial diseases | 4 (3.5) | 2 (2.1) | 5 (4.0) |
| retransplant/graft failure | 0 (0.0) | 2 (2.1) | 0 (0) |
| other | 14 (12.3) | 12 (12.4) | 12 (9.6) |
| Categorized PRA, n (%) | |||
| <20% | 106 (93.0) | 87 (89.7) | 117 (93.6) |
| ≥20% | 7 (6.1) | 9 (9.3) | 4 (3.2) |
| missing | 1 (0.9) | 1 (1.0) | 4 (3.2) |
CsA, cyclosporine; LI, less intensive; MI, more intensive; PRA, panel reactive antibody; RTR-PD, renal transplant recipients with preexisting diabetes.
Cyclosporine Trough Levels
Mean cyclosporine trough levels (SD) in RTR-PD patients were 344 (221), 252 (227), 201 (115), 180 (75), and 161 (78) ng/ml at 1, 3, 6, 9, and 12 months posttransplantation, respectively.
Patients Surviving with a Functioning Graft
The 12-month composite endpoint of patient/graft survival was numerically higher, with both belatacept regimens versus cyclosporine: 90.4% MI (103 of 114), 92.8% LI (90 of 97), and 80.8% cyclosporine (101 of 125); weighted differences from cyclosporine (97.3% CI) were 8.5 (−0.4 to 17.4) and 10.2 (1.5 to 18.9) for MI and LI, respectively (Table 2). Most of the difference between groups was due to a higher rate of graft loss in the cyclosporine group (4.4% MI, 3.1% LI, and 12.8% cyclosporine) (Table 2). All ITT RTR-PD patients were included in the analysis. Belatacept LI patients had numerically lower mortality than cyclosporine and MI patients (6.1% MI, 3.1% LI, and 5.6% cyclosporine) (Table 2). Adjudicated cause of graft loss is given in Table 3.
Table 2.
Outcomes: patient/graft survival, renal function and structure, and acute rejection at month 12 in RTR-PD subpopulation
| Outcome | Belatacept MI (n = 114) | Belatacept LI (n = 97) | CsA (n = 125) |
|---|---|---|---|
| Patient/graft survival | |||
| patients surviving with functioning graft, n (%) | 103 (90.4) | 90 (92.8) | 101 (80.8) |
| 95% CI | 84.9 to 95.8 | 87.6 to 97.9 | 73.9 to 87.7 |
| weighted difference from CsA (97.3% CI)a | 8.5 (−0.4 to 17.4) | 10.2 (1.5 to 18.9) | |
| graft loss or death, n (%) | 11 (9.6) | 7 (7.2) | 24 (19.2) |
| graft loss, n (%) | 5 (4.4) | 3 (3.1) | 16 (12.8) |
| death, n (%) | 7 (6.1) | 3 (3.1) | 7 (5.6) |
| death with functioning graft, n (%) | 5 (4.4) | 3 (3.1) | 6 (4.8) |
| imputed as graft loss or death | 1 (0.9) | 1 (1.0) | 2 (1.6) |
| Renal function and structure | |||
| composite renal impairment endpoint, n (%) | 65 (58.6) | 57 (64.0) | 102 (87.2) |
| 95% CI | 49.4 to 67.7 | 54.1 to 74.0 | 81.1 to 93.2 |
| weighted difference from CsA (97.3% CI)a | −27.0 (−39.2 to −14.8) | −21.8 (−35.1 to −8.6) | |
| mean iothalamate GFR (measured),b ml/min per 1.73 m2 (SE) | 59.8 (4.5) | 62.5 (4.6) | 45.4 (4.5) |
| estimated difference from CsA (97.3% CI)c | 14.4 (6.0 to 22.7) | 17.1 (8.2 to 25.9) | |
| CAN, n (% [95% CI]) | 28 (24.6 [16.7 to 22.5]) | 32 (33.3 [23.9 to 42.8]) | 56 (45.2 [36.4 to 3.9]) |
| weighted difference from CsA (97.3% CI)a | −18.9 (−31.3 to −6.4) | −8.2 (−22.4 to 5.9) | |
| Acute rejection | |||
| acute rejection, n (%) | 26 (22.8) | 20 (20.6) | 18 (14.4) |
| 95% CI | 15.1 to 30.5 | 12.6 to 28.7 | 8.2 to 20.6 |
| weighted difference from CsA (97.3% CI)a | 9.7 (−1.1 to 20.5) | 7.6 (−4.8 to 20.0) | |
| Banff grade, n (%) | |||
| mild acute (1A) | 1 (0.9) | 2 (2.1) | 0 (0.0) |
| mild acute (1B) | 1 (0.9) | 2 (2.1) | 1 (0.8) |
| moderate acute (IIA) | 11 (9.6) | 8 (8.2) | 13 (10.4) |
| moderate acute (IIB) | 12 (10.5) | 7 (7.2) | 4 (3.2) |
| severe acute (III) | 1 (0.9) | 1 (1.0) | 0 (0.0) |
CAN, chronic allograft nephropathy; CI, confidence interval; CsA, cyclosporine; LI, less intensive; MI, more intensive; RTR-PD, renal transplant recipients with preexisting diabetes.
The DerSimonian-Laird model was used to assess risk difference with a random effect of study.
Measured GFR analysis with imputation.
Estimated difference evaluated using an analysis of covariance with a random effect of study.
Table 3.
Adjudicateda cause of graft loss across treatment arms in RTR-PD patients
| Adjudicated Cause of Graft Loss | Belatacept MI | Belatacept LI | CsA |
|---|---|---|---|
| Infection | 1 | 0 | 2 |
| Primary graft thrombosis | 2 | 1 | 4 |
| Primary nonfunction | 0 | 0 | 2 |
| Rejection | 0 | 0 | 2 |
| Parenchymal disease | 0 | 0 | 1 |
| Other/technical | 2 | 1 | 4 |
| Unknown | 0 | 1 | 1 |
| Total | 5 | 3 | 16 |
The cause of graft loss was adjudicated by an independent Event Adjudication Committee (EAC) comprising four nephrologists/renal transplant surgeons. Only events meeting the protocol-defined criteria for graft loss were submitted to the EAC to assess cause of graft loss. CsA, cyclosporine; LI, less intensive; MI, more intensive; RTR-PD, renal transplant recipients with preexisting diabetes.
Renal Function and Structure
Of RTR-PD patients, 12-month renal function was better with belatacept versus cyclosporine, as demonstrated by fewer patients meeting the composite renal impairment endpoint (percentage of patients with impaired renal function): 58.6% MI, 64.0% LI, and 87.2% cyclosporine; weighted differences from cyclosporine (97.3% CI) were −27.0 (−39.2 to −14.8) and −21.8 (−35.1 to −8.6) for MI and LI, respectively (Table 2). Of the ITT RTR-PD population, 94% of patients were included in the analysis. The percentages of patients who were imputed as meeting composite renal impairment endpoint because of graft loss or death were 8.1% MI, 6.7% LI, and 17.9% cyclosporine. The percentages of patients excluded from this analysis because of missing GFR at months 3 and/or 12 who could not be imputed were 2.6% MI, 8.2% LI, and 6.4% cyclosporine.
Numerically higher renal function in belatacept RTR-PD patients was also demonstrated by mean mGFR alone. Of the ITT RTR-PD population, 83.6% of patients were included in this analysis. Mean mGFR values at 12 months (with imputation) were 59.8 ml/min per 1.73 m2 MI, 62.5 ml/min per 1.73 m2 LI, and 45.4 ml/min per 1.73 m2 cyclosporine. There was no imputation for patients with graft loss or death. For other patients with missing data, mGFR at other time points or cGFR at the same time points was used to impute missing values. The estimated differences from cyclosporine (97.3% CI) were 14.35 (5.99 to 22.71) and 17.08 (8.24 to 25.91) for MI and LI, respectively (Table 2). Observed mGFR was available for 77.2%, 72.2%, and 66.4% of the MI, LI, and cyclosporine arms, respectively. The corresponding percentages of patients included in this analysis with imputed GFR were 12.3%, 13.4%, and 10.4%, for the MI, LI, and cyclosporine arms, respectively. The values were missing and not applicable to be imputed for 10.5%, 14.4%, and 23.2% of patients in the respective treatment arms.
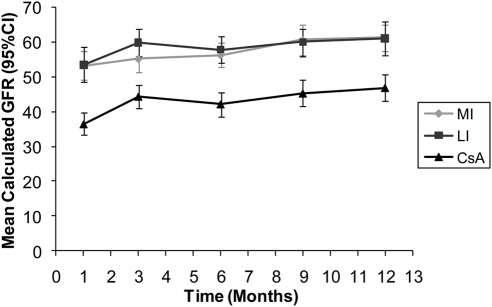
Numerically higher renal function in belatacept RTR-PD patients was also demonstrated by mean cGFR (Modification of Diet in Renal Disease) values. Figure 2 shows mean cGFR and 95% CI values for all treatment groups at various time points over 12 months. Belatacept mean cGFR in both the MI and LI arms was consistently higher than observed in the cyclosporine group from month 1 through month 12.
Figure 2.
Mean calculated GFR over 1 year in renal transplant recipients with pretransplant diabetes. CI, confidence interval; CsA, cyclosporine; LI, less intensive; MI, more intensive.
Chronic allograft nephropathy at 12 months is shown in Table 2: 24.6% MI, 33.3% LI, and 45.2% cyclosporine; weighted differences from cyclosporine (97.3% CI) were −18.9 (−31.3 to −6.4) and −8.2 (−22.4 to 5.9) for MI and LI, respectively. Of the ITT RTR-PD population, 99% of patients were included in the analysis.
Acute Rejection
The 12-month incidence of AR was higher with belatacept versus cyclosporine (22.8% MI, 20.6% LI, and 14.4% cyclosporine); weighted differences from cyclosporine (97.3% CIs) were 9.7 (−1.1 to 20.5) and 7.6 (−4.8 to 20.0) for MI and LI, respectively (Table 2). Higher grades of AR (Banff grade ≥ IIB rejection) were also observed in belatacept versus cyclosporine patients. All RTR-PD patients from the ITT population were included in the analysis.
Cardiovascular and Metabolic Outcomes
Mean 12-month BPs were lower with belatacept versus cyclosporine (systolic BP [SD]: 139.1 [18.5] mmHg MI, 139.5 [19.3] mmHg LI, and 145.3 [23.8] mmHg cyclosporine; diastolic BP: 76.7 [12.5] mmHg MI, 77.0 [9.1] mmHg LI, and 79.1 [10.7] mmHg cyclosporine) (Table 4). Of the ITT RTR-PD population, 80% MI, 76% LI, and 69% cyclosporine patients were included in the analysis. The proportion of patients using ≥3 antihypertensive medications at 12 months was lower with belatacept versus cyclosporine (40.9% MI, 35.8% LI, and 46.3% cyclosporine) (Table 4). Of the ITT RTR-PD population, 96% MI, 98% LI, and 97% cyclosporine patients were included in the analysis.
Table 4.
Secondary outcomes: cardiovascular endpoints at month 12 in RTR-PD subpopulation
| Parameter | Belatacept MI (n = 114) | Belatacept LI (n = 97) | CsA (n = 125) |
|---|---|---|---|
| Serum lipids | |||
| non-HDL cholesterol | |||
| mean change from baseline, mg/dl (SE) | 7.9 (4.2) | 11.3 (4.7) | 14.9 (4.3) |
| estimated difference from CsA (97.3% CI) | −6.9 (−20.3 to 6.4) | −3.6 (−17.8 to 10.6) | |
| triglycerides | |||
| mean change from baseline, mg/dl (SE) | −26.5 (13.8) | −25.6 (14.7) | −9.9 (14.3) |
| estimated difference from CsA (97.3% CI) | −16.7 (−60.7 to 27.4) | −15.7 (−61.0 to 29.6) | |
| blood pressure (mmHg) | |||
| mean systolic BP (SD) | 139.1 (18.5) | 139.5 (19.3) | 145.3 (23.8) |
| mean diastolic BP (SD) | 76.7 (12.5) | 77.0 (9.1) | 79.1 (10.7) |
| Patients receiving medications | |||
| ≥1 antihyperlipidemic medication, n (%) | 54 (47.4) | 48 (49.5) | 67 (53.6) |
| 95% CI | 38.2 to 56.5 | 39.5 to 59.4 | 44.9 to 62.3 |
| ≥1 antihypertensive medication, n (%) | 98 (89.1) | 85 (89.5) | 113 (93.4) |
| 95% CI | 83.3 to 94.9 | 83.3 to 95.6 | 89.0 to 97.8 |
| ≥3 antihypertensive medications, n (%) | 45 (40.9) | 34 (35.8) | 56 (46.3) |
CsA is the referent group in this analysis. CI, confidence interval; CsA, cyclosporine; LI, less intensive; MI, more intensive; RTR-PD, renal transplant recipients with preexisting diabetes.
Mean non-HDL level increase from baseline was lower with belatacept versus cyclosporine (7.9 mg/dl MI, 11.3 mg/dl LI, and 14.9 mg/dl cyclosporine) (Table 4). Of the ITT RTR-PD population, 78% MI, 72% LI, and 65% cyclosporine patients were included in the analysis. Mean decrease from baseline in triglyceride levels was greater with belatacept versus cyclosporine (−26.5 mg/dl MI, −25.6 mg/dl LI, and −9.9 mg/dl cyclosporine) (Table 4). Of the ITT RTR-PD population, 55% MI, 58% LI, and 46% cyclosporine patients were included in the analysis. The proportion of patients using ≥1 antihyperlipidemic medication at 12 months was lower with belatacept versus cyclosporine (47.4% MI, 49.5% LI, and 53.6% cyclosporine) (Table 4). All of the ITT patients were included in the analysis.
Safety
Adverse events (AEs) for RTR-PD patients are listed in Table 5. Those of particular interest in RTR are: total serious AEs (60.5% MI, 54.6% LI, and 67.2% cyclosporine); serious infections (29.8% MI, 20.6% LI, and 30.4% cyclosporine); neoplasms (3.5% MI, 3.1% LI, and 5.6% cyclosporine); and serious cardiac disorders (8.8% MI, 4.1% LI, and 10.4% cyclosporine). Three cases of posttransplant lymphoproliferative disorder (PTLD) occurred in this subpopulation, all in the arm receiving the belatacept LI regimen. One case involved the central nervous system.
Table 5.
Patients with serious adverse events in RTR-PD subpopulation
| Serious Adverse Events, n (%) | Belatacept MI (n = 114) | Belatacept LI (n = 97) | CsA (n = 125) |
|---|---|---|---|
| All adverse events | 113 (99.1) | 97 (100) | 124 (99.2) |
| Total serious adverse events | 69 (60.5) | 53 (54.6) | 84 (67.2) |
| infections and infestations | 34 (29.8) | 20 (20.6) | 38 (30.4) |
| injury, poisoning, and procedural complications of transplanted kidney | 14 (12.3) | 16 (16.5) | 19 (15.2) |
| renal and urinary disorders | 13 (11.4) | 9 (9.3) | 26 (20.8) |
| gastrointestinal disorders | 12 (10.5) | 6 (6.2) | 10 (8.0) |
| general disorders and administration site conditions | 11 (9.6) | 5 (5.2) | 13 (10.4) |
| cardiac disorders | 10 (8.8) | 4 (4.1) | 13 (10.4) |
| investigations | 9 (7.9) | 6 (6.2) | 12 (9.6) |
| vascular disorders | 9 (7.9) | 6 (6.2) | 17 (13.6) |
| blood and lymphatic system disorders | 8 (7.0) | 2 (2.1) | 7 (5.6) |
| metabolism and nutrition disorders | 5 (4.4) | 4 (4.1) | 7 (5.6) |
| nervous system disorders | 5 (4.4) | 0 (0) | 4 (3.2) |
| respiratory, thoracic, and mediastinal disorders | 5 (4.4) | 4 (4.1) | 5 (4.0) |
| neoplasms: benign, malignant, and unspecified (includes cysts and polyps) | 4 (3.5) | 3 (3.1) | 7 (5.6) |
| reproductive system and breast disorders | 3 (2.6) | 0 (0) | 2 (1.6) |
| eye disorders | 2 (1.8) | 0 (0) | 0 (0) |
| musculoskeletal and connective tissue disorders | 2 (1.8) | 0 (0) | 0 (0) |
| skin and subcutaneous tissue disorders | 2 (1.8) | 1 (1.0) | 2 (1.6) |
| congenital, familial, and genetic disorders | 1 (0.9) | 0 (0) | 0 (0) |
| endocrine disorders | 0 (0) | 0 (0) | 1 (0.8) |
| hepatobiliary disorders | 0 (0) | 1 (1.0) | 0 (0) |
There were no significant differences between belatacept and cyclosporine groups. CsA, cyclosporine; LI, less intensive; MI, more intensive; RTR-PD, renal transplant recipients with preexisting diabetes.
Comparison of Outcomes in Patients with or without Pre-existing Diabetes
The general observation of poorer outcomes in RTR-PD merited a comparison of the subpopulations with or without PD from BENEFIT and BENEFIT-EXT trials. For belatacept-treated patients, comparable rates of composite patient/graft survival and of graft loss alone were observed in the subpopulations with or without PD (Table 6). For cyclosporine-treated patients, the rate of patient/graft survival was lower, and the rate of graft loss was higher in the RTR-PD subpopulation versus without (Table 6). Across all treatment groups, death rates were higher in the RTR-PD subpopulation relative to the subpopulation without (Table 6).
Table 6.
Comparison of efficacy and safety in patients with or without preexisting diabetes
| Outcomes | Belatacept MI | Belatacept LI | CsA |
|---|---|---|---|
| Patient/graft survival | |||
| diabetes, %; difference from CsA (97.3% CI)a | 90.4; 8.5 (−0.4 to 17.4) | 92.8; 10.2 (1.5 to 18.9) | 80.8 |
| 95% CI (%) | 84.9 to 95.8 | 87.6 to 97.9 | 73.9 to 87.7 |
| no diabetes, %; difference from CsA (97.3% CI)a | 91.3; −0.70 (−5.4 to 4.0) | 92.8; 0.52 (−3.9 to 5.0) | 92.9 |
| 95% CI (%) | 88.1 to 94.6 | 89.9 to 95.7 | 89.8 to 95.9 |
| Graft loss | |||
| diabetes, % | 4.4 | 3.1 | 12.8 |
| no diabetes, % | 5.5 | 5.6 | 3.9 |
| Death | |||
| diabetes, % | 6.1 | 3.1 | 5.6 |
| no diabetes, % | 2.4 | 1.6 | 2.9 |
| Composite renal impairment endpoint | |||
| diabetes, %; difference from CsA (97.3% CI)a | 58.6; −27.0 (−39.2 to −14.8) | 64.0; −21.8 (−35.1 to −8.6) | 87.2 |
| 95% CI (%) | 49.4 to 67.7 | 54.1 to 74.0 | 81.1 to 93.2 |
| no diabetes, %; difference from CsA (97.3% CI)a | 63.5; −14.7 (−28.0 to −1.4) | 63.9; −13.5 (−39.3 to 12.3) | 78.5 |
| 95% CI (%) | 57.8 to 69.2 | 58.5 to 69.4 | 73.6 to 83.3 |
| Mean iothalamate GFR (measured)b | |||
| diabetes, mean; difference from CsA (97.3% CI)c | 59.8; 14.3 (6.0 to 22.7) | 62.5; 17.1 (8.2 to 25.9) | 45.4 |
| no diabetes, mean; difference from CsA (97.3% CI)c | 58.4; 10.2 (5.4 to 14.9) | 55.0; 6.8 (2.2 to 11.4) | 48.2 |
| Total SAEs | |||
| diabetes, % | 60.5 | 54.6 | 67.2 |
| no diabetes, % | 59.5 | 52.6 | 61.4 |
| Serious cardiac disorders | |||
| diabetes, % | 8.8 | 4.1 | 10.4 |
| no diabetes, % | 5.9 | 3.6 | 4.6 |
| Serious infections | |||
| diabetes, % | 29.8 | 20.6 | 30.4 |
| no diabetes, % | 26.3 | 25.0 | 26.4 |
| Neoplasms (total) | |||
| diabetes, % | 3.5 | 3.1 | 5.6 |
| no diabetes, % | 3.5 | 2.3 | 1.4 |
For patients with preexisting diabetes: n = 114 (MI), n = 97 (LI), n = 125 (CsA). For patients without preexisting diabetes: n = 289 (MI), n = 304 (LI), n = 280 (CsA). CI, confidence interval; CsA, cyclosporine; LI, less intensive; MI, more intensive; SAEs, serious adverse events.
The DerSimonian-Laird model was used to assess risk difference with a random effect of study.
Measured GFR analysis with imputation.
Estimated difference evaluated using an analysis of covariance with a random effect of study.
Similar proportions of belatacept-treated patients with or without PD reached the composite renal impairment endpoint (Table 6). The proportion of cyclosporine patients with impaired renal function was numerically higher in the RTR-PD subpopulation versus without (Table 6). The composite renal endpoint was defined by mGFR but also incorporates imputation because of death or graft loss. The differences observed between cyclosporine patients with or without PD are driven by a greater number of diabetic patients with graft loss. Measured 1-year GFR was similar for diabetic and nondiabetic patients treated with belatacept MI or cyclosporine (Table 6). In contrast, mGFR was numerically higher for diabetic versus nondiabetic patients treated with the belatacept LI regimen (Table 6).
Patients treated with belatacept LI regimen had comparable rates of AEs of interest in the diabetic and nondiabetic subpopulations: total serious AEs (54.6%, 52.6%), including cardiac disorders (4.1%, 3.6%), infections (20.6%, 25.0%), and neoplasms (3.1%, 2.3%) (Table 6). Patients treated with cyclosporine had somewhat higher rates of AEs in the diabetic versus nondiabetic subpopulation: total serious AEs (67.2%, 61.4%) including cardiac disorders (10.4%, 4.6%), infections (30.4%, 26.4%), and neoplasms (5.6%, 1.4%) (Table 6).
Discussion
The favorable efficacy and safety profile observed with belatacept in BENEFIT and BENEFIT-EXT extends to the RTR-PD subpopulation. The proportion of RTR-PD patients surviving with a functioning graft was numerically higher for belatacept versus cyclosporine at 1 year, particularly for the LI regimen, whereas similar composite patient/graft survival was observed in the belatacept and cyclosporine arms in the overall ITT population (16,17). Retrospective studies involving RTR-PD show 1-year patient and graft-survival rates in the approximate range of 85% to 95% (5,6,20) and 80% to 90% (4–6), respectively. In this study, the 1-year patient survival rates were at the higher end of the range previously reported (5,6,20), and 1-year survival rates were slightly higher for belatacept LI-treated patients compared with cyclosporine-treated patients. Belatacept patients in this analysis also had higher graft-survival rates than previously reported, whereas the rate for cyclosporine patients was within the range previously reported (4–6). Most of the difference between groups for the composite endpoint was due to a higher rate of graft loss with cyclosporine. The most common cause of graft loss with cyclosporine was primary graft thrombosis, followed by primary nonfunction, infection, and rejection. This was also true for nondiabetic patients. Graft loss in cyclosporine RTR-PD patients occurred at a higher rate in the BENEFIT-EXT population (extended criteria donors) (18.2%) compared with the BENEFIT population (living and standard criteria donors) (6.8%).
Better renal function was observed in belatacept versus cyclosporine RTR-PD patients, consistent with results observed in the ITT population. The renal function benefit in belatacept RTR-PD patients is evidenced by a lower proportion of patients meeting the composite renal impairment endpoint, and numerically higher mGFR and cGFR values at 1 year. RTR-PD patients responded well to belatacept LI regimen, as illustrated by a mean mGFR value that corresponds to the upper end of the range observed in belatacept LI patients from the ITT population at 1 year (16,17). Furthermore, the data presented here suggest that cyclosporine patients in this subgroup may have worse renal function than those in the ITT population. Mean mGFR for cyclosporine patients in this analysis falls at the lower end of the range observed in cyclosporine patients from the ITT population at 1 year (16,17). Lastly, a reduced prevalence of chronic allograft nephropathy and higher rates and grades of AR were noted in the diabetic subpopulation treated with belatacept versus cyclosporine.
An improved cardiovascular profile was observed for belatacept versus cyclosporine, consistent with results observed in the ITT population. Cardiovascular risk factors and outcomes are worse in RTR-PD (2,3), and improvements in these outcomes may enhance survival in RTR-PD patients. In this analysis, lower BP, smaller increases in non-HDL cholesterol, larger decreases in triglyceride levels from baseline, and less frequent use of antihypertensive and antilipidemic medications were noted with belatacept versus cyclosporine. The selective mechanism of action of belatacept may allow transplant recipients to avoid the adverse cardiovascular events associated with calcineurin inhibitors, such as hypertension and hyperlipidemia.
Safety observed with belatacept in the ITT population was maintained in RTR-PD patients. Clinical data from the BENEFIT and BENEFIT-EXT studies have indicated that the belatacept LI regimen is associated with a more favorable safety profile, supporting it as the recommended regimen moving forward (18). Thus, to investigate the safety of belatacept in the RTR-PD subpopulation, patients treated with the LI regimen were compared with patients treated with cyclosporine. Patients receiving the belatacept LI regimen had fewer serious AEs and serious infections than those receiving the cyclosporine or belatacept MI regimens. Of particular interest to RTR, serious cardiac disorders were less frequent in belatacept LI than in belatacept MI or cyclosporine patients. Additional follow-up is required to determine whether these 1-year differences translate to long-term survival benefits for belatacept LI RTR-PD patients. In general, RTR-PD patients demonstrated a more favorable safety profile when treated with the LI versus MI regimen, a result also observed in the ITT population (16–18,21).
Comparison of the RTR-PD and non-RTR-PD pooled subpopulations indicated that belatacept RTR-PD patients, in particular those treated with the LI regimen, had comparable outcomes to the non-RTR-PD patients treated with belatacept. Patient/graft survival, graft loss, renal function, rates of serious AEs, serious cardiac disorders, serious infections, and neoplasms were similar. The same directional findings were not observed in cyclosporine RTR-PD patients. These observations suggest that the suboptimal outcomes typically expected for RTR-PD may be avoided with belatacept therapy.
Mean cyclosporine trough levels for the RTR-PD subpopulation generally mirrored that of the ITT population. As recently reported, patients within quartiles of cyclosporine levels exhibited a similar degree of renal dysfunction in the BENEFIT trial (22). This suggests that cyclosporine effects may not be solely dose dependent. Patients treated with lower doses of cyclosporine exhibited poorer outcomes in previously reported trials (23), although calcineurin-inhibitor–sparing regimens have been shown to significantly improve GFR (24).
No controlled studies have assessed comparative outcomes of different immunosuppressant regimens in RTR-PD patients. This analysis used data from two large, randomized trials, permitting comparisons between belatacept and cyclosporine. However, this was a post hoc analysis, limited to 1-year follow-up, so the interpretation of data are descriptive rather than inferential, imposing limitations on conclusions drawn. Additionally, this post hoc analysis was not powered to detect differences between treatment arms for any of the endpoints, including cardiovascular parameters. This analysis was potentially limited by the definition of pre-existing diabetes, which relied on reported patient history of diabetes and use of anti-diabetic medications at baseline. World Health Organization criteria were not required for a definition of diabetes, so the pooled analysis did not capture fasting or 2-hour plasma glucose levels (25). Other studies examining the effect of PD have relied on historical definitions of diabetes (e.g., United States Renal Data System database or United Network for Organ Sharing database) (26,27). Prospective studies based on stringent World Health Organization definitions of diabetes may be incrementally beneficial in studying the specific effects of belatacept in this patient population. However, the definition of diabetes used in this study is not expected to over- or underestimate the number of patients for whom diabetes represented a clinical concern and thus, for whom belatacept might provide some positive effect.
The results of these analyses suggest that RTR-PD patients may experience particular benefit from the belatacept LI regimen versus the cyclosporine regimen. Further study is needed to extend and confirm the positive findings from these analyses, with special emphasis on evaluation of long-term patient and graft survival in this subpopulation.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge Anju Anne Roy for writing assistance in the preparation of this manuscript, with funding from Bristol-Myers Squibb.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J: Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13: 1084–1090, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Kasiske BL, Chakkera HA, Roel J: Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 11: 1735–1743, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y: Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant 8: 593–599, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Orsenigo E, Socci C, Carlucci M, Zuber V, Fiorina P, Gavazzi F, Secchi A, Di Carlo V, Staudacher C: Multivariate analysis of factors affecting patient and graft survival after renal transplant. Transplant Proc 37: 2461–2463, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM: Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant 15: 89–94, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Posada JM, Hernandez D, Bayes GB, Garcia PJ, Rivero SM: Impact of diabetes mellitus on kidney transplant recipients in Spain. Nephrol Dial Transplant 19[Suppl 3]: iii57–iii61, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Kronson JW, Gillingham KJ, Sutherland DE, Matas AJ: Renal transplantation for type II diabetic patients compared with type I diabetic patients and patients over 50 years old: A single-center experience. Clin Transplant 14: 226–234, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Miller LW: Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2: 807–818, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El Shahawy M, Budde K, Goto N: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007. 17359512 [Google Scholar]
- 12. Roland M, Gatault P, Doute C, Buchler M, Al Najjar A, Barbet C, Chatelet V, Marliere JF, Nivet H, Lebranchu Y, Halimi JM: Immunosuppressive medications, clinical and metabolic parameters in new-onset diabetes mellitus after kidney transplantation. Transpl Int 21: 523–530, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Joss N, Staatz CE, Thomson AH, Jardine AG: Predictors of new onset diabetes after renal transplantation. Clin Transplant 21: 136–143, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrom J, Leivestad T, Egeland T, Fauchald P: Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation 64: 979–983, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Johnston O, Rose CL, Webster AC, Gill JS: Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 19: 1411–1418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP: A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 10: 535–546, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial MC, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyo J: A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 10: 547–557, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Grinyo J, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti F, Reyes-Acevedo R, Apanovitch AM, Gujrathi S, Agarwal M, Thomas D, Larsen CP: An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation 90: 1521–1527, 2010 [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 20. David KM, Morris JA, Steffen BJ, Chi-Burris KS, Gotz VP, Gordon RD: Mycophenolate mofetil vs. azathioprine is associated with decreased acute rejection, late acute rejection, and risk for cardiovascular death in renal transplant recipients with pre-transplant diabetes. Clin Transplant 19: 279–285, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Shen J, Block A, Townsend R, You X, Shen Y, Zhou S, Geng D, McGirr N, Soucek K, Pursley J, DiRusso G, Kaul S: Pharmacokinetics, pharmacodynamics, and immunogenicity of belatacept in renal transplant recipients. [Abstract]. Presented at the XXIII International Congress of the Transplantation Society, August 15–19, 2010; Vancouver, Canada Abstract 1870 2010 [Google Scholar]
- 22. Vincenti F: Yes there is a benefit to BENEFIT. Am J Transplant 11: 634, 2011 [Google Scholar]
- 23. Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, Margreiter R, Hugo C, Grinyo JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF: Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Moore J, Middleton L, Cockwell P, Adu D, Ball S, Little MA, Ready A, Wheatley K, Borrows R: Calcineurin inhibitor sparing with mycophenolate in kidney transplantation: A systematic review and meta-analysis. Transplantation 87: 591–605, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Report of a WHO/IDFConsultation 2006. International Diabetes Federation 2011 [Google Scholar]
- 26. Irish WD, McCollum DA, Tesi RJ, Owen AB, Brennan DC, Bailly JE, Schnitzler MA: Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol 14: 2967–2974, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Parekh J, Bostrom A, Feng S: Diabetes Mellitus: A risk factor for delayed graft function after deceased donor kidney transplantation. Am J Transplant 10: 298–303, 2010 [DOI] [PubMed] [Google Scholar]