Abstract
Objective:
To compare the prevalence and type of early developmental lesions in patients with a clinical presentation consistent with electrical status epilepticus in sleep either with or without prominent sleep-potentiated epileptiform activity (PSPEA).
Methods:
We performed a case-control study and enrolled patients with 1) clinical features consistent with electrical status epilepticus in sleep, 2) ≥1 brain MRI scan, and 3) ≥1 overnight EEG recording. We quantified epileptiform activity using spike percentage, the percentage of 1-second bins in the EEG tracing containing at least 1 spike. PSPEA was present when spike percentage during non-REM sleep was ≥50% than spike percentage during wakefulness.
Results:
One hundred patients with PSPEA (cases) and 47 patients without PSPEA (controls) met the inclusion criteria during a 14-year period. Both groups were comparable in terms of clinical and epidemiologic features. Early developmental lesions were more frequent in cases (48% vs 19.2%, p = 0.002). Thalamic lesions were more frequent in cases (14% vs 2.1%, p = 0.037). The main types of early developmental lesions found in cases were vascular lesions (14%), periventricular leukomalacia (9%), and malformation of cortical development (5%). Vascular lesions were the only type of early developmental lesions that were more frequent in cases (14% vs 0%, p = 0.005).
Conclusions:
Patients with PSPEA have a higher frequency of early developmental lesions and thalamic lesions than a comparable population of patients without PSPEA. Vascular lesions were the type of early developmental lesions most related to PSPEA.
Non-REM sleep potentiates epileptiform activity1,2 and this influence is most prominently expressed in electrical status epilepticus in sleep (ESES), a condition in which there is a marked activation of epileptiform activity in the transition from wakefulness to sleep, leading to a near-continuous bilateral (or occasionally lateralized) slow spike and wave pattern occupying a significant proportion of non-REM sleep.3–5 Mechanisms underlying potentiation of epileptiform activity during non-REM sleep are largely unknown but pathologic disruption of the corticothalamic circuitry by early developmental lesions is thought to contribute to potentiation and generalization of epileptiform activity during non-REM sleep.4,6–8
However, data on occurrence of early developmental lesions in patients with and without prominent sleep-potentiated epileptiform activity (PSPEA) are lacking. The present study was designed to address this gap. Our working hypothesis was that early developmental lesions are more frequent in patients with PSPEA than in patients without PSPEA. Our specific aims are 1) to systematically compare the frequency of early developmental lesions in patients with and without PSPEA and 2) to describe the specific types of early developmental lesions that are found in patients with PSPEA.
METHODS
Protocol approval.
This study was approved by the Institutional Review Board of Children's Hospital Boston.
Study design.
We performed a case-control study at a tertiary pediatric epilepsy center.
Patients.
We included patients with the clinical suspicion of ESES between 0 and 21 years 1) with at least 1 overnight EEG recorded at our pediatric epilepsy monitoring unit between 1996 and 2009, and 2) at least 1 brain MRI study results available for review. We based our clinical suspicion of ESES on an age-related neuropsychological regression in at least 1 domain of development that was accompanied in most cases by a seizure disorder consistent with the evolution over time of seizure onset in infancy to early childhood, worsening of seizures (more frequent, more difficult to control, and with a typical evolution of seizure types) during childhood, and eventual improvement or remission around puberty.4 We considered acquired epileptic aphasia as one of the clinical presentations of ESES and defined it as an aphasic disorder with subacute onset that occurred in children with previously normal age-appropriate speech, with a clinical evolution that was progressive but with spontaneous fluctuations and a tendency to improve around puberty; seizures were not present in most of these patients.5 EEG and MRI reviewers were blinded to details of the patients' history and imaging or EEG findings, respectively.
EEG analysis.
All patients underwent a specific continuous EEG monitoring protocol to diagnose or exclude ESES. We monitored patients for a period of 12 to 96 hours. Scalp-EEG recordings were placed according to the 10–20 international system of electrode placement. EEG recordings were continuously monitored throughout the day and night by dedicated EEG technologists.
For the purposes of this study, 2 clinical neurophysiologists scored the EEG data. We defined “spike percentage” as the percentage of 1-second bins in the EEG tracing containing at least 1 spike during the first 5 minutes of every hour of slow wave sleep, and based on this assessment, we calculated the spike percentage. The same amount of non-REM sleep was sampled in cases and controls to prevent confounding. We considered unilateral or lateralized spike-waves to be equivalent to generalized bilateral discharges. In order to study a homogeneous group of patients with symmetric and synchronous SPEA and exclude patients with multifocal origin of epileptiform discharges, we excluded EEG tracings with independent left and right spike-waves. For the purpose of this study, a patient was considered to have PSPEA when spike percentage during sleep was ≥50% as compared to wakefulness, as we considered this to be a significant potentiation of epileptiform activity from wakefulness to sleep. We enrolled patients in the study because of a clinical presentation consistent with ESES and we reviewed their PSPEA (cases) or non-PSPEA (controls) status only after inclusion in the study.
Analysis of clinical data.
We extracted all clinical variables by retrospective chart review, including gender, age at overnight EEG monitoring, and clinical seizure types, as well as associated clinical conditions including cerebral palsy, autistic behavior, attention deficit hyperactivity disorder, developmental regression, and language regression. We also collected information on family history of epilepsy.
Analysis of neuroimaging features.
The neuroimaging features were the main variable compared between cases and controls. All patients had at least 1 brain MRI. For the purposes of this study, a pediatric neuroradiologist (S.P.P.) and a pediatric neurophysiologist (M.T.) blinded to the clinical diagnosis reviewed all MRIs. All patients had a minimum of axial T2-weighted and axial fluid-attenuated inversion recovery sequences reviewed. We considered the lack of an acute diffusion abnormality as a proof that there were no acute lesions superimposed upon the early onset lesions. For the purposes of this study, we considered as early developmental lesions any brain structural lesion, either malformative or destructive, that occurred prenatally or during the first 2 years of life. The type of early developmental lesions and the involvement of the thalamus in the early developmental lesions were specifically analyzed.
Comparison of the groups.
We compared the frequency of early developmental lesions and involvement of the thalamus in cases and controls. In the subgroup of cases, we also compared the types and frequency of early developmental lesions and the thalamic involvement between cases with spike percentage above and below 85%.
Statistical analysis.
We performed statistical analysis using SPSS 19 (SPSS Inc., Chicago, IL). We compared means using t test. For comparison of frequencies of nominal variables we used a 2 × 2 table and either Pearson χ2 or Fisher exact test as appropriate.
RESULTS
Patients.
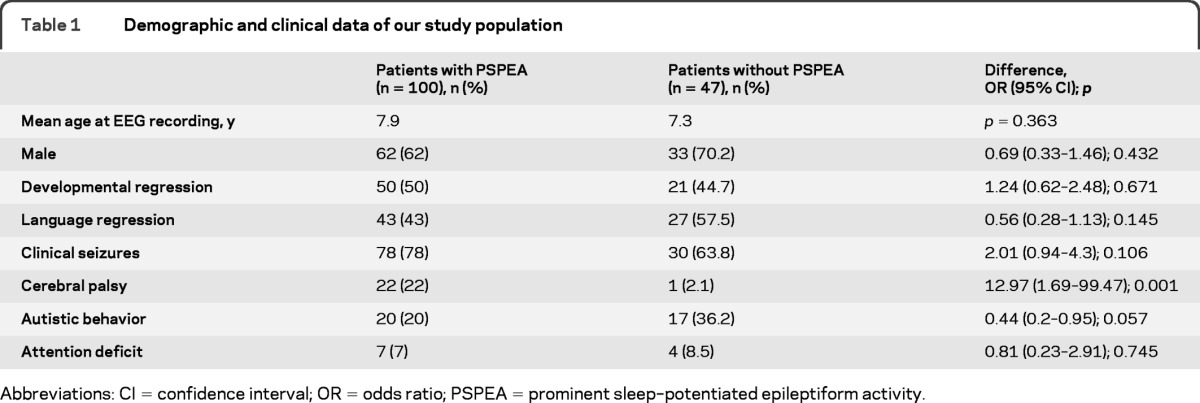
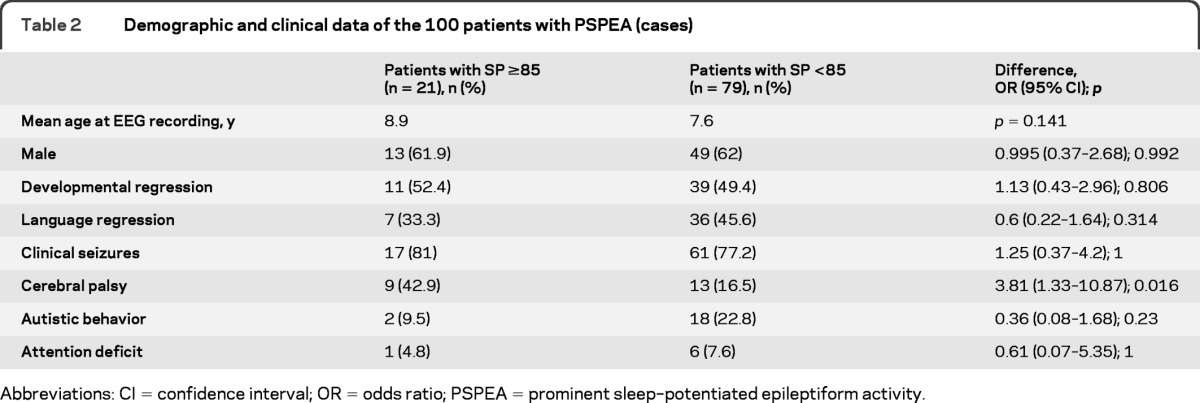
A total of 147 patients were assessed for a clinical presentation consistent with ESES, and based on subsequent EEG results, 100 of those had PSPEA (cases). Cases and controls did not differ regarding age, gender, or presence of clinical seizures, autistic behavior, attention deficit, developmental regression, or language regression. Cerebral palsy was more frequent in cases (table 1), and in the subgroup of cases, it was more frequent in patients with spike percentage ≥85% (table 2).
Table 1.
Demographic and clinical data of our study population
Abbreviations: CI = confidence interval; OR = odds ratio; PSPEA = prominent sleep–potentiated epileptiform activity.
Table 2.
Demographic and clinical data of the 100 patients with PSPEA (cases)
Abbreviations: CI = confidence interval; OR = odds ratio; PSPEA = prominent sleep–potentiated epileptiform activity.
Early developmental lesions in cases and controls.
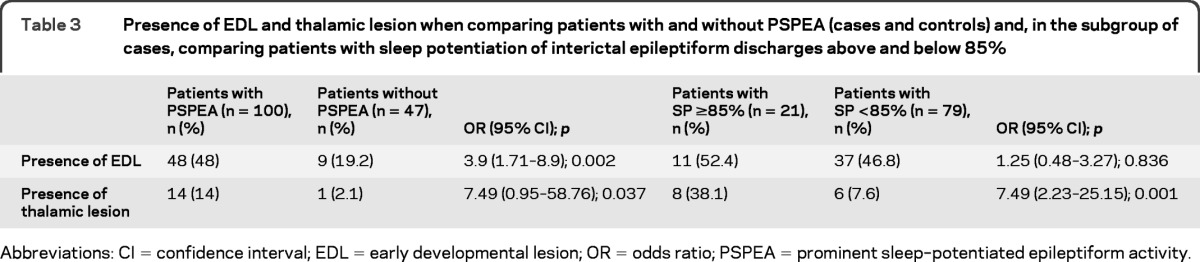
Early developmental lesions were more frequent in cases (table 3; figure e-1 on the Neurology® Web site at www.neurology.org). Additional subgroup analysis of the cases revealed that the frequency of early developmental lesions was not different between patients above and below a spike percentage during non-REM sleep of 85% (table 3).
Table 3.
Presence of EDL and thalamic lesion when comparing patients with and without PSPEA (cases and controls) and, in the subgroup of cases, comparing patients with sleep potentiation of interictal epileptiform discharges above and below 85%
Abbreviations: CI = confidence interval; EDL = early developmental lesion; OR = odds ratio; PSPEA = prominent sleep–potentiated epileptiform activity.
Thalamic involvement in cases and controls.
Thalamic lesions were more frequently seen in cases (table 3; figure e-2). Except for 3 patients with bilateral thalamic involvement, the rest had a unilateral thalamic lesion with a normal contralateral thalamus. Subgroup analysis revealed that in cases the frequency of thalamic lesions was higher in patients with spike percentage ≥85% (table 3).
Type of early developmental lesions.
The types of early developmental lesions found in cases and controls can be found in table 4. Illustrative examples can be found in figure 1. Early strokes were the only type of early developmental lesions that was more frequent in cases (table 4). In the subgroup of cases, vascular lesions were more frequent in patients with spike percentage ≥85 than in patients with spike percentage <85 (table e-1).
Table 4.
Types of EDL found in our population
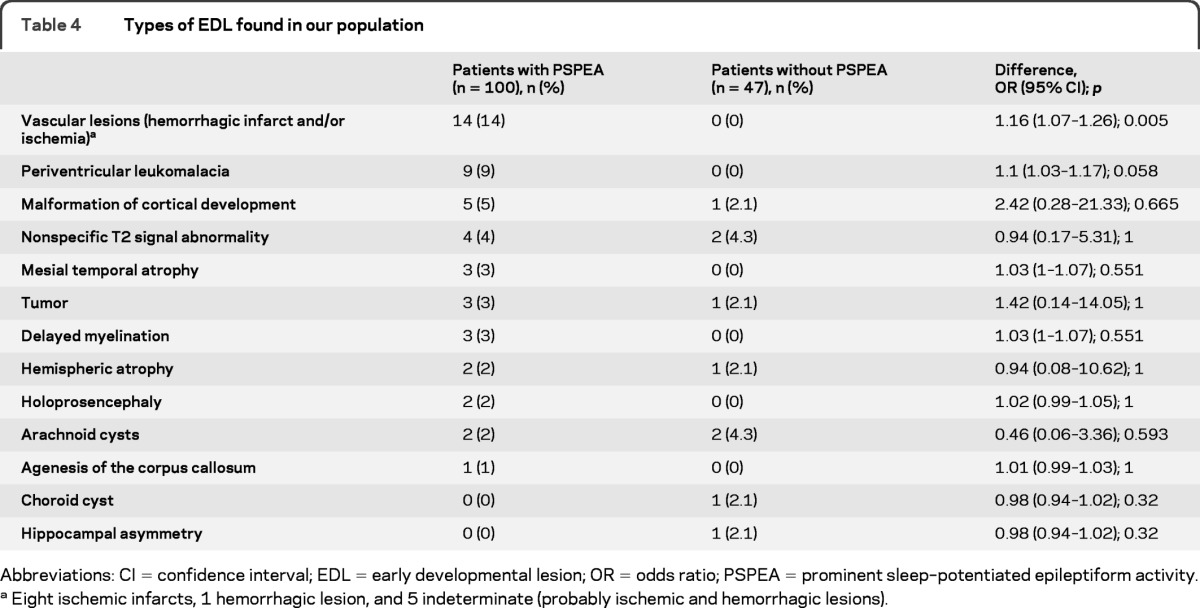
Abbreviations: CI = confidence interval; EDL = early developmental lesion; OR = odds ratio; PSPEA = prominent sleep–potentiated epileptiform activity.
Eight ischemic infarcts, 1 hemorrhagic lesion, and 5 indeterminate (probably ischemic and hemorrhagic lesions).
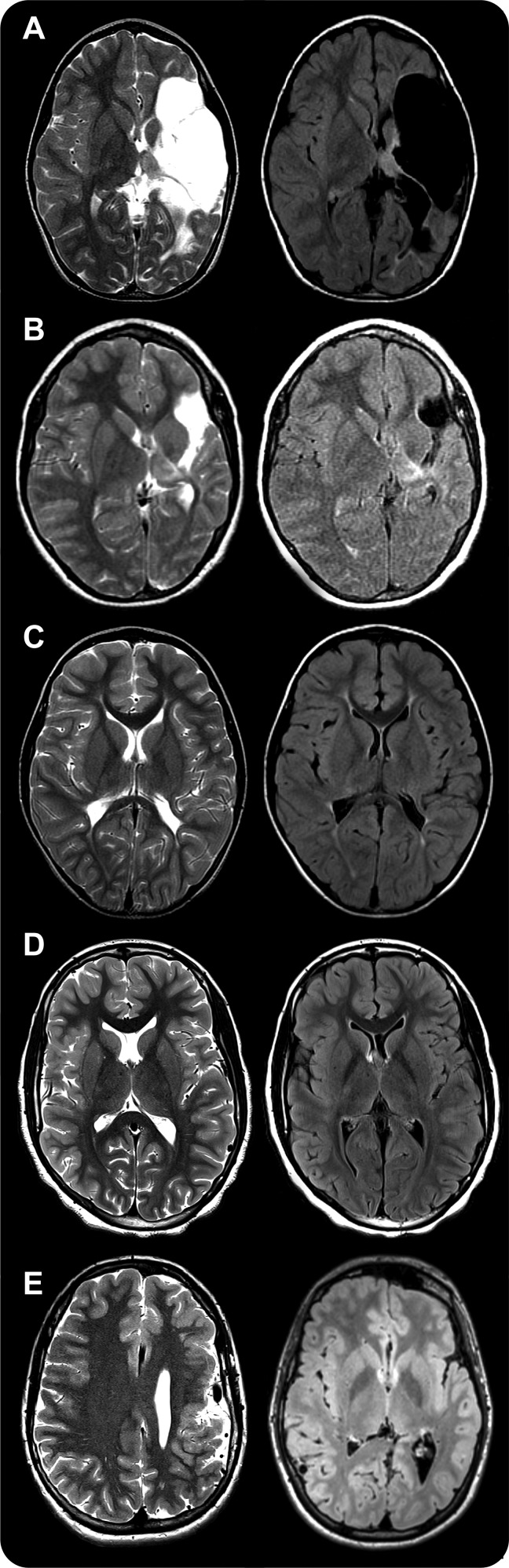
Figure 1. MRI of some of the early developmental lesions found in our series.
Axial view. T2-weighted in the left column and fluid-attenuated inversion recovery in the right column. (A) Extensive cystic encephalomalacia affecting the whole left middle cerebral artery distribution consistent with remote left middle cerebral artery infarct. (B) Localized cystic encephalomalacia found in left middle cerebral artery distribution consistent with remote left middle cerebral artery infarct. (C) Bilateral periventricular leukomalacia. (D) Right perisylvian polymicrogyria. (E) Left frontoparietal polymicrogyria. The left thalamus is involved in patients A and B; thalamic volume was small bilaterally in patient C and no thalamic involvement was seen in patients D and E.
Family history of seizures in cases and controls.
The presence of seizures in siblings, parents, and grandparents was lower in cases (5/95 patients [5.3%]) than in controls (8/45 patients [17.8%]), odds ratio (OR) 0.26 (95% confidence interval [CI] 0.079–0.837), Fisher exact test p = 0.027. There was no difference in the frequency of family history of epilepsy when comparing spike percentage during sleep ≥85% (3/21 cases [14.3%]) and <85% (14/74 cases [19%]), Fisher exact test p = 0.756.
Other clinical factors.
We did not find other differences in clinical presentation. Specifically, cases did not have a different frequency of prematurity (17/98 cases [17.4%]) than controls (4/47 cases [8.5%]), Fisher exact test p = 0.21.
DISCUSSION
Our data demonstrate that patients with epilepsy and PSPEA have a higher frequency of early developmental lesions, especially of vascular origin, and these lesions more often involve the thalamus as compared to a group of patients without PSPEA.
Cases and controls presented similar clinical and epidemiologic features. The higher frequency of cerebral palsy in cases may reflect the higher frequency of early developmental lesions in this group and may be considered more as an expression of the structural lesion of the brain than an independent factor predisposing to PSPEA.
The exact mechanism of PSPEA during non-REM sleep is unknown but the main hypothesis involves disruption of the cortico-thalamic neuronal network. The cyclical interaction between glutaminergic excitatory thalamocortical neurons in the dorsal thalamic nuclei and inhibitory GABAergic reticular thalamic neurons, located in the reticular nucleus, is the basis for the oscillating properties of the thalamus.6,8 A functional or structural lesion may lead to a switch from physiologic oscillations to pathologic epileptic discharges that may appear as generalized spike and wave complexes over the cerebral cortex.6,9,10 While the oscillating properties of this thalamic reverberating circuit are tonically inhibited by the input of the reticular activating system during wakefulness, the lack of input through the reticular activating system activity during non-REM sleep is thought to release the reverberating properties of this neuronal network.6,11,12
Several patients with thalamic lesions, in particular strokes, have developed EEG patterns consistent with ESES.13–17 In a series of 32 patients with early acquired lesions involving the thalamus ESES or PSPEA developed in 29 cases (90.6%).13 Additionally, early developmental lesions and thalamic lesions were frequently seen in patients with ESES: in a series of 67 patients with ESES, 33 patients (49.3%) had early developmental lesions,18 and in a series of 44 patients with ESES, 18 patients (41%) had early developmental lesions.19 In our series, the frequency of early developmental lesions in cases fell within the previously reported 41%–49.3% range. Furthermore, our results provide the answer to an unresolved question in previous literature: whether patients with PSPEA (cases) have a higher frequency of early developmental lesions than patients with interictal epileptiform discharges without PSPEA (controls). Our results demonstrate that cases have a higher frequency of early developmental lesions than controls, and this difference becomes even more marked when considering patients with early developmental lesions that involve the thalamus.
Because epileptiform activity occupying a minimum of 85% of the non-REM sleep tracing is by definition the most important element for the classic diagnosis of ESES,5,20 we aimed to detect whether there were imaging differences in the subgroups of cases and spike percentage during sleep above and below 85%. The higher frequency of thalamic lesions in cases with PSPEA ≥85% underlines the importance of thalamic involvement for development of the most extreme forms of PSPEA.
Early developmental lesions related to ESES are mainly of vascular origin. The first 2 case reports of early developmental lesions and ESES were described in the setting of thalamic hemorrhages.14,16 Additionally, in another series 29 out of 32 patients (90.6%) had ischemic and/or hemorrhagic prenatal or perinatal insults (stroke, infarction, periventricular leukomalacia, and/or hypoxic-ischemic encephalopathy).13 In a series of 18 patients with ESES and early developmental lesions, 14 (77.8%) had a vascular and/or infectious neonatal injury and the remaining 4 had malformations of cortical development.19 Vascular lesions were also the most frequent imaging findings (46.2%) in a series of 13 patients with surgically treated ESES and early developmental lesions.21 In another series of 33 patients with ESES and early developmental lesions, congenital stroke was the second most frequent imaging finding (21.2%) after cortical dysplasia (24.2%).18 Our data support the notion that early life strokes and periventricular leukomalacia are the main findings related to the subsequent development of PSPEA. The control group allowed us to expand these findings and demonstrate that vascular lesions are more frequent in patients with PSPEA than in a comparable population of patients with interictal epileptiform discharges during wakefulness without PSPEA.
The importance of prematurity or familial history of seizures has not been systematically studied. Based on our dataset we did not find an important contribution of prematurity or familial history of seizures to the development of PSPEA. In fact, family history of seizures was lower in cases than in a comparable population of controls. This is in accordance with previous observations that “in general, genetic factors seem to play a minor role in ESES syndrome.”5
Our data shed light on the etiology of PSPEA by demonstrating a higher frequency of early developmental lesions, especially of vascular origin, in patients with PSPEA. This finding may fuel further speculation that early disruption of the cortico-thalamic circuitry can contribute to the development of PSPEA in selected patients. Our results set the ground for future prospective studies that should follow patients with early developmental lesions and quantify the risk of developing PSPEA and ESES. The impact of our findings on immediate clinical practice is that a lower threshold for performing an MRI should be considered in pediatric patients with PSPEA as they have a higher frequency of early developmental lesions.
Our results need to be interpreted in the clinical context of the data acquisition. Data collection at a tertiary referral center may have selected a subpopulation of patients with a more severe presentation, and this may limit the overall generalizability of our results to less severely affected patients. The addition of a control group allowed us to compare patients with similar baseline characteristics except for the presence or absence of PSPEA, and therefore selection bias may not significantly affect the results as the control group has also been selected from the same tertiary referral center population.
Retrospective data collection may have been subject to information bias. We tried to overcome this bias by reviewing the original EEG tracings and MRI scans in order to standardize the data acquisition from the original studies and to reduce observer variability.
Following the basic and clinical research literature, we considered thalamic lesions as the main factor leading to PSPEA and, therefore, we focused our study on considering the presence and type or absence of early developmental lesions and the presence or absence of thalamic involvement. Other factors that can also contribute to PSPEA such as specific age at insult, location, and extension of the early developmental lesions were not specifically studied.
Selecting patients for a study on PSPEA based on their clinical presentation only without considering their EEG findings may also limit generalizability. Inclusion criteria in previous literature considered clinical and EEG findings. ESES is an EEG pattern with different clinical presentations,22 but all of them have in common an age-specific appearance of regression in, at least, one aspect of development. A proportion as high as 40%–50% of patients with ESES or PSPEA had early developmental lesions18,19 and a high proportion of patients with early developmental lesions affecting the thalamus developed ESES or PSPEA,13,14,16 but the question of whether patients with PSPEA have a higher frequency of early developmental lesions than comparable patients without PSPEA remained unanswered. We considered that the best way to obtain a control group for patients with PSPEA was to gather a population of patients with a similar clinical presentation and then determine sleep potentiation. An alternative approach of our study enrolling only patients with EEG-proven PSPEA or ESES would not have provided a control group, leaving the main question of our study unanswered.
The classic definition of ESES requires a generalized spike-wave index of at least 85% of the slow wave sleep tracing.5,20 Our study considered this definition, but also looked at patients with lower percentages of sleep potentiation. The 85% threshold is an arbitrarily set value in the initial definition of ESES5,20 and it has been largely followed.5,23–25 However, the International League Against Epilepsy (ILAE) criteria do not provide a cutoff value and only require “continuous diffuse spike-waves during slow wave sleep.”3 Other authors also consider that a lower percentage may also be consistent with ESES.26,27 We decided to include this cutoff value, but not to exclude patients with lower percentages in order to include patients along the continuum of this entity according to the ILAE definition.3 Comparing patients with and without PSPEA, a basic mechanism with different intensity of expression may shed additional light on the basic pathophysiology leading to ESES than comparing patients above and below an arbitrary threshold. In order to permit comparability with classic studies, we provided a comparison in the subgroup of cases comparing patients with a spike percentage during non-REM sleep above and below 85%.
In order to study a homogeneous population we have excluded patients with multifocal origin of epileptiform discharges, but a similar study of early developmental lesions in patients with multifocal PSPEA is under way.
Our series demonstrate that patients with PSPEA present with a higher frequency of early developmental lesions, and a higher frequency of thalamic lesions is seen in these patients than in those without PSPEA. The most frequent early developmental lesions were strokes. Early developmental lesions, especially those affecting the thalamus, and particularly in the setting of an early stroke, may play a role in the development of PSPEA.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Blaise Bourgeois for reading the manuscript and for comments during the study completion and Carmen Montilla Vallejo and Carlos Alaez Vasconcellos for technical support during the preparation of the figure.
GLOSSARY
- CI
confidence interval
- ESES
electrical status epilepticus in sleep
- ILAE
International League Against Epilepsy
- OR
odds ratio
- PSPEA
prominent sleep-potentiated epileptiform activity
Footnotes
Editorial, page 1708
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Iván Sánchez Fernández participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination. Masanori Takeoka participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, and study supervision or coordination. Emir Tas participated in drafting and revising the manuscript for content, including medical writing for content and acquisition of data. Jurriaan M. Peters participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, study supervision or coordination. Sanjay P. Prabhu participated in drafting and revising the manuscript for content, including medical writing for content, in analysis and interpretation of data, acquisition of data, and study supervision or coordination. Karen M. Stannard participated in study concept and design, in analysis and interpretation of data, and study supervision or coordination. Matt Gregas participated in statistical analysis. Yaman Eksioglu participated in drafting and revising the manuscript for content, including medical writing for content, acquisition of data, and study supervision or coordination. Alexander Rotenberg participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data. James J. Riviello participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, and study supervision or coordination. Sanjeev V. Kothare participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, and study supervision or coordination. Tobias Loddenkemper participated in drafting and revising the manuscript for content, including medical writing for content, in study concept and design, in analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination.
DISCLOSURE
Iván Sánchez Fernández is funded by a grant for the study of epileptic encephalopathies from “Fundación Alfonso Martín Escudero.” Tobias Loddenkemper is supported by a Career Development Fellowship Award from Harvard Medical School and Children's Hospital Boston, by the Program for Quality and Safety at Children's Hospital Boston, and receives funding from the Epilepsy Foundation of America. The rest of the authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Loddenkemper T, Lockley SW, Kaleyias J, Kothare SV. Chronobiology of epilepsy: diagnostic and therapeutic implications of chrono-epileptology. J Clin Neurophysiol 2011; 28: 146– 153 [DOI] [PubMed] [Google Scholar]
- 2. Quigg M. Circadian rhythms: interactions with seizures and epilepsy. Epilepsy Res 2000; 42: 43– 55 [DOI] [PubMed] [Google Scholar]
- 3. Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30: 389– 399 [DOI] [PubMed] [Google Scholar]
- 4. Loddenkemper T, Sánchez Fernández I, Peters JM. Continuous spike and waves during sleep and electrical status epilepticus in sleep. J Clin Neurophysiol 2011; 28: 154– 164 [DOI] [PubMed] [Google Scholar]
- 5. Tassinari CA, Rubboli G, Volpi L, et al. Encephalopathy with electrical status epilepticus during slow sleep or ESES syndrome including the acquired aphasia. Clin Neurophysiol 2000; 111: S94– S102 [DOI] [PubMed] [Google Scholar]
- 6. Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 2009; 62: 612– 632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sánchez-Vives M, McCormick D. Functional properties of perigeniculate inhibition of dorsal lateral geniculate nucleus thalamocortical neurons in vitro. J Neurosci 1997; 17: 8880– 8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262: 679– 685 [DOI] [PubMed] [Google Scholar]
- 9. Blumenfeld H, McCormick D. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci 2000; 20: 53– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci 2006; 26: 8633– 8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science 1997; 278: 130– 134 [DOI] [PubMed] [Google Scholar]
- 12. Sanchez-Vives MV, McCormick DA. Functional properties of perigeniculate inhibition of dorsal lateral geniculate nucleus thalamocortical neurons in vitro. J Neurosci 1997; 17: 8880– 8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guzzetta F, Battaglia D, Veredice C, et al. Early thalamic injury associated with epilepsy and continuous spike-wave during slow sleep. Epilepsia 2005; 46: 889– 900 [DOI] [PubMed] [Google Scholar]
- 14. Incorpora G, Pavone P, Smilari PG, Trifiletti R, Parano E. Late primary unilateral thalamic hemorrhage in infancy: report of two cases. Neuropediatrics 1999; 30: 264– 267 [DOI] [PubMed] [Google Scholar]
- 15. Kelemen A, Barsi P, Gyorsok Z, Sarac J, Szucs A, Halasz P. Thalamic lesion and epilepsy with generalized seizures, ESES and spike-wave paroxysms: report of three cases. Seizure 2006; 15: 454– 458 [DOI] [PubMed] [Google Scholar]
- 16. Monteiro JP, Roulet-Perez E, Davidoff V, Deonna T. Primary neonatal thalamic haemorrhage and epilepsy with continuous spike-wave during sleep: a longitudinal follow-up of a possible significant relation. Eur J Paediatr Neurol 2001; 5: 41– 47 [DOI] [PubMed] [Google Scholar]
- 17. Tezer FI, Saygi S. Unilateral thalamic lesions and generalized or lateralized spike wave discharges. Epilepsy Res 2009; 86: 228– 231 [DOI] [PubMed] [Google Scholar]
- 18. Van Hirtum-Das M, Licht EA, Koh S, Wu JY, Shields WD, Sankar R. Children with ESES: variability in the syndrome. Epilepsy Res 2006; 70 (suppl 1): S248– S258 [DOI] [PubMed] [Google Scholar]
- 19. Buzatu M, Bulteau C, Altuzarra C, Dulac O, Van Bogaert P. Corticosteroids as treatment of epileptic syndromes with continuous spike-waves during slow-wave sleep. Epilepsia 2009; 50 (suppl 7): 68– 72 [DOI] [PubMed] [Google Scholar]
- 20. Patry G, Lyagoubi S, Tassinari CA. Subclinical “electrical status epilepticus” induced by sleep in children: a clinical and electroencephalographic study of six cases. Arch Neurol 1971; 24: 242– 252 [DOI] [PubMed] [Google Scholar]
- 21. Peltola ME, Liukkonen E, Granstrom ML, et al. The effect of surgery in encephalopathy with electrical status epilepticus during sleep. Epilepsia 2011; 52: 602– 609 [DOI] [PubMed] [Google Scholar]
- 22. Veggiotti P, Beccaria F, Guerrini R, Capovilla G, Lanzi G. Continuous spike-and-wave activity during slow-wave sleep: syndrome or EEG pattern? Epilepsia 1999; 40: 1593– 1601 [DOI] [PubMed] [Google Scholar]
- 23. Loddenkemper T, Cosmo G, Kotagal P, et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery 2009; 64: 328– 337 [DOI] [PubMed] [Google Scholar]
- 24. Saltik S, Uluduz D, Cokar O, Demirbilek V, Dervent A. A clinical and EEG study on idiopathic partial epilepsies with evolution into ESES spectrum disorders. Epilepsia 2005; 46: 524– 533 [DOI] [PubMed] [Google Scholar]
- 25. Tassinari CA, Rubboli G, Volpi L, Billard C, Bureau M. Electrical status epilepticus during slow sleep (ESES or CSWS) including acquired epileptic aphasia (Landau-Kleffner syndrome). In: Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P.eds. Epileptic Syndromes in Infancy, Childhood and Adolescence, 4th ed. London: John Libbey; 2005: 295– 314 [Google Scholar]
- 26. Inutsuka M, Kobayashi K, Oka M, Hattori J, Ohtsuka Y. Treatment of epilepsy with electrical status epilepticus during slow sleep and its related disorders. Brain Dev 2006; 28: 281– 286 [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi K, Hata H, Oka M, et al. Age-related electrical status epilepticus during sleep and epileptic negative myoclonus in DRLPA. Neurology 2006; 66: 772– 773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.