Abstract
Summary
Background and objectives
Pregnant women with chronic kidney disease (CKD) are at risk of adverse maternal and fetal outcomes. We conducted a systematic review of observational studies that described this risk.
Design, setting, participants, & measurements
We searched several databases from their date of inception through June 2010 for eligible articles published in any language. We included any study that reported maternal or fetal outcomes in at least five pregnant women in each group with or without CKD. We excluded pregnant women with a history of transplantation or maintenance dialysis.
Results
We identified 13 studies. Adverse maternal events including gestational hypertension, pre-eclampsia, eclampsia, and maternal mortality were reported in 12 studies. There were 312 adverse maternal events among 2682 pregnancies in women with CKD (weighted average of 11.5%) compared with 500 events in 26,149 pregnancies in normal healthy women (weighted average of 2%). One or more adverse fetal outcomes such as premature births, intrauterine growth restriction, small for gestational age, neonatal mortality, stillbirths, and low birth weight were reported in nine of the included studies. Overall, the risk of developing an adverse fetal outcome was at least two times higher among women with CKD compared with those without.
Conclusions
This review summarizes current available evidence to guide physicians in their decision-making, advice, and care for pregnant women with CKD. Additional studies are needed to better characterize the risks.
Introduction
Recent guidelines stage chronic kidney disease (CKD) according to levels of kidney function irrespective of the type of kidney disease (1). Pregnancy in women with CKD is considered high risk (2,3). Diseased kidneys may be unable to adapt to the normal physiologic changes of pregnancy leading to perinatal complications (4,5). Knowledge of this risk guides patient counseling and follow-up. Although a number of observational studies have shown that women with CKD have an increased risk of developing adverse maternal and fetal outcomes, a robust synthesis of this information is lacking (2,6–13). We conducted this systematic review to determine the following: (1) the risk of adverse maternal outcomes in women with CKD compared with women without CKD (comparator group) and (2) the risk of adverse fetal outcomes comparing the two groups of women.
Materials and Methods
We conducted and reported this systematic review according to published guidelines using a prespecified protocol (14,15).
Eligibility Criteria
We included any observational study that reported maternal or fetal outcomes in five or more pregnant women with CKD and five or more pregnant women without CKD as a comparator group. Primary studies defined CKD as any of the following: abnormal serum creatinine/abnormal GFR and/or proteinuria with a specific primary or secondary kidney disease. The comparator group consisted of women without CKD and these women may or may not have had other comorbidities (such as diabetes mellitus or systemic lupus erythematosus). Adverse maternal outcomes were as defined by the primary study authors and included gestational hypertension, pre-eclampsia, eclampsia, and maternal mortality. Adverse fetal outcomes included premature births, intrauterine growth restriction (IUGR), small for gestational age (SGA), neonatal mortality, stillbirths, and low birth weight. We included full text papers and abstracts published in any language that reported at least one outcome of interest. We excluded studies of women with CKD with a history of kidney transplantation or maintenance dialysis, as well as studies of women with acute kidney injury or a single kidney.
Information Sources
We designed and implemented a systematic literature search with the help of an experienced librarian. We searched the following electronic databases from the date of inception up to June 2010: MEDLINE and PreMedline (OVID, 1966 through 2010), EMBASE (OVID, 1980 through 2010), BIOSIS Previews (1969 through 2010), the ISI Science Citation Index Expanded (1981 through 2010), Cochrane Controlled Trials Register (Wiley InterScience, all years), SCOPUS (1966 through 2010), and specialty search engines Google Scholar and Elsevier's Scirus. The search strategy included a combination of key words and MeSH terms and was adapted for each database to account for differences in indexing. We imposed no language restrictions or other limits in the search process. We also searched gray literature sources (nephrology conference proceedings and Web of Science database) and conference abstracts. We conducted citation tracking using SCOPUS and the ISI Science Citation Index, and used related articles features in PubMed, OVID, Elsevier's Scirus, and Google Scholar.
Study Selection
Two reviewers (I.F.N., A.R.) independently screened titles and abstracts. We retrieved the full text for any article considered potentially relevant by at least one reviewer. To ensure accuracy, two reviewers then independently screened full texts articles for inclusion in this review. We resolved disagreements by discussion or with the help of a third reviewer (A.D.). We reviewed all non-English citations with the help of translators.
Data Abstraction and Analysis
Two reviewers (I.F.N., A.R.) independently abstracted data using a standardized form that proved robust in pilot testing. This was done in duplicate to increase accuracy and reduce measurement bias (16). We resolved any disagreements with the help of a third reviewer (A.D.). We abstracted the following data: (1) study characteristics such as year of publication, country where study was conducted, study design, sample size, year of study, and funding sources; (2) methodological characteristics such as definitions of CKD and outcomes used, whether confounding variables were accounted for in the study, and whether the studies reported loss to follow-up; (3) patient characteristics including the number of women and pregnancies in each group, mean age, race, and whether the control group was normal healthy women or women with other comorbidities but normal kidney function; and (4) the number of adverse events (both individual and as a composite) and any adjusted measures of association. Finally, we contacted the authors of the studies included in the review for any missing data. We assessed agreement between two reviewers using the κ statistic. Kappa was calculated for full text eligibility (17). We entered all data into Review Manager Version 5 (18).
Results
Study Selection
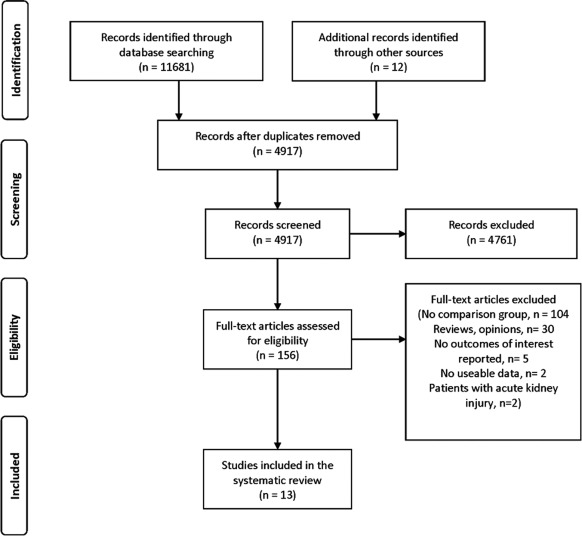
We screened and evaluated 4917 citations and assessed 156 full text articles for eligibility. The chance-corrected agreement for full text eligibility was good (estimated κ = 0.88). We excluded 104 studies because they had no comparator group, 30 were reviews, five had no outcomes of interest, two had no useable data, and two studies included women with acute kidney injury (Figure 1). Thirteen studies were eligible for review (2,7,8,12,19–27). Twelve of the 13 studies described maternal outcomes and all 13 reported at least one fetal outcome of interest. We contacted six primary authors and two confirmed or provided additional data (20,25).
Figure 1.
Study selection.
Description of Studies, Methods, and Participants
Thirteen studies from seven countries reported at least one outcome of interest and followed a total of 28,917 pregnancies of whom 26,192 (range 8 to 20,034 across studies) had normal kidney function and 2725 (range 7 to 1257 across studies) had CKD (2,7,8,12,19–24,26,27) (Table 1). Most studies were done in North America (n = 7), followed by Europe (n = 3), Japan (n = 2), and South America (n = 1). Of the 13 studies, eight provided a definition for CKD (8,12,20,22,24–27), two identified CKD through medical coding (2,19), and two defined their CKD population through kidney biopsy (21,23). One study did not report their definition of CKD (7). Many of the studies did not provide a clear definition of the maternal outcomes studied (2,7,19–21,26,27). Some women with CKD had a history of hypertension or proteinuria before pregnancy, which may have influenced the ascertainment of outcomes such as pre-eclampsia (2,7,19,21,26,27). Seven studies had a control group of normal healthy women (2,7,19,22,23,25,27) and the remaining six studies had a control group of women with other comorbidities but with normal kidney function (8,12,20,21,24,26). These comorbidities included diabetes mellitus, hypertension in patients with IgA nephropathy, and lupus nephritis (all with normal kidney function as defined by the primary authors). Two large retrospective studies included over 25,000 pregnancies (2,7). The first of these studies included approximately 21,000 pregnancies but did not define CKD or account for other variables that may have confounded the relationship between CKD and outcome (7). The other large retrospective cohort study used an administrative database (2). Of the 13 studies, seven collected data prospectively (8,12,20,23–26) and six used pre-existing data from health records (2,7,19,21,22,27). Seven studies accounted for potential confounding factors such as maternal age, parity, race, socioeconomic status, diabetes status, trimester of first antenatal visit, smoking, year of delivery, marital status, place of childbirth, hospital, attending clinician, maternal education, alcohol use, medication used during the study period, and early antenatal referral (2,8,19,20,22,25,27). Of the seven studies, three studies used matching to control for confounding (19,22,27) whereas the remaining adjusted for potential confounders in multivariable analysis (2,8,20,25) (Table 1). Loss to follow-up was reported in only one study and was <5% (25).
Table 1.
Study characteristics
| Study, Year | Country of Study | Study Design | Total No. Pregnancies Studied, na | Year of Study | Definition of Chronic Kidney Disease | Defined Maternal Outcomes | Defined Fetal Outcomes | Accounted for Potential Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Gazarek F, 1966 (7) | Czechoslovakia | Retrospective cohort | 21,291 | Not reported | Not reported | Maternal mortality not defined | Premature births not defined | No |
| Houser MT, 1979 (21)b | United States | Retrospective cohort | 16 | Not reported | Biopsy | Toxemia not defined | Premature births not defined | No |
| Leppert P, 1979 (22) | United States | Retrospective cohort | 145 | 1974 through 1976 | Biopsy, sr.cr >1.2 mg/dl, persistent proteinuria + 1 | Gestational hypertension defined as BP ≥140/85 mmHg at the 3rd trimester; preeclampsia defined as BP ≥140/90 mmHg or ≥2 + proteinuria and edema occurring beyond the 20th week of pregnancy | Preterm defined as <37-wk of gestation at child birth, stillbirths defined as fetal death occurring beyond 28-wk of gestation; SGA, neonatal mortality, low birth weight not defined | Matched on age ±5 yr, race, SES, hospital |
| Nagai Y, 1989 (24) | Japan | Prospective cohort | 19 | Not reported | Biopsy, Sr.cr, 24 h Cr.cl, proteinuria | Hypertension defined as BP of ≥140/90 mmHg at the time of delivery | Low birth weight defined as <2500 g birth weight | No |
| Kimmerle R, 1995 (12) | Germany | Prospective cohort | 146 | 1982 through 1992 | Cr. cl <80 mg/ml, proteinuria >400 mg/day or ≥1 + dipstick in absence of urinary tract infection and other causes of kidney disease | Pre-eclampsia defined as acute worsening of hypertension (>15% diastolic pressure) in the presence of proteinuria >3 g/d and generalized edema | Preterm birth defined as <34 wk of gestation, small for gestational age defined as birth weight less than the 10th percentile; stillbirth not defined | No |
| Holley JL, 1996 (8) | United States | Prospective cohort | 86 | 1991 through 1993 | Sr.cr ≥0.8 mg/dl in the first trimester, or proteinuria ≥300 mg/24 h, with known kidney disease | Not reported | Premature births <36 wk of gestation; intrauterine growth restriction and neonatal mortality not defined | Age, race, parity, diabetes status |
| Rosenn B, 1997 (26)b | United States | Prospective cohort | 408 | 1978 through 1993 | Diabetic nephropathy defined as proteinuria >500 mg in 24 hours prior to 16 weeks of gestation with no bacteriuria | Pre-eclampsia not defined | Premature births, IUGR/SGA, neonatal mortality, low birth weight not defined | No |
| Fink J, 1998 (19) | United States | Retrospective cohort | 675 | 1987 through 1993 | ICD-9 codes including diabetic and hypertensive nephropathy, acute and chronic glomerulonephritis, nephrotic syndrome, acute and chronic renal failure, disorders with impaired renal function, small kidneys of unknown cause, renal agenesis, and cystic diseases | Pre-eclampsia and eclampsia not defined | Premature births <37 wk of gestation; SGA defined using William et al. (33) method; neonatal death defined as infant death within 28 days of birth | Adjusted for maternal age, trimester of first prenatal visit, parity, and smoking; year of delivery was matched |
| Murakami S, 2000 (23) | Japan | Prospective cohort study | 86 | 1980 through 1999 | Biopsy | Pre-eclampsia defined as proteinuria >300 mg in 24-hour urine collection and BP >160/110 mmHg | SGA not defined | No |
| Fischer M, 2004 (2) | United States | Retrospective cohort | 5517 | 1989 through 2001 | Medical coding | Pre-eclampsia, eclampsia or abruptio placenta not defined | Prematurity <37 wk of gestation, neonatal mortality death of newborn <28 days, low birth weight <2500 g | Maternal age, parity, race, marital status, place of birth, attending clinician, maternal education, cigarette and alcohol use |
| Trevisan G, 2004 (27) | Brazil | Retrospective cohort | 75 | 1989 through 1999 | Sr.cr ≥1.5 mg/dl | Pre-eclampsia not defined | Stillbirth defined as dead fetus | Maternal age, gestational age, and time of delivery were matched between the comparison groups |
| Gladman D, 2010 (20) | Canada | Prospective study | 120 | 1970 through 2003 | Sr.cr >120 mmol/L 6 months prior to pregnancy until outcomes, proteinuria >500 mg/24 h, nephrotic syndrome | Gestational hypertension, pre-eclampsia, and maternal mortality not defined | Low birth weight defined as below the 10th percentile for sex and gestational age; stillbirth as death of fetus in utero past 20-wk of gestation; perinatal death defined as neonate death within 7 days of birth | Adjusted for medication used in the study period (if sample size is large enough), otherwise adjusted only for repeated measures |
| Piccoli G, 2010 (25) | Italy | Prospective study | 333 | 1999 through 2007 | As defined by the KDOQI guidelines | Pre-eclampsia defined as the appearance of hypertension (≥140/90 mmHg) with proteinuria ≥300 mg/24 h after 20-wk of gestation in a previously normotensive woman; maternal mortality not defined | Small for gestational age defined as birth weight below the 10th percentile according to Italian birth weight references; neonatal mortality not defined | Maternal age, parity, race, early antenatal referral |
Studies ordered as per year of publication. Loss to follow-up was reported in one study as <5% (5). Only two studies reported funding of which one was funded by the U.S. public health service grant, NIH Clinical Research grant, Ciba pharmaceutical company (22), and the other by NIH, clinical research training in kidney disease (19). Abbreviations: ICD-9, international classification of diseases-9; Sr.cr, serum creatinine; Cr.cl, creatinine clearance; SES, socioeconomic status; IUGR, intrauterine growth restriction; SGA, small for gestational age. Conversion factors for units: serum creatinine in mg/dl to μmol/L, × 88.4.
Number of pregnancies studied in women with and without chronic kidney disease.
Published abstracts.
The mean age of women included in the studies was 28 years. In studies where race was reported, more than half of the women were white (2,8,19,22,25,27) (Tables 2 and 3). Serum creatinine levels were reported in five studies and ranged from 0.8 to 4.61 mg/dl (70 to 407 μmol/L) in women with CKD (8,20,22,24,27). All studies except four reported outcomes on singleton pregnancies (7,21,23,26). One study included a small percentage of women (5%) who had acute glomerulonephritis/acute renal failure (19). Another study included a small percentage of renal transplant patients (<1%) (25). We included these studies in the review as we deemed these numbers of ineligible women to be too small to have a significant influence on the results obtained.
Table 2.
Baseline characteristics: comparator group, healthy women with normal kidney function
| Study, Year | Women with Chronic Kidney Disease |
Healthy Women with Normal Kidney Function |
||||||
|---|---|---|---|---|---|---|---|---|
| No. Women Studied, n | No. Pregnancies Studied, N | Age in years, Mean (SD) | White Race (%) | No. Women Studied, n | No. Pregnancies Studied, N | Age in years, Mean (SD) | White Race (%) | |
| Gazarek F, 1966 (7) | 1257 | 1257 | (… ) | (… ) | 20034 | 20034 | (… ) | (… ) |
| Leppert P, 1979 (22)a | 7 | 9 | (… ) | 54 | 39 | 80 | (… ) | 54 |
| Fink J, 1998 (19)a | 169 | 169 | (… ) | 73 | 506 | 506 | (… ) | 83 |
| Murakami S, 2000 (23) | 19 | 19 | (… ) | (… ) | 67 | 67 | (… ) | (… ) |
| Fischer M, 2004 (2)a | 911 | 911 | (… ) | 93 | 4606 | 4606 | (… ) | 92 |
| Trevisan G, 2004 (27)a | 25 | 25 | 29 (5) | 92 | 50 | 50 | 29 (6) | 87 |
| Piccoli G, 2010 (25)a | 91 | 91 | 31 (5) | 89 | 267 | 267 | 29 (5) | 77 |
(… ), not reported or reported in a way from which data cannot be extracted. Abbreviations: SD, standard deviation; %, percentage; N, n = number.
Multiple gestations (twins, triplets, etc.) were excluded from the study.
Table 3.
Baseline characteristics: comparator group, women with normal kidney function but other comorbidities
| Study, Year | Women with Chronic Kidney Disease |
Women with Normal Kidney Function but with Other Comorbidities |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Women Studied, n | No. Pregnancies Studied, N | Age in years, Mean (SD) | White Race (%) | Comorbidity | No. Women Studied, n | No. Pregnancies Studied, N | Age in years, Mean (SD) | White Race (%) | |
| Houser MT, 1979 (21) | 6 | 7 | (… ) | (… ) | Lupus nephritis | 5 | 9 | (… ) | (… ) |
| Nagai Y, 1989 (24)a | 10 | 11 | (… ) | (… ) | IgA nephropathy | 7 | 8 | (… ) | (… ) |
| Kimmerle R, 1995 (12)a | 33 | 36 | 29 (5) | (… ) | Diabetes | 91 | 110 | 28 (4) | (… ) |
| Holley JL, 1996 (8)a | 40 | 43 | 29 (6) | 84 | Diabetes | 43 | 43 | 28 (5) | 84 |
| Rosenn B, 1997 (26) | 73 | 73 | 27 (5) | (… ) | Diabetes | 335 | 335 | 25 (5) | (… ) |
| Gladman D, 2010 (20)a | 81 | 81 | 28 (5) | (… ) | Lupus nephritis | 112 | 112 | 31 (5) | (… ) |
(… ), not reported or reported in a way from which data cannot be extracted. Abbreviations: SD, standard deviation; %, percentage; N, n = number.
Multiple gestations (twins, triplets, etc.) were excluded from the study.
Adverse Maternal Outcomes
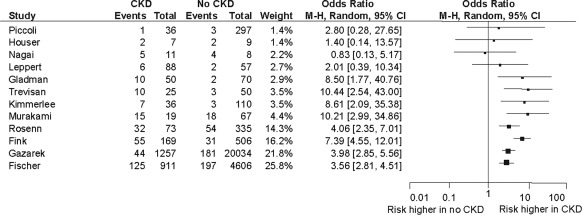
Twelve studies reported adverse maternal outcomes including gestational hypertension, pre-eclampsia, eclampsia, and maternal mortality (Figure 2, Table 4). Most studies demonstrated at least a twofold increase in the risk of adverse maternal outcomes in women with CKD compared with those without (Figure 2). The overall adverse maternal events were fivefold higher in women with CKD compared with women without CKD. There were 312 adverse events among 2682 pregnancies in women with CKD (weighted average 11.5%) compared with 500 events in 26,149 pregnancies in women with no CKD (weighted average 2%). Two studies each followed over 5000 women with CKD and non-CKD groups combined (2,7). All studies except one reported hypertensive disorders of pregnancy (7).
Figure 2.
Adverse maternal outcomes (gestational hypertension, pre-eclampsia, eclampsia, and maternal mortality). CKD, chronic kidney disease; CI, confidence interval.
Table 4.
Adverse maternal and fetal outcomes
| Study Year | Maternal Hypertensiona |
Maternal Mortality |
Premature Birthsb |
IUGR |
SGAc |
Neonatal Mortalityd |
Stillbirthse |
Low Birth Weightf |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CKD | No CKD | CKD | No CKD | CKD | No CKD | CKD | No CKD | CKD | No CKD | CKD | No CKD | CKD | No CKD | CKD | No CKD | |
| Gazarek F, 1966 (7) | — | — | 44/1257 (4)g | 181/20034 (1)g | 111/1257 (8)g | 1165/20034 (6)g | — | — | — | — | — | — | — | — | — | — |
| Houser MT, 1979 (21) | 2/7 (29)g | 2/8 (25)g | — | — | 2/7 (29)g | 0/9 (0)g | — | — | — | — | — | — | — | — | — | — |
| Leppert P, 1979 (22) | 6/88 (7) | 2/57 (4) | — | — | 2/114 (2) | 1/80 (1) | 1/114 (1)g | 0/80 (0)g | 0/114 (0)g | 0/80 (0)g | 0/114 (0) | 2/80 (3) | 3/114 (3)g | 0/80 (0)g | ||
| Nagai Y, 1989 (24) | 5/11 (45) | 4/8 (50) | — | — | 1/11 (9) | 0/8 (0) | — | — | — | — | — | — | — | — | 1/11 (9) | 1/8 (13) |
| Kimmerle R, 1995 (12) | 7/36 (19) | 3/110 (3) | — | — | 11/36 (30) | 3/110 (3) | — | — | 8/36 (22) | 2/110 (2) | — | — | 0/36 (0)g | 0/110 (0)g | — | — |
| Holley JL, 1996 (8) | — | — | — | — | 7/43 (16) | 4/43 (9) | 2/43 (5)g | 0/43 (0)g | — | — | 3/43 (7)g | 0/43 (0)g | — | — | — | — |
| Rosenn B, 1997 (26) | 32/73 (44)g | 54/335 (16)g | — | — | 38/73 (52)g | 74/335 (22)g | — | — | 8/73 (11)g | 13/335 (4)g | 4/73 (5)g | 3/335 (1)g | — | — | 31/73 (42)g | 27/335 (8)g |
| Fink J, 1998 (19) | 55/169 (33) | 31/506 (6) | — | — | 37/169 (22) | 27/506 (5) | — | — | 34/169 (20)g | 25/506 (5)g | 5/169 (3) | 1/506 (0) | — | — | — | — |
| Murakami S, 2000 (23) | 15/19 (79) | 18/67 (27) | — | — | — | — | — | — | 4/19 (21)g | 34/67 (51)g | — | — | — | — | — | — |
| Fischer M, 2004 (2) | 125/911 (14) | 197/4606 (4) | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Trevisan G, 2004 (27) | 10/25 (40)g | 3/50 (6)g | — | — | — | — | — | — | — | — | — | — | 9/25 (36) | 2/50 (4) | — | — |
| Gladman D, 2010 (20) | 10/70 (14)g | 2/50 (4)g | 0/70 (0)g | 0/50 (0)g | 20/50 (40) | 16/70 (23) | — | — | — | — | 1/47 (2) | 2/67 (3) | 3/50 (6) | 3/70 (4) | 18/47 (38) | 13/67 (19) |
| Piccoli G, 2010 (25) | 1/36 (3) | 3/97 (3) | 0/36 (0)g | 0/97 (0)g | — | — | — | — | 15/91 (16) | 28/267 (10) | 0/91 (0)g | 0/267 (0)g | — | — | — | — |
All numbers in Table 4 are reported as follows: No. events/No. pregnancies (percentage); percentages are rounded to the nearest whole number. (—), data not reported in primary article or not extractable. Abbreviations: IUGR, intrauterine growth restriction; SBA, small for gestational age; CKD, chronic kidney disease.
Maternal hypertension defined as ≥140/85 mmHg in the third trimester (22), ≥140/90 mmHg at the time of delivery (24), accelerated hypertension, edema, or hypoalbuminemia (12), ICD-9 medical coding (19), onset of pre-eclampsia before 30 weeks of gestation and proteinuria at 6 weeks postpartum (23), a composite of pre-eclampsia, eclampsia, and abruptio placenta (2), pre-eclampsia defined as appearance of hypertension with systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg with proteinuria ≥300 mg/24 h after 20 weeks of gestation (25).
Premature births defined as <34 weeks of gestation (12,24), <36 weeks of gestation (8), and <37 weeks of gestation (19, 20, 22) at the time of childbirth.
SGA defined as birth weight lower than the 10th percentile (12), and birth weight below 10th percentile according to Italian birth weight references (25).
Neonatal mortality defined as neonate death within 7 days of birth (20); infant death within 28 days of birth (19).
Stillbirths defined as fetal death (27), fetal death occurring beyond 28 weeks of gestation (22), death of fetus in utero past 20 weeks of gestation (20).
Low birth weight defined as <2500-g birth weight (24), defined as below the 10th percentile for gender and gestational age (20).
Outcomes not defined in primary article.
Adverse Fetal Outcomes
Fetal outcomes of interest such as premature births, IUGR, SGA, neonatal mortality, stillbirths, and low birth weight were variably defined and reported in the studies (2,8,12,19,20,22–27) (Table 4). Nine studies reported premature births (7,8,12,19,21,22,24,26). Premature birth was defined as birth <34 weeks of gestation (12,24), <36 weeks of gestation (8), and <37 weeks of gestation (19,20,22), and was not defined in three of the studies (7,21,26). In one study, premature birth was defined as birth <37 weeks as a component in a composite outcome (2). All nine studies reported 229 premature births among 1760 pregnancies (weighted average 13%) in women with CKD, and 1290 among 21,195 pregnancies (weighted average 6%) of women with no kidney disease. Compared with controls, the incidence of premature birth in women with CKD was consistently higher across all nine studies (and was statistically different in most studies). Compared with controls, the risk of IUGR in women with CKD was observed to be five times higher in one study (8) and the risk of SGA at least threefold higher in two studies (12,26). The risk of neonatal mortality was fivefold higher in two studies (8,26), the risk of stillbirth was ninefold higher in one study (27), and the risk of low birth weight was fivefold higher in one study (26). However, the total number of events in many studies was small, and the results across studies were not consistent (Table 4).
Discussion
Over the past 4 decades, 13 studies have described the association between CKD and adverse maternal and fetal outcomes with the use of an internal comparator group. Women with CKD appear to have at least a twofold higher risk of developing adverse maternal outcomes compared with women without CKD. Similarly, premature births occurred at least twice as often in women with CKD compared with women without CKD. However, these data are derived mostly from small studies performed in a single center and the studies were overall of low methodological quality. Definitions of CKD were quite variable and many studies did not report the most meaningful outcome such as maternal mortality. Although there is consensus that women with CKD have a higher risk of adverse maternal and fetal outcomes compared with women without CKD, the magnitude of this risk is not clear. There is also no clear evidence as to the degree of risk at various stages of CKD, information needed for informed consent in women considering the risk benefits of pregnancy. These results provide an impetus for future high-quality, large multicenter studies of pregnancy outcomes to better quantify risks in women with CKD (recognizing the time, effort, and funding involved in such studies). There is a need to characterize the risk associated with low renal function separately from the comorbid conditions that can occur with CKD which also modify risk.
Our review has a number of strengths. It is the first systematic review and complements previous narrative reviews on this topic (28,29). Many of the previous narrative reviews did not include studies with an internal comparator group (6). We also identified two additional studies not included in previous reviews (20,25). We did a comprehensive search to identify relevant literature in accordance with published guidelines and a prespecified protocol. Two reviewers independently identified, selected, and abstracted data from articles to avoid potential biases. We considered studies published in any language. In addition, we were able to confirm data from some of the primary authors.
The quality of the primary studies inherently limits the conclusions that can be drawn from this review. Thus, the review serves to efficiently summarize past studies, but is not definitive as to what risks should be quoted to women with CKD. Confounding factors that may distort the association between CKD and adverse pregnancy outcomes were not addressed in six studies (7,12,21,23,24,26) and were inadequately addressed in the remaining studies. Studies that included multiple pregnancies within the same women did not describe statistical techniques to account for correlated observations (8,12,21,22,24).
Often studies were retrospective and used administrative data where there was a possibility of misclassification, surveillance, selection, and ascertainment bias (2,19). Only one of the included studies reported loss to follow-up (25). Also, although one would expect a dose-response relationship with worse pregnancy outcomes in more advanced CKD (30,31), this was not considered in most prior studies. CKD was defined using different criteria in the included studies. Only one study used the modern classification of CKD (25). Other studies used serum creatinine and/or proteinuria, 24-hour creatinine clearance, and kidney biopsy to define their population of interest (8,12,20–27) and two studies used medical coding (2,19). One of the studies did not explicitly define the criteria for inclusion (7).
Another limitation of the included studies is that women were not completely free of the outcomes of interest before becoming pregnant. According to the International Society for the Study of Hypertension in Pregnancy (ISSHP), a research definition of pre-eclampsia is newly diagnosed hypertension after 20 weeks of pregnancy with well documented proteinuria (32). Many of the women included in the studies had hypertension or proteinuria before pregnancy (2,8,12,19,22,24,26,27). Finally, in addition to comparing maternal and fetal outcomes between women with and without CKD, there is a clear need to compare maternal outcomes of CKD progression in women with CKD who do and do not become pregnant. The latter information is also central to informing the pregnancy choices of women with CKD.
Given the potential for risk, women with CKD who wish to become pregnant should have preconception counseling and antenatal care with a multidisciplinary “high-risk pregnancy” team. This review summarizes key published information on the estimated risk of adverse maternal and fetal outcomes in women with CKD compared with women without CKD. This review is an efficient way for clinicians to become aware of the current published literature and to understand the limitations of available literature. They can integrate the information with their clinical expertise when counseling women with CKD about pregnancy. The results of this review provide a foundation for future studies to better characterize the risks.
Conclusions
This systematic review of observational studies highlights a higher risk of adverse maternal and fetal outcomes in women with CKD compared with women without CKD. However, well designed and methodologically rigorous studies are needed to better estimate the magnitude of this risk. Such studies could provide important insights into ways of counseling women with CKD. In the meantime, it would be rational to use the findings of our review to design such robust studies.
Disclosures
None.
Acknowledgments
Dr. Nevis is supported by an Ontario Graduate Scholarship. Dr. Nevis had primary access to the data and takes responsibility for the presented results. Dr. McDonald is supported by a Canadian Institutes of Health Research New Investigator Salary Award. Dr. Garg is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate online is available for additional clinical information at www.cjasn.org.
References
- 1. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 2. Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR: Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis 43: 415–423, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Hou S: Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 33: 235–252, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Epstein FH: Pregnancy and renal disease. N Engl J Med 335: 277–278, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Lindheimer MD, Davison JM, Katz AI: The kidney and hypertension in pregnancy: Twenty exciting years. Semin Nephrol 21: 173–189, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Fischer MJ: Chronic kidney disease and pregnancy: Maternal and fetal outcomes. Adv Chronic Kidney Dis 14: 132–145, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Gazarek F: Influence of chronic pyelonephritis on pregnancy. Acta Univ Palackianae Olomucensis Fac Med 40: 303–306, 1966 [Google Scholar]
- 8. Holley JL, Bernardini J, Quadri KH, Greenberg A, Laifer SA: Pregnancy outcomes in a prospective matched control study of pregnancy and renal disease. Clin Nephrol 45: 77–82, 1996 [PubMed] [Google Scholar]
- 9. Imbasciati E, Gregorini G, Cabiddu G, Gammaro L, Ambroso G, Del Giudice A, Ravani P: Pregnancy in CKD stages 3 to 5: Fetal and maternal outcomes. Am J Kidney Dis 49: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Jones DC, Hayslett JP: Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med 335: 226–232, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Kimmerle R, Zass R, Cupisti S, Somville T, Berger M: Pregnancies in women with diabetic nephropathy 1982–92 - Status of women and children 1993. Diabetologia 1993 [PubMed] [Google Scholar]
- 12. Kimmerle R, Zass RP, Cupisti S, Somville T, Bender R, Pawlowski B, Berger M: Pregnancies in women with diabetic nephropathy: Long-term outcome for mother and child. Diabetologia 38: 227–235, 1995 [PubMed] [Google Scholar]
- 13. Mostello D, Catlin TK, Roman L, Holcomb WL, Jr., Leet T: Preeclampsia in the parous woman: Who is at risk? Am J Obstet Gynecol 187: 425–429, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol 62: 1006–1012, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP: Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol 59: 697–703, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Cyr L, Francis K: Measures of clinical agreement for nominal and categorical data: The kappa coefficient. Comput Biol Med 22: 239–246, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008 [Google Scholar]
- 19. Fink JC, Schwartz SM, Benedetti TJ, Stehman-Breen CO: Increased risk of adverse maternal and infant outcomes among women with renal disease. Paediatr Perinat Epidemiol 12: 277–287, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Gladman DD, Tandon A, Ibanez D, Urowitz MB: The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol 37: 754–758, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Houser MT, Fish AJ, Michael AF: Lupus nephropathy (Ln) and pregnancy. Kidney Int 16: 931, 1979 [Google Scholar]
- 22. Leppert P, Tisher CC, Cheng SC, Harlan WR: Antecedent renal disease and the outcome of pregnancy. Ann Intern Med 90: 747–751, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Murakami S, Saitoh M, Kubo T, Koyama T, Kobayashi M: Renal disease in women with severe preeclampsia or gestational proteinuria. Obstet Gynecol 96: 945–949, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Nagai Y, Waschizawa Y, Suzuki T, Fushimi T, Hirata K, Kawamura S, Schiina K, Tanaka M, Maeda M: Influence of gestation on renal function in gravida with IgA nephropathy. Nippon Jinzo Gakkai Shi 31: 635–641, 1989 [PubMed] [Google Scholar]
- 25. Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D'Amico F, Consiglio V, Bontempo S, Todros T: Pregnancy and chronic kidney disease: A challenge in all CKD stages. Clin J Am Soc Nephrol 5: 844–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenn B, Miodovik M, Khoury J, Hsu H, Kopernik G, Siddiqui T: Outcome of pregnancy in women with diabetic nephropathy [abstract]. Am J Obstet Gynecol 176: S179 1997 [Google Scholar]
- 27. Trevisan G, Ramos JG, Martins-Costa S, Barros EJ: Pregnancy in patients with chronic renal insufficiency at Hospital de Clinicas of Porto Alegre, Brazil. Ren Fail 26: 29–34, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Blowey DL, Warady BA: Outcome of infants born to women with chronic kidney disease. Adv Chronic Kidney Dis 14: 199–205, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Maynard SE, Thadhani R: Pregnancy and the kidney. J Am Soc Nephrol 20: 14–22, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Cunningham FG, Cox SM, Harstad TW, Mason RA, Pritchard JA: Chronic renal disease and pregnancy outcome. Am J Obstet Gynecol 163: 453–459, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Katz AI, Davison JM, Hayslett JP, Singson E, Lindheimer MD: Pregnancy in women with kidney disease. Kidney Int 18: 192–206, 1980 [DOI] [PubMed] [Google Scholar]
- 32. Brown MA, Lindheimer MD, de SM, Van AA, Moutquin JM: The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 20: IX–XIV, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M: Fetal growth and perinatal viability in California. Obstet Gynecol 59: 624–632, 1982 [PubMed] [Google Scholar]