Abstract
Summary
Background and objectives
Serum phosphorus levels are associated with mortality, cardiovascular disease, and renal function loss in individuals with and without chronic kidney disease. The association of pretransplant serum phosphorus levels with transplant outcomes is not clear.
Design, setting, participants, & measurements
Data of the Scientific Registry of Transplant Recipients (SRTR) up to June 2007 were linked to the database (2001 through 2006) of one of the U.S.-based large dialysis organizations (DaVita). The selected 9384 primary kidney recipients were divided into five groups according to pretransplant serum phosphorus levels (mg/dl): <3.5, 3.5 to <5.5 (reference group), 5.5 to <7.5, 7.5 to <9.5, and ≥9.5. Unadjusted and multivariate adjusted risks for transplant outcomes were compared.
Results
Patients were 48 ± 14 years old and included 37% women and 27% African Americans. After multivariate adjustment, all-cause and cardiovascular death hazard ratios were 2.44 (95% confidence interval: 1.28 to 4.65) and 3.63 (1.13 to 11.64), respectively, in recipients in the ≥9.5 group; allograft loss hazard ratios were 1.42 (1.04 to 1.95) and 2.36 (1.33 to 4.17) in recipients with 7.5 to >9.5 and ≥9.5, respectively. No significant association with delayed graft function was found.
Conclusions
Pretransplant phosphorus levels 7.5 to <9.5 mg/dl and ≥9.5 mg/dl were associated with increased risk of functional graft failure and increased risk of all-cause and cardiovascular deaths, respectively, when compared with 3.5 to <5.5 mg/dl. Additional studies are needed to examine whether more aggressive control of pretransplant serum phosphorus may improve posttransplant outcomes.
Introduction
Serum levels of phosphorus are associated with mortality (1,2), cardiovascular disease (CVD) (3), and renal function loss in chronic kidney disease (CKD) patients (4–6). Both low and high serum phosphorus levels are associated with an increased risk of death (7,8), whereas risks of a cardiovascular event and kidney dysfunction increase linearly with serum phosphorus levels (4,7). In CKD patients on dialysis (dialysis-CKD), phosphorus levels over 6.5 to 7.0 mg/dl are significantly associated with increased mortality (1,9,10), and therapeutic guidelines recommend maintaining serum phosphorus in the 3.5 to 5.5 mg/dl range (11). Despite this, 50 to 60% of the U.S. dialysis-CKD patients who are potential kidney transplant candidates have serum phosphorus over 5.5 mg/dl.
The reasons for an inferior survival among low and high phosphorus dialysis-CKD patients are different. Hypophosphatemia, in the majority of the cases, is a marker of a poor nutritional status, whereas hyperphosphatemia can induce vascular calcification. High phosphorus levels can stimulate differentiation of vascular smooth muscle cells into osteoblastic-like cells, with bone matrix production and mineralization (12,13). As a consequence of stiffer arteries, pulse wave velocity, arterial sheer stress, and cardiac output resistance increase and may cause vascular intima damage and left ventricular hypertrophy (14–16). Coronary artery calcification may also contribute to coronary insufficiency and myocardial ischemia (17). Therefore, hyperphosphatemia is considered a nontraditional risk factor for CVD in CKD patients, and for many kidney recipients vascular disease is an undesired heritage from the dialysis period (18,19).
Increased phosphorus wasting and hypophosphatemia are described in the posttransplant period (20,21). In native kidneys phosphaturia can cause calcium-phosphorus deposition in the tubules, which may trigger a local inflammatory response and lead to tubular obstruction, and ultimately to kidney failure (22). These mechanisms may be reproducible in the renal allograft and may contribute to graft dysfunction (23).
The effect of pretransplant recipients' serum phosphorus level on transplant outcomes is unclear; however, a recent study showed in living kidney donor transplants that a higher donor phosphorus level was an independent risk factor for early allograft dysfunction (24). We sought to examine the association of pretransplant serum phosphorus levels with all-cause and CVD mortality, graft failure, and delayed graft function (DGF) in a U.S.-based renal transplant population and we hypothesized that higher phosphorus levels are incrementally associated with poor graft and patient outcomes.
Materials and Methods
Patients
We linked data on all kidney transplant recipients listed in the Scientific Registry of Transplant Recipients (SRTR) up to June 2007 to a list of individuals with CKD who underwent maintenance hemodialysis treatment from July 2001 to June 2006 in one of the outpatient dialysis facilities of a U.S.-based large dialysis organization (DaVita Inc., before its acquisition of former Gambro dialysis facilities) using patients' social security numbers. The study was approved by the Institutional Review Boards of both Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research.
Clinical and Demographic Measures
The creation of the national DaVita dialysis patient cohort has been described previously (25–31). To minimize measurement variability, all repeated measures for each patient during any given calendar quarter (i.e., over a 13-week interval) were averaged and the summary estimate was used in all models. Average values were obtained from up to 20 calendar quarters (q1 through q20) for each laboratory and clinical measure for each patient for up to 6 years of follow-up. The first (baseline) studied quarter for each patient was the calendar quarter in which the patient's dialysis vintage was >90 days. Demographic data and details of medical history were collected, with information on age, gender, race, type of insurance, marital status, presence of diabetes, height, posthemodialysis dry weight (to calculate averaged body mass index [BMI]), and dialysis vintage. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day of kidney transplantation.
Laboratory Measures
Blood samples were drawn using uniform techniques in all of the DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida, typically within 24 hours. All laboratory values were measured by automated and standardized methods in the DaVita Laboratory. Most laboratory values were measured monthly, including serum urea, creatinine, albumin, calcium, phosphorus, bicarbonate, and total iron binding capacity (TIBC). Serum ferritin and intact parathyroid hormone (PTH) were measured at least quarterly. Hemoglobin was measured at least monthly in essentially all patients and weekly to biweekly in most patients. Most blood samples were collected predialysis with the exception of postdialysis serum urea nitrogen to calculate urea kinetics. Kt/V (single pool) was calculated using urea kinetic modeling equations as described elsewhere (27). To examine the “dose-response” association between pretransplant serum phosphorus categories and outcomes risk, we divided patients into five a priori defined categories based on pretransplant serum phosphorus level: <3.5, 3.5 to <5.5, 5.5 to <7.5, 7.5 to <9.5, and ≥9.5 mg/dl values.
Statistical Analyses
Data were summarized using proportions, means (±SD). We examined P values for trends across pretransplant serum phosphorus categories. Time-to-event survival analyses were done to determine association of serum phosphorus with all-cause and cardiovascular mortality and graft failure (defined as reinitiation of dialysis treatment or retransplantation). For delayed graft function (DGF), defined as the need for any dialysis therapy in the first week after transplantation (32), time to event was not accounted for. Survival analyses to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of death or graft failure employed Cox proportional hazards regression. In mortality analyses patients were followed until event (death) or censoring (graft failure or end of follow-up period), whichever happened first. In graft failure analyses patients were followed until event (graft failure) or censoring (death or end of follow-up period), whichever happened first. In the combined outcome analyses patients were followed until event (death or graft failure) or censoring (end of follow-up period), whichever happened first. Proportional hazard assumption was tested using log(−log) against survival plots. Logistic regression models were used to estimate the odds ratio (OR) and 95% CI of posttransplant DGF.
For each regression analysis, four levels of multivariate adjustment were examined: (1) an unadjusted model that included pretransplant serum phosphorus categories (3.5 to <5.5 mg/dl as reference) as the predictor; (2) case-mix adjusted models that included the above plus age, gender, recipient race-ethnicity (African Americans and other self-categorized blacks, non-Hispanic whites, Asians, Hispanics, and others), diabetes mellitus, dialysis vintage (<6 months, 6 months to 2 years, 2 to <5 years, and ≥5 years), primary insurance (Medicare, Medicaid, private, and others), marital status (married, single, divorced, widowed, and other or unknown), standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities (atherosclerotic heart disease, congestive heart failure, cancer, chronic obstructive pulmonary disease, CVD, hypertension, peripheral vascular disease, and tobacco use); (3) malnutrition-inflammation-complex syndrome (MICS) adjusted models which included all of the above covariates plus nine surrogates of nutritional status and inflammation measured during the last calendar quarter before transplantation including BMI and eight laboratory variables (i.e., normalized PCR [nPCR] as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance [nPNA] [33], and serum or blood concentrations of TIBC, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, and albumin); and (4) case-mix, MICS, and transplant data adjusted models included all of the above plus eight transplant-related variables: (1) donor type (deceased or living), (2) donor age, (3) donor gender, (4) panel reactive antibody (PRA) titer (last value before transplant), (5) number of HLA mismatches, (6) cold ischemia time, (7) DGF (except when DGF was a dependent variable in logistic regression models), and (8) extended donor criteria using standard definition (donor history of hypertension and/or serum creatinine of donor >1.5 mg/dl and/or cause of death in donor is cerebrovascular event). All analyses were carried out with SAS version 9.1 (SAS Institute Inc., Cary, NC) and STATA version 11.1 (STATA Corporation, College Station, TX).
Results
The original 5-year (July 2001 through June 2006) national database of all DaVita patients included 164,789 adult patients. Out of 65,386 DaVita patients who were identified in the SRTR database, 17,629 had undergone one or more kidney transplantations during their lifetime, but only 14,508 dialysis patients had undergone kidney transplantation for the first time. From these 14,508 dialyzed patients we excluded patients on chronic peritoneal dialysis (n = 2092) and patients who did not have serum phosphorus measurements (n = 3032). The analytic cohort consisted of the remaining 9384 patients who underwent first kidney transplantation during the observation period and who were followed until death, graft failure, loss of follow-up, or survival until June 30, 2007 (Supplemental Figure S1). There were 737 deaths (7.9%) and 811 graft failures (8.6%) irrespective of subsequent deaths. The median cohort time was 803 days (interquartile range was 384 to 1330 days). The basic characteristics of waitlisted, but nontransplanted, patients have been described elsewhere (34). Tables 1 and 2 show the clinical, demographic, and laboratory data of the 9384 transplanted patients across five pretransplant serum phosphorus categories.
Table 1.
Baseline characteristics of 9384 dialysis patients who underwent renal transplantation between July 2001 and June 2006
| Pretransplant Serum Phosphorus Level (mg/dl) | <3.5 | 3.5 to <5.5 | 5.5 to <7.5 | 7.5 to <9.5 | ≥9.5 | P for Trend |
|---|---|---|---|---|---|---|
| N [%] | 270 [3] | 3700 [40] | 3980 [42] | 1229 [13] | 205 [2] | N/A |
| Age (years) | 53 ± 14 | 51 ± 13 | 47 ± 13 | 42 ± 13 | 37 ± 12 | <0.001 |
| Gender (% women) | 34 | 40 | 36 | 35 | 37 | 0.003 |
| Race (% African American) | 32 | 28 | 28 | 24 | 16 | <0.001 |
| Diabetes mellitus (%) | 35 | 39 | 35 | 30 | 22 | <0.001 |
| BMI (kg/m2) | 25.3 ± 5.3 | 26.2 ± 5.8 | 26.8 ± 5.8 | 27.0 ± 5.7 | 27.0 ± 6.9 | <0.001 |
| Presence of ischemic heart disease (%) | 12 | 10 | 8 | 6 | 7 | <0.001 |
| Presence of congestive heart failure (%) | 14 | 10 | 10 | 9 | 8 | 0.02 |
| Presence of hypertension (%) | 76 | 77 | 77 | 74 | 71 | 0.03 |
| Presence of cerebrovascular events (%) | 4 | 3 | 3 | 2 | 1 | 0.005 |
| Presence of peripheral vascular disease (%) | 6 | 5 | 4 | 3 | 2 | 0.002 |
| Presence of chronic obstructive pulmonary disease (%) | 2 | 1 | 1 | 1 | 0 | 0.10 |
| Presence of cancer (%) | 4 | 2 | 1 | 2 | 2 | 0.05 |
| Tobacco use (%) | 4 | 3 | 4 | 5 | 5 | 0.01 |
| Dialysis vintage (%) | ||||||
| 0 to 6 months | 9 | 11 | 12 | 11 | 13 | 0.51 |
| 6 to 24 months | 25 | 29 | 26 | 23 | 26 | <0.001 |
| 2 to 5 years | 33 | 37 | 36 | 39 | 37 | 0.49 |
| >5 years | 32 | 22 | 25 | 28 | 25 | 0.01 |
| Kt/V | 1.71 ± 0.38 | 1.65 ± 0.36 | 1.59 ± 0.35 | 1.53 ± 0.33 | 1.46 ± 0.31 | <0.001 |
| nPCR (g/kg per day) | 0.98 ± 0.29 | 1.01 ± 0.26 | 1.06 ± 0.25 | 1.12 ± 0.25 | 1.15 ± 0.25 | <0.001 |
| Serum creatinine (mg/dl) | 8.9 ± 3.5 | 9.6 ± 3.1 | 10.9 ± 3.0 | 12.1 ± 3.0 | 12.9 ± 3.2 | <0.001 |
| Serum albumin (mg/dl) | 3.9 ± 0.5 | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.4 | <0.001 |
| Serum calcium (mg/dl) | 9.4 ± 0.7 | 9.5 ± 0.7 | 9.5 ± 0.8 | 9.3 ± 0.9 | 9.0 ± 0.9 | <0.001 |
| Blood hemoglobin (g/dl) | 11.8 ± 1.5 | 12.1 ± 1.3 | 12.3 ± 1.2 | 12.3 ± 1.3 | 12.3 ± 1.4 | <0.001 |
| iPTH (pg/ml) | 293 ± 277 | 305 ± 314 | 411 ± 394 | 552 ± 549 | 425 ± 303 | <0.001 |
| WBC (× 103/L) | 6.9 ± 2.3 | 6.8 ± 2.1 | 6.8 ± 2.0 | 7.2 ± 2.1 | 7.5 ± 2.4 | <0.001 |
| Number of HLA mismatch | 3.9 ± 1.8 | 3.6 ± 1.9 | 3.6 ± 1.8 | 3.5 ± 1.8 | 3.2 ± 1.9 | 0.01 |
| Number of HLA-DR mismatch | 1.16 ± 0.74 | 1.06 ± 0.74 | 1.06 ± 0.74 | 1.07 ± 0.74 | 0.96 ± 0.71 | 0.14 |
| PRA (%) | 10 ± 24 | 9 ± 22 | 10 ± 24 | 12 ± 27 | 13 ± 28 | 0.01 |
| PRA >20% (%) | 14 | 13 | 14 | 18 | 17 | 0.002 |
| Donor age (years) | 39 ± 15 | 40 ± 15 | 39 ± 15 | 37 ± 15 | 38 ± 13 | <0.001 |
| Donor gender (% women) | 49 | 46 | 48 | 47 | 51 | 0.30 |
| Donor type (% living) | 26 | 32 | 34 | 34 | 47 | <0.001 |
| EDC kidney (%)a | 23 | 21 | 18 | 14 | 9 | <0.001 |
| Cold ischemia time (hours)a | 17.8 ± 8.3 | 18.1 ± 8.3 | 17.9 ± 8.3 | 17.6 ± 7.7 | 17.6 ± 8.3 | 0.25 |
Data are presented mean ± SD. N/A, not applicable. BMI, body mass index; nPCR, normalized protein catabolic rate; iPTH, intact parathyroid hormone; WBC, white blood cell count; RA, panel reactive antibody (last value prior to transplant). EDC, extended donor criteria.
In recipients who received a kidney from deceased donors.
Table 2.
Posttransplant outcomes of 9384 dialysis patients who underwent renal transplantation between July 2001 and June 2006
| Pretransplant Serum Phosphorus (mg/dl) | <3.5 | 3.5 to <5.5 | 5.5 to <7.5 | 7.5 to <9.5 | ≥9.5 | P for trend |
|---|---|---|---|---|---|---|
| N [%] | 270 [3] | 3700 [40] | 3980 [42] | 1229 [13] | 205 [2] | N/A |
| Deaths (n) [crude death rate %] | 37 [14] | 305 [8] | 292 [7] | 81 [7] | 22 [11] | 0.02 |
| Crude all-cause mortality rate (/1000 patient-years) [95% CI] | 57.5 [41.7 to 79.4] | 34.9 [31.2 to 39.0] | 30.1 [26.9 to 33.8] | 25.0 [20.1 to 31.1] | 46.1 [30.4 to 70.0] | N/A |
| Cardiovascular deaths (n) [crude CV death rate %] | 12 [4] | 69 [2] | 74 [2] | 23 [2] | 8 [4] | 0.82 |
| Crude CV mortality rate (/1000 patient-years) [95% CI] | 18.6 [10.6 to 32.8] | 7.9 [6.2 to 10.0] | 7.6 [6.1 to 9.6] | 7.1 [4.7 to 10.7] | 16.8 [8.4 to 33.5] | N/A |
| Graft failure (n) [crude graft failure rate %] | 28 [10] | 288 [8] | 324 [8] | 137 [11] | 34 [17] | <0.001 |
| Crude graft failure rate (/1000 patient-years) [95% CI] | 43.5 [30.0 to 63.0] | 32.9 [29.3 to 36.9] | 33.4 [30.0 to 37.3] | 42.3 [35.8 to 50.4] | 71.2 [50.9 to 99.7] | N/A |
| DGF (n) [crude DGF %] | 54 [21] | 720 [20] | 791 [21] | 258 [23] | 38 [20] | 0.24 |
| History of acute rejection (%) | 3 | 4 | 4 | 5 | 7 | 0.16 |
Values in brackets indicate the crude death and cardiovascular death rate, crude graft failure rate, and crude DGF rate in the indicated group during the 6 years of observation. Abbreviations: CI, confidence interval; CV, cardiovascular; DGF, delayed graft function.
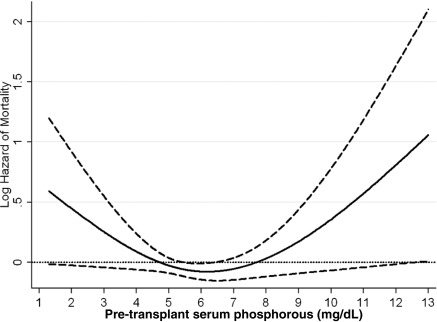
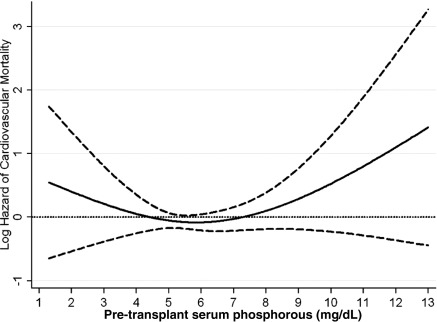
The crude all-cause mortality rate was 32.3/1000 patient-years (95% CI: 30.1 to 34.8). The associations of pretransplant phosphorus categories with the posttransplant risk of death, cardiovascular death, graft failure, or the composite of graft failure or death and delayed graft function are shown in Table 3. Compared with recipients with pretransplant phosphorus 3.5 to <5.5 mg/dl, recipients with pretransplant phosphorus of <3.5 and ≥9.5 mg/dl phosphorus had 66% (HR: 1.66, 95% CI: 1.18 to 2.33) and 32% (HR: 1.32, 95% CI: 0.85 to 2.03) higher all-cause mortality; and recipients with pretransplant phosphorus of 7.5 to <9.5 had 27% (HR: 0.73, 95% CI: 0.57 to 0.93) lower unadjusted death risk. After additional adjustment for case-mix and MICS and transplant-related variables, recipients with pretransplant phosphorus of ≥9.5 mg/dl phosphorus had 2.4-fold (HR: 2.44, 95% CI: 1.28 to 4.65) higher death risk compared with recipients with pretransplant phosphorus 3.5 to <5.5 mg/dl (Table 3A). Figures 1, 2, 3, and 4 show the cubic spline models for the association of the entire range of pretransplant serum phosphorus level with posttransplant outcomes. The association of pretransplant serum phosphorus level (Figure 1) with all-cause mortality was U-shaped; both very low and very high phosphorus levels were associated with higher mortality risk. However, in the low range the association was not statistically significant. A similar association pattern was found for cardiovascular death (Figure 2). After adjustment for case-mix and MICS and transplant-related variables recipients with pretransplant phosphorus levels of ≥9.5 mg/dl had 3.6-fold (HR: 3.63, 95% CI: 1.13 to 11.64) higher cardiovascular death risk compared with recipients with pretransplant phosphorus 3.5 to <5.5 mg/dl (Table 3B).
Table 3.
Hazard ratio (95% confidence intervals) of posttransplant death (all-cause or cardiovascular) or graft failure or delayed graft function comparing pretransplant serum phosphorus categories (3.5 to <5.5 mg/dl the reference) using Cox regression and logistic regression analyses in 9384 dialysis patients who underwent renal transplantation and were observed for up to 6 years (July 2001 through June 2007)
| Pretransplant Serum Phosphorus Level (mg/dl) | Unadjusted (n = 9384) |
+Case-mix Adjusteda (n = 8469) |
+MICS Adjustedb (n = 8073) |
+Transplant Data Adjustedc (n = 4830) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| A. Graft failure censored all-cause death | ||||||||
| <3.5 | 1.66 (1.18 to 2.33) | 0.004 | 1.61 (1.10 to 2.36) | 0.01 | 1.46 (0.98 to 2.17) | 0.06 | 1.26 (0.77 to 2.04) | 0.36 |
| 3.5 to <5.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 5.5 to <7.5 | 0.87 (0.74 to 1.02) | 0.09 | 0.96 (0.81 to 1.14) | 0.62 | 0.97 (0.81 to 1.16) | 0.75 | 0.89 (0.72 to 1.10) | 0.29 |
| 7.5 to <9.5 | 0.73 (0.57 to 0.93) | 0.01 | 0.92 (0.70 to 1.21) | 0.55 | 0.94 (0.70 to 1.25) | 0.67 | 1.09 (0.77 to 1.52) | 0.84 |
| ≥9.5 | 1.32 (0.85 to 2.03) | 0.21 | 2.25 (1.41 to 3.59) | 0.001 | 2.21 (1.36 to 3.59) | 0.001 | 2.44 (1.28 to 4.65) | 0.007 |
| B. Graft failure censored cardiovascular death | ||||||||
| <3.5 | 2.39 (1.30 to 4.42) | 0.005 | 2.40 (1.22 to 4.71) | 0.01 | 2.18 (1.10 to 4.32) | 0.03 | 1.72 (0.71 to 4.19) | 0.23 |
| 3.5 to <5.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 5.5 to <7.5 | 0.98 (0.70 to 1.36) | 0.89 | 1.00 (0.71 to 1.42) | 0.99 | 1.04 (0.72 to 1.52) | 0.81 | 1.15 (0.74 to 1.78) | 0.54 |
| 7.5 to <9.5 | 0.93 (0.58 to 1.49) | 0.75 | 1.04 (0.67 to 1.48) | 0.81 | 1.25 (0.73 to 2.13) | 0.42 | 1.14 (0.56 to 2.33) | 0.72 |
| ≥9.5 | 2.12 (1.02 to 4.41) | 0.04 | 2.66 (1.18 to 6.01) | 0.02 | 2.61 (1.07 to 6.38) | 0.04 | 3.63 (1.13 to 11.64) | 0.04 |
| C. All-cause death censored graft failure | ||||||||
| <3.5 | 1.33 (0.90 to 1.96) | 0.15 | 1.19 (0.77 to 1.84) | 0.45 | 1.18 (0.76 to 1.83) | 0.47 | 1.06 (0.58 to 1.92) | 0.85 |
| 3.5 to <5.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 5.5 to <7.5 | 1.02 (0.87 to 1.20) | 0.79 | 0.94 (0.79 to 1.11) | 0.46 | 0.98 (0.82 to 1.17) | 0.79 | 1.10 (0.88 to 1.39) | 0.40 |
| 7.5 to <9.5 | 1.32 (1.07 to 1.61) | 0.01 | 1.10 (0.88 to 1.37) | 0.43 | 1.26 (0.96 to 1.55) | 0.10 | 1.42 (1.04 to 1.95) | 0.03 |
| ≥9.5 | 2.16 (1.51 to 3.08) | <0.001 | 2.06 (1.42 to 2.99) | <0.001 | 2.24 (1.50 to 3.35) | <0.001 | 2.36 (1.33 to 4.17) | 0.003 |
| D. Combined all-cause death or graft failure | ||||||||
| <3.5 | 1.46 (1.11 to 1.92) | 0.01 | 1.42 (1.05 to 1.93) | 0.02 | 1.32 (0.97 to 1.81) | 0.08 | 1.13(0.75 to 1.70) | 0.56 |
| 3.5 to <5.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 5.5 to <7.5 | 0.95 (0.84 to 1.07) | 0.42 | 0.94 (0.83 to 1.07) | 0.35 | 0.96 (0.84 to 1.10) | 0.57 | 0.95 (0.81 to 1.13) | 0.59 |
| 7.5 to <9.5 | 1.04 (0.89 to 1.23) | 0.61 | 1.03 (0.86 to 1.23) | 0.77 | 1.09 (0.90 to 1.32) | 0.37 | 1.11 (0.87 to 1.42) | 0.40 |
| ≥9.5 | 1.71 (1.28 to 2.29) | <0.001 | 2.08 (1.52 to 2.83) | <0.001 | 2.15 (1.54 to 2.98) | <0.001 | 1.99 (1.23 to 3.21) | 0.01 |
| Unadjusted (n = 8924) | +Case-mix Adjusteda (n = 8069) | +MICS Adjustedb (n = 7692) | +Transplant Data Adjustedc (n = 4830) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| E. Delayed graft function | ||||||||
| <3.5 | 1.05 (0.77 to 1.43) | 0.76 | 0.85 (0.59 to 1.21) | 0.36 | 0.85 (0.59 to 1.23) | 0.39 | 0.80 (0.53 to 1.22) | 0.31 |
| 3.5 to <5.5 | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A |
| 5.5 to <7.5 | 1.04 (0.92 to 1.16) | 0.55 | 1.06 (0.93 to 1.19) | 0.39 | 1.00 (0.88 to 1.14) | 0.97 | 1.01 (0.87 to 1.17) | 0.90 |
| 7.5 to <9.5 | 1.15 (0.98 to 1.35) | 0.09 | 1.21 (1.01 to 1.44) | 0.04 | 1.08 (0.89 to 1.31) | 0.44 | 1.06 (0.84 to 1.33) | 0.63 |
| ≥9.5 | 0.99 (0.69 to 1.42) | 0.95 | 1.23 (0.83 to 1.82) | 0.31 | 1.03 (0.67 to 1.57) | 0.90 | 1.02 (0.60 to 1.73) | 0.93 |
Abbreviations: HR, hazard ratio; CI, confidence interval; N/A, not applicable; OR, odds ratio.
Adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter and eight comorbidities.
Adjusted for all of the covariates plus body mass index, normalized protein nitrogen appearance, serum or blood concentrations of total iron binding capacity, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, and albumin.
Adjusted for all of the above plus donor type, donor age, donor gender, panel reactive antibody titer (last value prior to transplant), number of HLA mismatches, cold ischemia time, delayed graft function (except when delayed graft function was a dependent variable in our logistic regression models), and extended donor criteria.
Figure 1.
Cubic spline model for the association of pre-transplant serum phosphorus levels with post-transplant all-cause mortality. This figure shows the hazard ratios with 95% confidence intervals of posttranplant graft failure censored all-cause death across the entire range of pretransplant serum phosphorus levels using Cox regression analyses in 9384 long-term hemodialysis transplant patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001 through June 2007). Model adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, nPNA, serum or blood concentrations of TIBC, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor gender, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, DGF, and extended donor criteria. Abbreviations: DGF, delayed graft function; nPNA, normalized protein nitrogen appearance; TIBC, total iron binding capacity.
Figure 2.
Cubic spline model for the association of pre-transplant serum phosphorus levels with post-transplant cardiovascular mortality. This figure shows the hazard ratios with 95% confidence intervals of posttranplant graft failure censored cardiovascular death across the entire range of pretransplant serum phosphorus levels using Cox regression analyses in 9384 long-term hemodialysis transplant patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001 through June 2007). Model adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, nPNA, serum or blood concentrations of TIBC, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor gender, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, DGF, and extended donor criteria. Abbreviations: DGF, delayed graft function; nPNA, normalized protein nitrogen appearance; TIBC, total iron binding capacity.
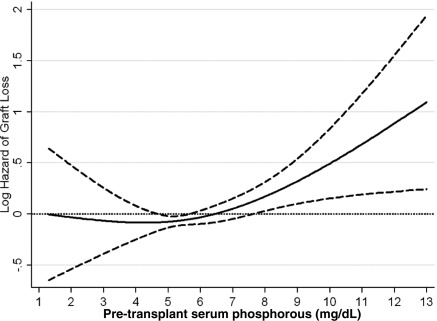
Figure 3.
Cubic spline model for the association of pre-transplant serum phosphorus levels with graft loss. This figure shows the hazard ratios with 95% confidence intervals of death censored graft failure across the entire range of pretransplant serum phosphorus levels using Cox regression analyses in 9384 long-term hemodialysis transplant patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001 through June 2007). Model adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, nPNA, serum or blood concentrations of TIBC, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor gender, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, DGF, and extended donor criteria. Abbreviations: DGF, delayed graft function; nPNA, normalized protein nitrogen appearance; TIBC, total iron binding capacity.
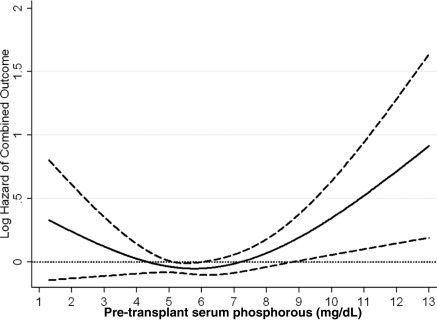
Figure 4.
Cubic spline model for the association of pre-transplant serum phosphorus levels with death or graft loss. This figure shows the hazard ratios with 95% confidence intervals of combined outcomes (all-cause of death or graft loss) across the entire range of pretransplant serum phosphorus levels using Cox regression analyses in 9384 long-term hemodialysis transplant patients who underwent renal transplantation and who were observed over a 6-year study period (July 2001 through June 2007). Model adjusted for age, gender, recipient race-ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, standardized mortality ratio of the dialysis clinic during entry quarter, dialysis dose as indicated by Kt/V (single pool), presence or absence of a hemodialysis catheter, and residual renal function during the entry quarter, eight comorbidities, body mass index, nPNA, serum or blood concentrations of TIBC, ferritin, calcium, bicarbonate, peripheral white blood cell count, lymphocyte percentage, albumin, donor type, donor age, donor gender, panel reactive antibody titer (last value before transplant), number of HLA mismatches, cold ischemia time, DGF, and extended donor criteria. Abbreviations: DGF, delayed graft function; nPNA, normalized protein nitrogen appearance; TIBC, total iron binding capacity.
The crude graft loss rate was 35.6/1000 patient-years (95% CI: 33.2 to 38.1). Pretransplant phosphorus levels and graft loss risk (Figure 3) became more closely associated starting at the level of 5 mg/dl and continuing up to extremely high levels. In our fully adjusted model, recipients with pretransplant phosphorus levels of 7.5 to >9.5 and ≥9.5 mg/dl phosphorus had 1.4-fold (HR: 1.42, 95% CI: 1.04 to 1.95) and 2.4-fold (HR: 2.36, 95% CI: 1.33 to 4.17) higher risk of graft loss compared with recipients with pretransplant phosphorus 3.5 to <5.5 mg/dl (Table 3C). The association of pretransplant serum phosphorus level (Figure 4) with the combined outcome was U-shaped. Both very low and very high phosphorus levels were associated with higher mortality risk; however, in the low range the association was not statistically significant.
We did not find significant associations between pretransplant phosphorus level and delayed graft function (Table 3E).
Discussion
In this retrospective analysis of 9384 primary kidney transplant recipients we describe an association between pretransplant serum phosphorus levels higher than 7.5 and 9.5 mg/dl with an increased risk of death-censored graft failure and death, respectively.
Our findings are noteworthy for a number of reasons. First, the association of phosphorus levels with all-cause mortality and with cardiovascular mortality was not entirely comparable to findings in dialysis-CKD patients. Pretransplant serum phosphorus was only significantly associated with mortality in renal transplant recipients at very high levels (>9.5 mg/dl), whereas in dialysis-CKD patients this association has been demonstrated at relatively lower phosphorus levels (>6.5 or 7.0 mg/dl) (1,10). This difference may in part be explained by the transplant selection process. Only the healthiest recipients among those with multiple dialysis-related complications, including severe hyperphosphatemia are eligible to receive a transplant. As an example, in our study, recipients in the 7.5 to <9.5 and >9.5 group were younger and had fewer cases of diabetes mellitus, and fewer pretransplant comorbidities when compared with the other groups. Moreover, the higher phosphorus levels in our population may represent a better nutritional status. When comparing baseline characteristics, those in the 7.5 to <9.5 and >9.5 mg/dl group had higher BMI (with fewer recipients with diabetes mellitus), higher nPCR, higher creatinine, and similar serum albumin compared with the other groups. These characteristics may have attenuated the association of moderately increased phosphorus levels with mortality.
Hypophosphatemia (<3.5 mg/dl) was associated with mortality in the unadjusted and case-mix models, but not in the fully adjusted model (case-mix, MICS, and transplant characteristics). However, this last model examined outcomes of fewer recipients, which could result in loss of statistical power, and hence small associations may not have been identified. Therefore, it is hard to assume that phosphorus <3.5 mg/dl is not associated with mortality based only on the fully adjusted model results, as cubic spline curves showed a crescent risk of death with decrease of phosphorus to this level. However, in this low range the association was not statistically significant. This particular group of recipients should be further analyzed.
A second noteworthy finding was the association of phosphorus levels >7.5 mg/dl with death censored graft failure, while not showing associations with mortality. It seems that relatively lower levels of pretransplant phosphorus may be more closely associated with graft function than with survival. In the majority of kidney transplant recipients both serum phosphorus and PTH are expected to normalize after transplant, abrogating the effect of phosphorus as a vascular toxin. This does not immediately correct pre-established vascular lesions, although some calcified lesions may be gradually reabsorbed after successful transplantation (35). As a result, it is possible that the mortality risk in those with moderate increases in serum phosphorus (>7.5 to 9.0 mg/dl) may be reduced after transplant. Elevated pretransplant phosphorus levels may represent the presence of large phosphorus reserves in the bones, which will usually be mobilized after transplantation. Increased renal phosphorus wasting with associated hyphosphatemia is often observed after transplantation (21). This is related to side effects of immunosuppressive drugs (calcineurin inhibitors [36], sirolimus [37], and steroids [20]) and to increased levels of PTH and fibroblast growth factor 23 (FGF-23) that persist at least in the first few months after transplantation (38). The presence of higher phosphorus concentrations in the tubules may lead to deposition of calcium-phosphorus crystals in the tubular epithelium, and contribute to the risk of graft failure (23). Molecules that facilitate crystal adhesion may be expressed in transplant tubular cells (22), which may potentiate crystal deposition in the allograft. The crystal deposition may create a focus of inflammation that may progress to fibrosis and nephron loss (39). Calcium oxalate, uric acid, and to a lesser extent calcium-phosphorus crystals increase production of monocyte-chemoattractant protein-1 and many other profibrotic and proinflammatory pathways (39,40). Therefore, a plausible hypothesis is that crystal deposition may contribute to graft loss either by triggering rejection episodes or by facilitating fibrosis.
The mechanism of increased graft loss in recipients with higher pretransplant phosphorus seems not to involve delayed graft function. In our population associations with DGF could be mitigated by the higher frequency of living donor transplants in the >9.5 mg/dl group. However, we examined the risk of DGF separately in living and deceased donor recipients and we did not find a significant association (data not shown).
Two previous studies examined the relationship of pretransplant abnormalities in serum phosphorus, calcium, and PTH levels with transplant outcomes. Roodnat et al. (41) reported an association of elevated PTH but not phosphorus with increased graft failure, and Calabia et al. (42) showed that pretransplant calcium, PTH, and phosphorus levels were not associated with DGF. Other studies reported associations of serum phosphorus concentration during the transplant follow-up period with outcomes. Schaeffner et al. (43) reported that higher phosphorus or calcium-phosphorus product levels during the transplant follow-up period were associated with increased risk of graft dysfunction. Connolly et al. (44) and Stevens et al. (45) showed that posttransplant hyperphosphatemia was associated with increased mortality. Interestingly, in pediatric and adult populations, elevated FGF-23 levels during the transplant follow-up period were associated with increased risk for deterioration of kidney function and mortality, more so than phosphorus or PTH levels (46,47). Also, in pediatric recipients increased FGF-23 was associated with a higher number of rejection episodes (46).
Our study has several limitations related to its retrospective nature. Data on induction and maintenance immunosuppression, cardiovascular medications (statins, β-blockers, etc.), and coronary artery calcification were not available. Pretransplant use of phosphorus binders and other dietary treatments (48–51), vitamin D analogs, or calcimimetics was not taken into consideration, and it is possible that recipients with similar phosphorus levels have different degrees of severity of metabolic bone disease, and therefore different levels of vascular damage. PTH levels were also not examined in conjunction with phosphorus levels, as data on this were missing in the majority of the patients. Moreover, uneven distribution of serum phosphate measurement frequency in the study sample (some may have more measurements than others) might also result in bias.
Conclusions
Individuals with pretransplant phosphorus levels >7.5 mg/dl have increased risk of functional graft failure and those with levels over 9.5 mg/dl are also at increased risk for all-cause and cardiovascular mortality. Recommendations to keep phosphorus levels between 3.5 and 5.5 mg/dl during the dialysis period are important not only to reduce dialysis-related mortality but also possibly for the success of kidney transplantation.
Disclosures
Drs. Nissenson and Krishnan are employees of DaVita. Dr. Kalantar-Zadeh is the medical director of DaVita Harbor-UCLA/MFI in Long Beach, California. Dr. Kovesdy received research grants from Shire, Inc.
Supplementary Material
Acknowledgments
We express our sincere appreciation to the teammates in nearly 1600 DaVita clinics who work every day, not only to take care of patients but also to ensure the extensive data collection on which our work is based. We thank DaVita Clinical Research (DCR) for providing the clinical data, analysis, and review for this research project and for advancing the knowledge and practice of kidney care. The study was supported by K.K.Z.'s research grant from the American Heart Association (0655776Y). K.K.Z.'s other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health (R01 DK078106), a research grant from DaVita Clinical Research, and a philanthropic grant from Mr. Harold Simmons. M.Z.M. received grants from the National Developmental Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund and was also supported by the Hungarian Kidney Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org
References
- 1. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Block GA: Prevalence and clinical consequences of elevated Ca x P product in hemodialysis patients. Clin Nephrol 54: 318–324, 2000 [PubMed] [Google Scholar]
- 3. Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW, group tPs: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825–831, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol 73: 268–275, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Block GA: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cunningham J, Silver J: CKD-MBD: Comfort in the trough of the U. Nephrol Dial Transplant 26: 1764–1766, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Qunibi W, Kalantar-Zadeh K: Target levels for serum phosphorus and parathyroid hormone. Semin Dial 24: 29–33, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 11. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 12. Cozzolino M, Dusso AS, Slatopolsky E: Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J Am Soc Nephrol 12: 2511–2516, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fujiu A, Ogawa T, Matsuda N, Ando Y, Nitta K: Aortic arch calcification and arterial stiffness are independent factors for diastolic left ventricular dysfunction in chronic hemodialysis patients. Circ J 72: 1768–1772, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H: Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 27: 47–52, 2004 [DOI] [PubMed] [Google Scholar]
- 16. London GM: Cardiovascular disease in chronic renal failure: Pathophysiologic aspects. Semin Dial 16: 85–94, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Fatica RA, Dennis VW: Cardiovascular mortality in chronic renal failure: Hyperphosphatemia, coronary calcification, and the role of phosphate binders. Cleve Clin J Med 69 [Suppl 3]: S21–S27, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Roe P, Wolfe M, Joffe M, Rosas SE: Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis 212: 589–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santos RD: Coronary artery calcification progression and cardiovascular events in renal transplant recipients, bad inheritance from previous kidney disease: Commentary on the study of Roe et al. Atherosclerosis 212: 390–391, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Levi M: Post-transplant hypophosphatemia. Kidney Int 59: 2377–2387, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Evenepoel P, Meijers BKI, de Jonge H, Naesens M, Bammens B, Claes K, Kuypers D, Vanrenterghem Y: Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol 3: 1829–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vervaet BA, Verhulst A, D'Haese PC, De Broe ME: Nephrocalcinosis: New insights into mechanisms and consequences. Nephrol Dial Transplant 24: 2030–2035, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Evenepoel P, Lerut E, Naesens M, Bammens B, Claes K, Kuypers D, Vermeersch P, Meijers B, Van Damme B, Vanrenterghem Y: Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant 9: 2470–2478, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Chang PC, Saha S, Gomes AM, Padiyar A, Bodziak KA, Poggio ED, Hricik DE, Augustine JJ: Donor phosphorus levels and recipient outcomes in living-donor kidney transplantation. Clin J Am Soc Nephrol 6: 1179–1184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, Kalantar-Zadeh K: Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 6: 1041–1048, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molnar MZ, Lukowsky LR, Streja E, Dukkipati R, Jing J, Nissenson AR, Kovesdy CP, Kalantar-Zadeh K: Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. J Hypertens 28: 2475–2484, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, Greenland S, Kalantar-Zadeh K: Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis 55: 100–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, Kalantar-Zadeh K: Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol 32: 403–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, Ricks J, Jing J, Nissenson AR, Greenland S, Norris KC: Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res 25: 2448–2458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD: The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc 85: 991–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Mehrotra R, Krishnan M, Nissenson AR, Kalantar-Zadeh K: Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 11: 1006–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, Parikh CR: Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol Dial Transplant 23: 2995–3003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, Krishnan M, Nissenson AR, Danovitch GM, Kalantar-Zadeh K: Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant 11: 725–736, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abedi SA, Tarzamni MK, Nakhjavani MR, Bohlooli A: Effect of renal transplantation on coronary artery calcification in hemodialysis patients. Transplant Proc 41: 2829–2831, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Yulia M, Ronen L, Vardit L-M, Keith BC, Jeffery DM, Justin S, Tally N-M: Calcineurin Abeta is central to the expression of the renal type II Na/Pi co-transporter gene and to the regulation of renal phosphate transport. J Am Soc Nephrol 15: 2972–2980, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Schwarz C, Böhmig GA, Steininger R, Mayer G, Oberbauer R: Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol Dial Transplant 16: 378–382, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Riella LV, Rennke HG, Grafals M, Chandraker A: Hypophosphatemia in kidney transplant recipients: Report of acute phosphate nephropathy as a complication of therapy. Am J Kidney Dis 57: 641–645, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Khan SR: Crystal-induced inflammation of the kidneys: Results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 8: 75–88, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Umekawa T, Chegini N, Khan SR: Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant 18: 664–669, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Roodnat JI, van Gurp EA, Mulder PG, van Gelder T, de Rijke YB, de Herder WW, Kal-van Gestel JA, Pols HA, Ijzermans JN, Weimar W: High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 82: 362–367, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Rodrigo Calabia E, Ruiz San Millan JC, Gago M, Ruiz Criado J, Pinera Haces C, Fernandez Fresnedo G, Palomar R, Gomez Alamillo C, Martin de Francisco AL, Arias M: [Changes in the pre-transplant bone-mineral metabolism do not affect the initial progress of the renal graft]. Nefrologia 29: 143–149, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Schaeffner ES, Fodinger M, Kramar R, Sunder-Plassmann G, Winkelmayer WC: Prognostic associations of serum calcium, phosphate and calcium phosphate concentration product with outcomes in kidney transplant recipients. Transpl Int 20: 247–255, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP: Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 87: 1040–1044, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Stevens KK, Morgan IR, Patel RK, Geddes CC, Mark PB, Jardine AG, Delles C: Serum phosphate and outcome at one year after deceased donor renal transplantation. Clin Transplant 25: E199–E204, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Wesseling-Perry K, Tsai EW, Ettenger RB, Jüppner H, Salusky IB: Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: A role for FGF-23? Nephrol Dial Transplant 2011, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K: Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis 56: 842–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shantouf R, Ahmadi N, Flores F, Tiano J, Gopal A, Kalantar-Zadeh K, Budoff MJ: Impact of phosphate binder type on coronary artery calcification in hemodialysis patients. Clin Nephrol 74: 12–18, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Taylor LM, Kalantar-Zadeh K, Markewich T, Colman S, Benner D, Sim JJ, Kovesdy CP: Dietary egg whites for phosphorus control in maintenance haemodialysis patients: A pilot study. J Ren Care 37: 16–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.