Abstract
In response to gonadectomy certain inbred mouse strains develop sex steroidogenic adrenocortical neoplasms. One of the hallmarks of neoplastic transformation is expression of GATA4, a transcription factor normally present in gonadal but not adrenal steroidogenic cells of the adult mouse. To show that GATA4 directly modulates adrenocortical tumorigenesis and is not merely a marker of gonadal-like differentiation in the neoplasms, we studied mice with germline or conditional loss-of-function mutations in the Gata4 gene. Germline Gata4 haploinsufficiency was associated with attenuated tumor growth and reduced expression of sex steroidogenic genes in the adrenal glands of ovariectomized B6D2F1 and B6AF1 mice. At 12 months after ovariectomy, wild-type B6D2F1 mice had biochemical and histological evidence of adrenocortical estrogen production, whereas Gata4+/− B6D2F1 mice did not. Germline Gata4 haploinsufficiency exacerbated the secondary phenotype of postovariectomy obesity in B6D2F1 mice, presumably by limiting ectopic estrogen production in the adrenal glands. Amhr2-cre-mediated deletion of floxed Gata4 (Gata4F) in nascent adrenocortical neoplasms of ovariectomized B6.129 mice reduced tumor growth and the expression of gonadal-like markers in a Gata4F dose-dependent manner. We conclude that GATA4 is a key modifier of gonadectomy-induced adrenocortical neoplasia, postovariectomy obesity, and sex steroidogenic cell differentiation.
Adrenocortical neoplasms exist in approximately 5% of people over the age of 50 yr (1). The majority of these tumors are nonfunctioning adenomas, but some secrete steroid hormones that cause Cushing syndrome or other complications (2). Adrenocortical carcinomas are rare (∼1 case per million per year) but carry a poor prognosis (3, 4). When functional, adrenocortical carcinomas tend to secrete cortisol or aldosterone; in rare cases these cancers produce androgens or estrogens (5). Despite extensive investigation, the factors accounting for the high incidence of adrenocortical adenomas and the low incidence of adrenocortical carcinomas are unknown (4).
The prevalence of adrenocortical neoplasia in certain animals can provide a foothold by which to study the molecular basis of tumorigenesis in humans (6). One genetically tractable model is postgonadectomy adrenocortical neoplasia in the mouse. In response to gonadectomy and the ensuing rise in serum gonadotropins, cells in the subcapsular region of the mouse adrenal cortex transform into sex steroid-producing neoplasms that are histologically and biochemically similar to normal gonadal tissue (7–12). Gonadectomy-induced adrenocortical neoplasia is strain dependent; susceptible strains include DBA/2J, CE/J, and hybrid C57Bl/6 × DBA/2J F1 (B6D2F1) mice (7–12). Genome-wide linkage analysis of inbred strain crosses reveals that postgonadectomy adrenocortical neoplasia is a complex trait influenced by multiple genetic loci, but the underlying genes remain enigmatic (11).
GATA4, a transcription factor normally found in sex steroid-producing cells of the gonads but not corticoid-producing cells of the adult adrenal gland, is present in gonadectomy-induced adrenocortical neoplasms (13, 14). Targeted mutagenesis of Gata4 has been linked to defects in the differentiation of sex steroidogenic lineages in the fetal mouse ovary and testis (15–22). By analogy, it has been hypothesized that GATA4 regulates the differentiation of gonadal-like sex steroidogenic cells in the adrenal glands of gonadectomized mice (7, 23).
Here, we assess the impact of loss-of-function mutations in murine Gata4 on postovariectomy adrenocortical neoplasia and the secondary phenotype of ovariectomy-induced obesity. Gata4−/− mice do not survive to term (24–28), precluding the use of these homozygotes in studies of adrenocortical tumorigenesis. Therefore we examine adrenocortical tumor formation in Gata4-haploinsufficient (29, 30) and conditional knockout (20, 21) mice. We show that constitutive and acquired mutations in Gata4 mitigate the accumulation of neoplastic cells and the expression of sex steroidogenic markers in the adrenal cortex of ovariectomized mice. We also demonstrate that germline Gata4 haploinsufficiency exacerbates postovariectomy obesity, presumably by constraining ectopic estrogen production by the adrenal glands. These results establish that GATA4 directly modulates postgonadectomy adrenocortical neoplasia and is not merely a marker of gonadal-like differentiation in the tumors.
Materials and Methods
Experimental mice
Procedures involving mice were approved by an institutional committee for laboratory animal care and were conducted in accordance with National Institutes of Health guidelines for the care and use of experimental animals.
C57Bl/6 Gata4+/− mice (also termed Gata4+/Δex2) were generated and genotyped as described elsewhere (29, 30). Gata4F/F mice (also termed Gata4tm1.1Sad/J), which are homozygous for a floxed allele of Gata4 (28, 31), and Amhr2cre/+ mice (also termed B6.129S7-Amhr2tm3(cre)Bhr/Mmnc) (32, 33) were genotyped as described. Some of the Amhr2cre/+ mice were maintained on a mixed B6.129 genetic background, whereas others were backcrossed onto a C57Bl/6 background.
Gata4+/− C57Bl/6 mice were crossed with DBA2/J or A/J mice to generate wild type (WT) and Gata4+/− B6D2F1 or B6AF1 hybrids, respectively. Amhr2cre/+ C57Bl/6 mice were crossed with DBA2/J mice to generate WT and Amhr2cre/+ B6D2F1 hybrid mice. To generate conditional knockout mice, Gata4F/F mice were mated with B6.129 Amhr2cre/+ mice, and the resultant Gata4F/+;Amhr2cre/+ mice were mated with Gata4F/F mice to produce Gata4F/F;Amhr2cre/+ mice (20, 21). To assess the pattern of expression of the Amhr2-cre transgene within the adrenal gland, B6.129 Amhr2cre/+ mice were crossed with ROSA26 flox-stop-flox LacZ reporter mice (also termed B6.129S4-Gt(ROSA)26Sortm1Sor/J) (34).
Mice were anesthetized and gonadectomized at 4 wk of age (12). At specified times, the mice were euthanized, blood was collected by cardiac puncture, and tissues were harvested. For the estrogen stimulation experiments, immature (19 d old) Gata4+/− mice were implanted sc with a 1.5-mg 17β-estradiol or placebo pellet (Innovative Research of America; Sarasota, FL) (35). Their uteri were harvested 7 d later after euthanasia. For the compensatory adrenal growth experiments, 6-wk-old male mice were anesthetized and subjected to left adrenalectomy (36). After 48 h, the mice were injected with bromodeoxyuridine (BrdU) (1 mg in 0.1 ml PBS ip). The right adrenal gland was harvested 1 d later after euthanasia.
Immunohistochemistry
Tissues were fixed overnight in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned, and subjected to immunoperoxidase staining (37). The primary antibodies were: 1) goat anti-GATA4 (sc-1237, Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:200), 2) goat antigremlin (sc-18274; 1:200), 3) goat antilactoferrin (sc-14434; 1:200), 4) mouse anti-BrdU (sc-51514; 1:200), 5) mouse antihuman estrogen receptor-α (ERα) (DAKO, Carpenteria, CA; 1:500), 6) mouse antihuman cytochrome P450 aromatase (Cyp19; sc-14245; 1:200), and 7) goat antihuman cytochrome P450 17α-hydroxylase/C17-C20 lyase (Cyp17; sc-46081; 1:200). Secondary antibodies were: 1) donkey antigoat biotinylated IgG (Jackson ImmunoResearch Laboratories, West Grove, PA; dilution 1:1000) and 2) the MOM kit (Vector Laboratories, Burlingame, CA; prediluted). The avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit, Vector Laboratories, Inc.) and diaminobenzidine were used to visualize the bound antibody. Our analysis included control studies in which the primary antibody was omitted.
Morphological analyses
Adrenocortical tumor cross-sectional area was measured (38) using hematoxylin and eosin (H&E) stained tissue sections. The analysis included a minimum of four sections from each adrenal, spaced evenly (every 100 μm) through the central portion of the gland; tangential sections lacking medulla were excluded. To detect extragonadal sex steroid production in ovariectomized mice, tissues sensitive to estrogens (uterus, vagina) and androgens (submaxillary gland) were analyzed (39).
X-gal staining
Adrenal glands were fixed with 0.2% glutaraldehyde in PBS for 15 min, permeabilized with 100 mm potassium phosphate (pH 7.4), 0.02% Nonidet P-40, and 0.01% sodium deoxycholate for 5 min, and then stained with X-gal at 37 C overnight (26). After staining, the glands were frozen in OCT cyropreservation media (Tissue-Tek, Torrance, CA), sectioned, and stained with nuclear fast red. Alternatively, the stained glands were postfixed with Karnovsky solution and processed for transmission electron microscopy (20, 21).
Serum hormone levels
Measurements were made using ELISA kits for FSH, LH, and estrone (E1) sulfate from Endocrine Technologies, Inc. (Newark, CA), estradiol (E2) from BioCheck (Foster City, CA), and prolactin (PRL) from Cusabio Biotech Co. (Newark, DE).
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from adrenal glands using TRIzol (Invitrogen, Carlsbad, CA). First-strand cDNA was produced with the SuperScript VILO cDNA Synthesis Kit (Invitrogen). An aliquot of cDNA was subjected to real time RT-PCR (20, 21) using the intron-spanning primers listed in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Target gene expression was normalized to the expression of three housekeeping genes: β-actin, ribosomal protein L19, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Qualitative RT-PCR for detection of Cre-mediated recombination in adrenal glands
Total RNA was isolated from adrenal gland tissue using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA). cDNA was synthesized using a QuantiTect Reverse Transcription Kit (QIAGEN, Valencia, CA). cDNA was subjected to RT-PCR using a forward primer from exon 2 of the Gata4 gene and a reverse primer from exon 7 (20, 21).
Statistical analysis
Numerical data are represented as mean ± sd. Except were indicated, differences were assessed for statistical significance (P < 0.05) with the Student's t test.
Results
Germline Gata4 haploinsufficiency impairs postgonadectomy adrenocortical tumor formation in B6D2F1 and B6AF1 mice
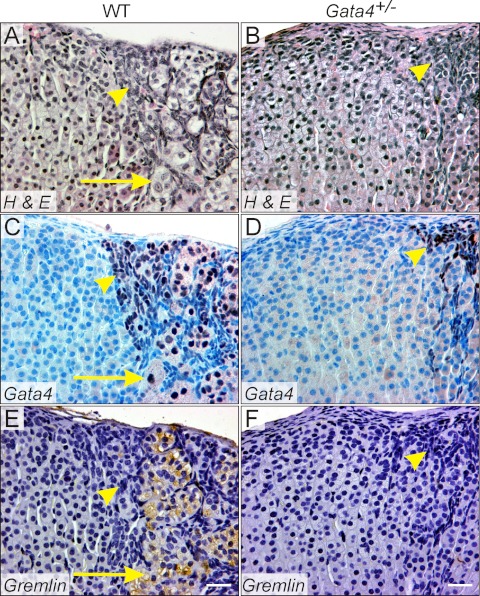
To determine the impact of GATA4 deficiency on adrenocortical tumorigenesis, we crossed Gata4+/− C57Bl/6 mice, which harbor a deletion in exon 2 of Gata4 that includes the translation start site (21, 29, 40), with DBA/2J mice and analyzed the resultant WT and Gata4+/− B6D2F1 offspring. B6D2F1 mice, like the parental DBA/2J strain, develop adrenocortical neoplasia with complete penetrance by 6 months after ovariectomy (11). H&E-stained sections of adrenal glands from ovariectomized WT B6D2F1 mice confirmed the presence of subcapsular neoplasms (Fig. 1A). The tumors were composed of spindle-shaped type A cells (Fig. 1A, arrowhead), which resemble stromal cells of the postmenopausal ovary, and sex steroidogenic type B cells (Fig. 1A, arrow), which express the gonadal-like markers LH chorionic gonadotropin receptor (Lhcgr), inhibin-α, Cyp17, and Cyp19 (7). Nuclear GATA4 antigen was evident in both the type A (Fig. 1C, arrowhead) and type B cells (Fig. 1C, arrow). Antibody against gremlin, a bone morphogenetic protein antagonist implicated in granulosa cell function (41), stained sex steroidogenic type B cells (Fig. 1E, arrow) but not type A cells (Fig. 1E, arrowhead). Histological analysis of adrenal glands from ovariectomized Gata4+/− B6D2F1 mice demonstrated only rare, small patches of GATA4-expressing type A cells that did not invade deeply into the cortex (Fig. 1, B and D). There was a paucity of morphologically recognizable type B cells in the adrenals of Gata4+/− mice (Fig. 1B) and a concomitant reduction in gremlin immunoreactivity (Fig. 1F).
Fig. 1.
Microscopic appearance of adrenal glands from ovariectomized WT and Gata4+/− B6D2F1 mice. Weanling WT (A, C, and E) or Gata4+/− (B, D, and F) B6D2F1 mice were ovariectomized, and adrenal glands were harvested 3 months later. Adjacent tissue sections were stained with H&E (A and B), anti-GATA4 antibody (C and D), or antigremlin antibody (E and F). Neoplastic type A cells (yellow arrowheads) expressed nuclear GATA4, whereas neoplastic type B cells (yellow arrows) expressed both GATA4 and gremlin. Note the absence of type B cells in the Gata4+/− adrenal gland. Bars, 40 μm.
qRT-PCR confirmed the reduced expression of Gata4 in the adrenal glands of gonadectomized B6D2F1 haploinsufficient mice. At 5 months after ovariectomy, the ratio of GATA4 mRNA levels in Gata4+/− vs. WT mice was 0.22 ± 0.03 (P < 0.05; results normalized to expression of the housekeeping gene β-actin).
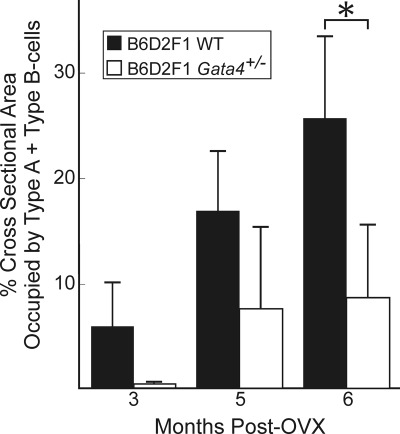
To quantify differences in adrenocortical tumor formation between the WT and Gata4+/− B6D2F1 mice, we measured the cross-sectional area of cortex occupied by neoplastic cells at varying times after ovariectomy (Fig. 2). By 6 months after ovariectomy, neoplastic cells occupied 27% of the cross-sectional area of adrenal cortex in WT mice. Gata4 haploinsufficiency was associated with a reduction in the area occupied by neoplastic cells at all time points examined; at 6 months after ovariectomy the difference was highly significant (P < 0.001). The reduction in tumor size reflected decreases in both type A cells and type B cells, but the decrease in the latter was particularly striking. At 6 months after ovariectomy, sections of WT adrenal glands contained an average of 132 ± 104 type B cells, whereas sections of Gata4+/− adrenal glands contained an average of 2 ± 1 type B cells (P < 0.05). The isolated type A cell hyperplasia seen in the adrenal glands of ovariectomized Gata4+/− mice is a feature of older nongonadectomized WT mice (42) and is not considered tumorous (43).
Fig. 2.
Comparison of postovariectomy adrenocortical tumor size in WT and Gata4+/− B6D2F1 mice. Weanling WT (black bars) or Gata4+/− (white bars) B6D2F1 female mice were subjected to ovariectomy (OVX), and adrenal glands were harvested 3, 5, or 6 months later. Individual adrenal glands were fixed, sectioned, and stained with H&E. The average cross-sectional area of cortex occupied by tumor cells (type A plus type B cells) was quantified. Values represent the mean ± sd of four to six adrenal glands. Note that the percentage of cortex occupied by tumor was greater in the WT than in the Gata4+/− adrenals at all time points examined, and at 6 months this difference was highly significant (*, P < 0.001).
GATA factors have been implicated in the development and function of the anterior pituitary gland (44, 45). To ensure that Gata4 haploinsufficient mice had intact gonadotrope function, we measured serum FSH and LH levels (Supplemental Fig. 1). Gata4 haploinsufficiency had no significant impact on either basal or postovariectomy gonadotropin levels, so the reduction in tumor formation in Gata4+/− mice cannot be ascribed to altered gonadotrope function.
To show that the Gata4 haploinsufficiency effect was not limited to a single hybrid strain, we examined postovariectomy adrenocortical neoplasia in C57Bl/6 × A/J F1 (B6AF1) mice. We found that WT B6AF1 mice, like the parental A/J strain (46), develop adrenocortical tumors after ovariectomy (Supplemental Fig. 2A). The tumors in adrenal glands of ovariectomized B6AF1 mice contained type A cells that expressed GATA4 antigen (Supplemental Fig. 2C) and type B cells that expressed both GATA4 (Supplemental Fig. 2C) and gremlin (Supplemental Fig. 2E). As in B6D2F1 mice, Gata4 haploinsufficiency mitigated ovariectomy-induced adrenocortical neoplasia in B6AF1 mice (Supplemental Fig. 2, B, D, and F).
Germline Gata4 haploinsufficiency impairs expression of gonadal-like markers in the adrenal glands of ovariectomized B6D2F1 and B6AF1 mice
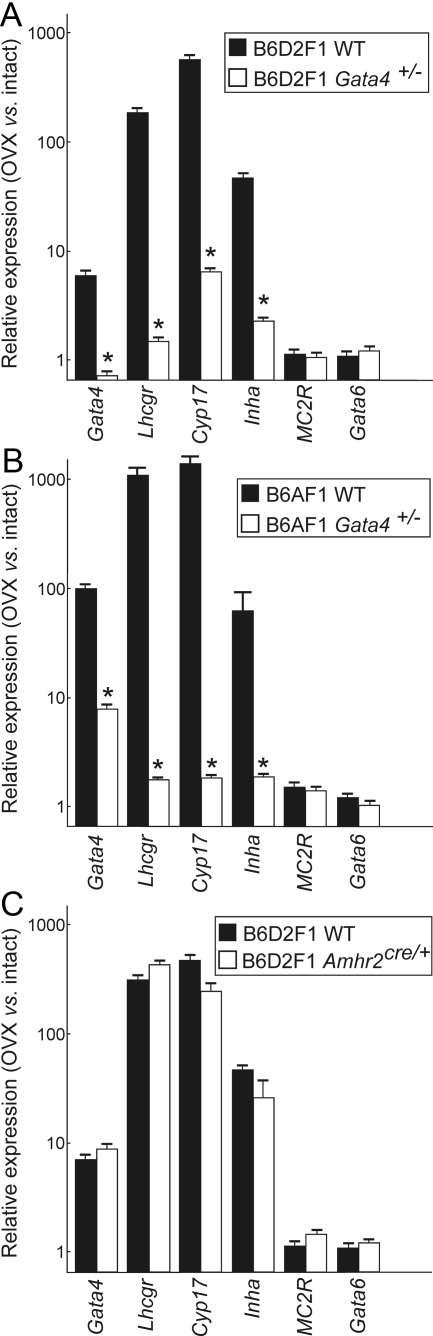
The effect of Gata4 haploinsufficiency on ovariectomy-induced changes in gonadal-like markers in the adrenal glands of B6D2F1 and B6AF1 mice was assessed by qRT-PCR 5 months after surgery. Results were normalized to expression of β-actin; normalization to two other housekeeping genes, L19 and GAPDH, yielded similar results. Consistent with studies on other susceptible strains (7, 8, 11–13), ovariectomy of WT B6D2F1 (Fig. 3A) and WT B6AF1 (Fig. 3B) mice was associated with increased adrenal expression of the sex steroidogenic differentiation markers GATA4, Cyp17, Lhcgr, and inhibin-α (Fig. 3, A and B). In contrast, ovariectomy of WT mice had no significant impact on expression of the prototypical adrenocortical markers M2CR and GATA6 (i.e. the ratio of mRNA levels in ovariectomized vs. intact mice was approximately 1) (Fig. 3, A and B). Gata4 haploinsufficiency markedly reduced the ovariectomy-dependent expression of sex steroidogenic transcripts in both B6D2F1 (Fig. 3A) and B6AF1 (Fig. 3B) mice.
Fig. 3.
Expression of steroidogenic differentiation markers in adrenal glands from ovariectomized WT, Gata4 haploinsufficient, and Amhr2 haploinsufficient mice. Weanling B6D2F1 (A and C) or B6AF1 (B) mice of the specified genotypes were subjected to ovariectomy (OVX) or left intact. Adrenal glands were harvested 5 months later and subjected to qRT-PCR analysis. Graphs show the relative expression (mean ± sd) of various transcripts in ovariectomized (n = 4) vs. intact mice (n = 4). Results were normalized to expression of β-actin. The y-axis is plotted on a logarithmic scale. Note that Gata4 haploinsufficiency attenuated the ovariectomy-dependent increase in expression of the sex steroidogenic differentiation markers Lhcgr, Cyp17, and inhibin-α (*, P < 0.001). In contrast, loss of one functional Amhr2 allele via knockin of cre had no significant impact on expression of steroidogenic cell markers in B6D2F1 mice.
As a control, we analyzed ovariectomy-dependent expression of steroidogenic genes in the adrenal glands of B6D2F1mice haploinsufficient for another gonadal marker, Amhr2, the anti-Müllerian hormone receptor gene. Amhr2 is expressed in murine postgonadectomy adrenocortical tumors but not in adjacent normal tissue (7, 8, 13). We performed qRT-PCR analysis on adrenal glands from ovariectomized Amhr2cre/+ mice, which harbor a knock-in cre allele that renders the mice Amhr2 haploinsufficient. Amhr2 haploinsufficiency had no impact on the expression of sex steroidogenic markers in the adrenal glands of ovariectomized B6D2F1 mice (Fig. 3C). The absence of a phenotype in the Amhr2 haploinsufficient mice underscores the significance of the Gata4 haploinsufficiency effect on sex steroidogenesis and constitutes an important negative control for the Amhr2-cre-mediated conditional mutagenesis studies described below.
Germline Gata4 haploinsufficiency impairs adrenocortical estrogen production and exacerbates obesity in ovariectomized B6D2F1 mice
The examination of tissues that undergo morphological changes in response to circulating estrogens (uterus, vagina) or androgens (submaxillary gland) offers evidence for adrenocortical sex steroid production in gonadectomized mice. Older ovariectomized DBA/2J mice exhibit estrogenic stimulation of the uterus and vagina, whereas aged ovariectomized CE/J mice display both estrogenic and androgenic stimulation of target tissues (39).
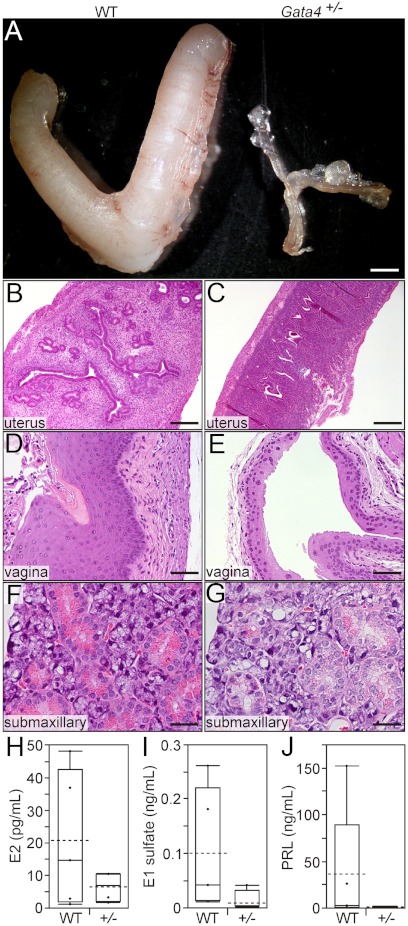
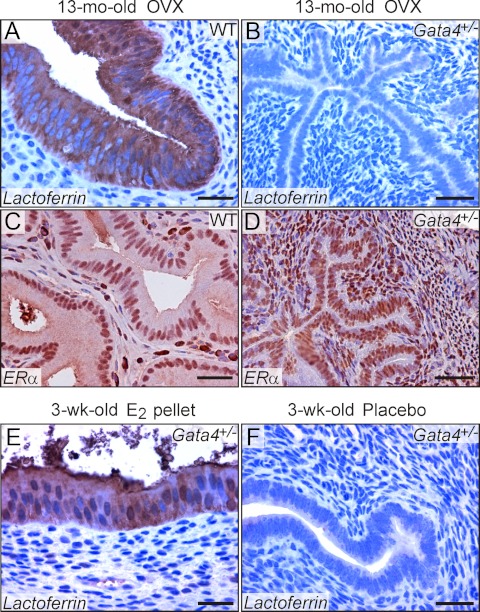
Older ovariectomized WT B6D2F1 mice show a prominent estrogenic phenotype resembling that of the parental DBA/2J strain. At 12 months after ovariectomy, every WT B6D2F1 mouse (n = 5) had grossly enlarged uterine horns (Fig. 4A) with histological evidence of estrogenic changes in the uterus (Fig. 4B) and vagina (Fig. 4D), including uterine epithelial immunoreactivity for lactoferrin, an estrogen-responsive gene (Fig. 5A) (47). By comparison, none of the age-matched ovariectomized Gata4+/− mice (n = 4) showed morphological evidence of extragonadal estrogen production (P < 0.005; two population proportions testing). Instead, the Gata4+/− mice had grossly atrophic uteri (Fig. 4A) and lacked estrogen-induced histological changes in target tissues (Fig. 4, C and E), including lactoferrin immunoreactivity in the uterus (Fig. 5B). In contrast, nuclear estrogen receptor (ER)α immunoreactivity was evident in uterine epithelial and stromal cells of both the WT (Fig. 5C) and Gata4+/− (Fig. 5D) mice. Uterine weights in the ovariectomized WT and Gata4+/− B6D2F1 mice were 98 ± 5 mg and 13 ± 0.3 mg, respectively (P < 0.01).
Fig. 4.
Gata4 haploinsufficiency abrogates extragonadal sex steroid production in older ovariectomized B6D2F1 mice. Weanling mice of the indicated genotypes were ovariectomized, and 12 months later tissues were analyzed for evidence of estrogen- or androgen-dependent stimulation. The uteri of WT mice were enlarged (A, left) and estrogenic (B), whereas the uteri of Gata4+/− mice were atrophic (A, right) and hypoestrogenic (C). Consistent with estrogen stimulation, the vaginal epithelium of WT mice was thick and cornified (D). In contrast, the vaginal mucosa of Gata4+/− mice appeared thin and hypoestrogenic (E). WT mice had evidence of androgen stimulation of the submaxillary gland, manifest as tall columnar acinar cells with basally located nuclei and abundant eosinophilic cytoplasmic granules (F). In Gata4+/− mice, however, the submaxillary glands had the typical appearance of female mice, with centrally located epithelial cell nuclei and only a few cytoplasmic granules (G). Bars, 2 mm (A), 300 μm (B and C), 75 μm (D and E), and 40 μm (F and G). Serum E2, E1 sulfate, and PRL levels in WT and Gata4+/− mice are shown in panels H, I, and J, respectively. In the box-and-whisker plots, dots represent individual measurements, boxes represent the interquartile range, whiskers indicate the first and fourth quartiles, solid lines indicate the median, and dashed lines represent the mean (63).
Fig. 5.
Lactoferrin and ERα immunoreactivity in the uteri of WT and Gata4+/− B6D2F1 mice. Weanling WT or Gata4+/− B6D2F1 mice were ovariectomized (OVX), and 12 months later uteri were subjected to immunoperoxidase staining for the estrogen response marker lactoferrin or for ERα. Lactoferrin immunoreactivity was seen in the uterine epithelial cells of ovariectomized WT mice (A) but not Gata4+/− mice (B). In contrast, nuclear immunoreactivity for ERα was evident in uterine epithelial and stromal cells of ovariectomized WT (C) and Gata4+/− (D) mice. To show that the uteri of Gata4+/− mice were capable of responding to estrogen, prepubertal Gata4+/− mice were implanted sc with an E2 pellet or a placebo. Lactoferrin immunoreactivity and other estrogenic changes were observed in the uteri of immature mice implanted with E2 (E) but not the placebo (F). Bars, 75 μm.
Because GATA4 is not expressed in the uterus or its precursor, the Müllerian duct, the differences in uterine morphology between ovariectomized WT and Gata4 haploinsufficient mice cannot be attributed to a direct effect of GATA4 on uterine responsiveness to estrogen (21). Reinforcing this premise, a robust estrogenic response, including increased lactoferrin immunoreactivity, was observed in the uteri of prepubertal Gata4+/− mice implanted sc with an E2 pellet (Fig. 5E) but not a placebo (Fig. 5F).
In addition to estrogenic changes, older ovariectomized WT mice (Fig. 4F), but not their Gata4+/− counterparts (Fig. 4G), had histological evidence of androgenic stimulation in the submaxillary glands. Such masculinization of the female submaxillary gland occurs during pregnancy and lactation as a result of increased androgen secretion (48).
At 12 months after ovariectomy, serum E2 and E1 sulfate levels were greater in WT than Gata4+/− B6D2F1 mice (Fig. 4, H and I). Estrogen induces PRL secretion (49, 50), and ovariectomized WT mice had higher serum PRL levels than their Gata4+/− counterparts (Fig. 4J). PRL can have direct effects on the adrenal gland (51) and estrogen-sensitive organs such as the uterus (49), so some of the changes in the ovariectomized WT mice could reflect PRL effects. Arguing against a direct effect of PRL on the uterus, Kiss et al. (52) found that administration of PRL + E2 to ovariectomized B6D2F1 mice elicited no more uterine epithelial proliferation than E2 alone.
Gross inspection of older ovariectomized WT B6D2F1 mice demonstrated large adrenal tumor nodules that distorted the surface of the glands (data not shown), supporting the notion that adrenocortical tumors were the source of extragonadal sex steroids. Such adrenal tumor nodules were not evident in aged ovariectomized Gata4+/− mice.
Estrogenic changes were not observed in reproductive organs of younger ovariectomized WT B6D2F1 mice (< 6 months after ovariectomy; data not shown). qRT-PCR analysis of adrenal glands from WT B6D2F1 mice of varying ages showed that the up-regulation of Cyp19 expression lagged that of other gonadal-like markers, such as Lhcgr and Cyp17 (Supplemental Fig. 3A). A marked increase in Cyp19 expression occurred between 5 and 6 months after ovariectomy. The late onset of Cyp19 expression may explain why estrogenic changes in the uterus and vagina are limited to older ovariectomized mice. Immunohistochemistry on adrenal glands from WT B6D2F1 mice 6 months after ovariectomy demonstrated Cyp17 (Supplemental Fig. 3B) and Cyp19 (Supplemental Fig. 3C) immunoreactivity in type B neoplastic cells, in agreement with studies of other ovariectomized strains of mice (7).
In addition to triggering adrenocortical tumorigenesis, ovariectomy is known to exacerbate obesity in B6D2F1 and other strains of mice (11). Postovariectomy obesity has been attributed to reduced energy expenditure (53), and estrogen repletion of ovariectomized mice has been shown to prevent gains in visceral adiposity (53). At 12 months after ovariectomy, WT B6D2F1 mice were significantly less obese than their Gata4+/− counterparts [body weights = 40.4 ± 4.7 g and 51.7 ± 4.9 g, respectively (P < 0.01)], consistent with extragonadal estrogen production in the former. Visual inspection confirmed that the differences in weight between WT and Gata4+/− mice reflected differences in abdominal adiposity.
Collectively, these observations suggest that Gata4 haploinsufficiency abrogates extragonadal sex steroid production in ovariectomized B6D2F1 mice by inhibiting adrenocortical tumor formation and that absence of ectopic estrogen production exacerbates postovariectomy obesity.
Germline Gata4 haploinsufficiency has no effect on the growth of nonneoplastic adrenocortical cells in B6D2F1 mice
Having demonstrated that constitutive Gata4 haploinsufficiency decreases postgonadectomy adrenocortical tumor development, we next assessed the impact of Gata4 haploinsufficiency on the growth of nonneoplastic adrenocortical cells, using the established model of compensatory adrenal hyperplasia/hypertrophy in mice subjected to left adrenalectomy (36). There was no difference in right adrenal gland weight between 6-wk-old WT and Gata4+/− B6D2F1 mice subjected to sham adrenalectomy (1.1 ± 0.2 mg vs. 1.2 ± 0.1 mg, respectively). Thus, even though Gata4 is transiently expressed in the adrenal during fetal development (54), Gata4 haploinsufficiency had no effect on adrenal gland size in intact adult mice. Likewise, Gata4 haploinsufficiency had no impact on compensatory adrenal growth after unilateral adrenalectomy (Supplemental Fig. 4A). Histological analysis demonstrated that the increase in adrenal gland weight reflected enlargement of the cortex (Supplemental Fig. 4, B–E), and BrdU-labeling studies showed that unilateral adrenalectomy elicited similar increases in adrenocortical cell proliferation in WT and Gata4+/− mice (Supplemental Fig. 4, B–E). We conclude that Gata4 haploinsufficiency, unlike steroidogenic factor-1 (Sf1) (Nr5a1, Ad4BP) haploinsufficiency (36, 55), has no effect on the growth of normal adrenocortical cells.
Conditional deletion of Gata4 in nascent adrenocortical neoplasms of ovariectomized B6.129 mice impairs sex steroidogenesis in a Gata4 dose-dependent manner
The Cre-loxP recombination system was used to conditionally ablate the Gata4 gene in nascent adrenocortical tumor cells of ovariectomized B6.129 mice. Like other mouse strains, the mixed B6.129 strain develops postgonadectomy adrenocortical tumors composed of type A and type B cells (Fig. 6A), although the postovariectomy adrenocortical tumors are smaller than those found in comparably aged B6D2F1or B6AF1 mice. To generate conditional knockout mice, we crossed mice harboring a floxed allele of (Gata4F) with mice bearing the Amhr2-cre knock-in allele. Cre-mediated recombination deletes exons 3–5 of Gata4, resulting in a null allele (28, 31). The Amhr2-cre transgene has been used extensively to target gene deletion in gonadal somatic cells (32, 33, 56–61). Gata4 conditional knockout mice generated using Amhr2-cre have reduced fertility due to defects in gonadal somatic cell function (20, 21). By analogy, we hypothesized that recombination of Gata4F by Amhr2-cre would impair the development of gonadal-like tumors in the adrenal glands of gonadectomized mice in a Gata4F dose-dependent manner.
Fig. 6.
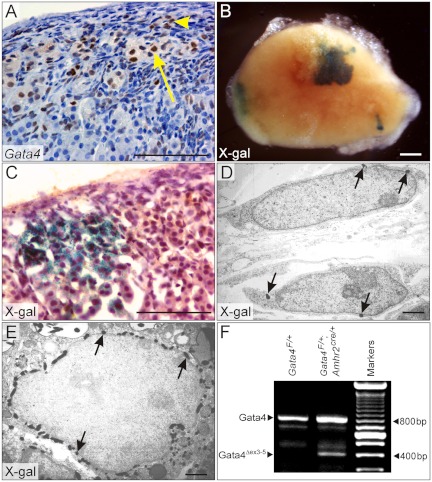
Ovariectomized B6.129 mice develop adrenocortical neoplasms that express GATA4 and Amhr2-cre. B6.129 mice were ovariectomized, and adrenal glands were harvested 5 months later. Immunohistochemical staining of adrenal tissue sections (A) showed nuclear GATA4 antigen in type A (yellow arrowhead) and type B (yellow arrow) cells. B–E, Mice harboring Amhr2-cre and R26R transgenes were ovariectomized, and after 5 months adrenal glands were subjected to whole-mount X-gal staining (B) to highlight cells expressing Cre. Sections of X-gal-stained adrenal tissue were processed for light (C) or transmission electron (D and E) microscopy. Note the presence of subcapsular neoplastic cells expressing Cre (C). Electron-dense crystalloids (black arrows) indicative of the X-gal reaction product were seen in both small, spindle-shaped, type A cells (D) and large, mitochondria-rich, type B cells (E). Weanling mice of the specified genotypes were ovariectomized, and adrenal RNA was isolated 3 months later. The RNA was subjected to qualitative RT-PCR analysis with primers that distinguish transcripts derived from the intact Gata4F allele vs. the recombined allele lacking exons 3 to 5 (Gata4Δex3–5) (F). Note that a transcript derived from the recombined allele is present in the adrenal glands of Gata4F/+;Amhr2Cre/+ mice. Bars, 100 μm (A–C), 2 μm (D and E).
A ROSA26 flox-stop-flox lacZ reporter (R26R), which indelibly expresses β-galactosidase in response to Cre recombinase (34), was used to assess the pattern of expression of the Amhr2-cre transgene within the adrenal glands of ovariectomized mice. Whole-mount X-gal staining of adrenal glands from ovariectomized R26R; Amhr2cre/+ mice demonstrated β-galactosidase activity in discrete patches of cells near the surface of the gland (Fig. 6B). Histological analysis of these adrenal glands showed X-gal staining in subcapsular neoplasms but not in adjacent normal adrenocortical cells (Fig. 6C). Transmission electron microscopy demonstrated the presence of electron-dense crystalloids indicative of the X-gal reaction product in both type A (Fig. 6D) and type B cells (Fig. 6E). Consistent with prior ultrastructural studies (62), type A cells contained abundant rough endoplasmic reticulum but few mitochondria and no lipid droplets (Fig. 6D), whereas type B cells contained prominent smooth endoplasmic reticulum, lipid droplets, and mitochondria (Fig. 6E). Control whole-mount X-gal stains of adrenal glands from nonovariectomized R26R; Amhr2cre/+ mice showed little or no staining (data not shown). These R26R lineage tracing experiments establish that the Amhr2-cre transgene is induced after ovariectomy and is expressed in type A and type B cells or their progenitor(s).
To verify Amhr2-cre-mediated deletion of the Gata4F allele in the adrenal gland of gonadectomized mice, we employed a qualitative RT-PCR assay that distinguishes transcripts derived from the intact and recombined alleles (20). RT-PCR analysis of adrenal glands from ovariectomized Gata4F/+ mice yielded a 782-bp fragment reflecting transcription of the intact allele (Fig. 6F). In contrast, the adrenal glands of ovariectomized Gata4F/+;Amhr2cre/+ mice contained both the 782-bp fragment and a 401-bp fragment derived from the recombined allele lacking exons 3–5 (Gata4Δex3–5).
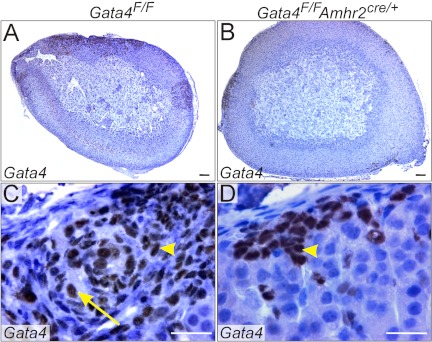
Adrenal glands from ovariectomized Gata4F/F mice contained tumors that invaded deeply into the cortex, as highlighted by GATA4 immunohistochemistry (Fig. 7, A and C). Both type A (Fig. 7C, arrowhead) and type B (Fig. 7C, arrow) cells were evident in the tumors. In contrast, adrenal glands from Gata4F/F;Amhr2cre/+ (conditional knockout) mice contained only small neoplasms composed exclusively of type A cells that did not invade deeply into the cortex (Fig. 7, B and D). We conclude that up-regulation of Amhr2-cre in nascent adrenocortical tumors reduces but does not entirely block the accumulation of GATA4-positive neoplastic cells. To confirm reduced expression of Gata4 in the adrenal glands of gonadectomized conditional knockout mice, we performed qRT-PCR using primers specific for the WT GATA4 mRNA. The ratio of GATA4 mRNA levels in ovariectomized Gata4F/F;Amhr2cre/+ vs. Gata4F/F mice was 0.20 ± 0.02 (P < 0.05, normalized to β-actin mRNA levels).
Fig. 7.
Microscopic appearance of adrenal glands from ovariectomized Gata4F/F and Gata4F/F;Amhr2cre/+ B6.129 mice. Weanling Gata4F/F (A and C) or Gata4F/F;Amhr2cre/+ (B and D) B6.129 mice were ovariectomized, and adrenal glands were harvested 3 months later. Tissue sections were stained with GATA4 antibody. Shown are low- (A and B) and high-magnification (C and D) views. Tumors composed of type A cells (yellow arrowheads) and type B cells (yellow arrows) invade deeply into the adrenal cortex of ovariectomized Gata4F/F mice. Note the absence of type B cells in the Gata4F/F;Amhr2cre/+ adrenal gland. Bars, 200 μm (A and B); 25 μm (C and D).
Previous studies have documented leaky expression of Amhr2-cre in the pituitary gland (63). To assess pituitary gonadotrope function in the conditional knockout animals, we measured basal and postovariectomy serum gonadotropin levels in mice of the following four genotypes: 1) Gata4F/+, 2) Gata4F/+;Amhr2cre/+, 3) Gata4F/F, and 4) Gata4F/F;Amhr2cre/+ (Supplemental Fig. 5, A and B). There were no significant differences in gonadotropin secretion among the four genotypes, so the reduction in tumor formation in the conditional knockout mice cannot be attributed to altered pituitary function.
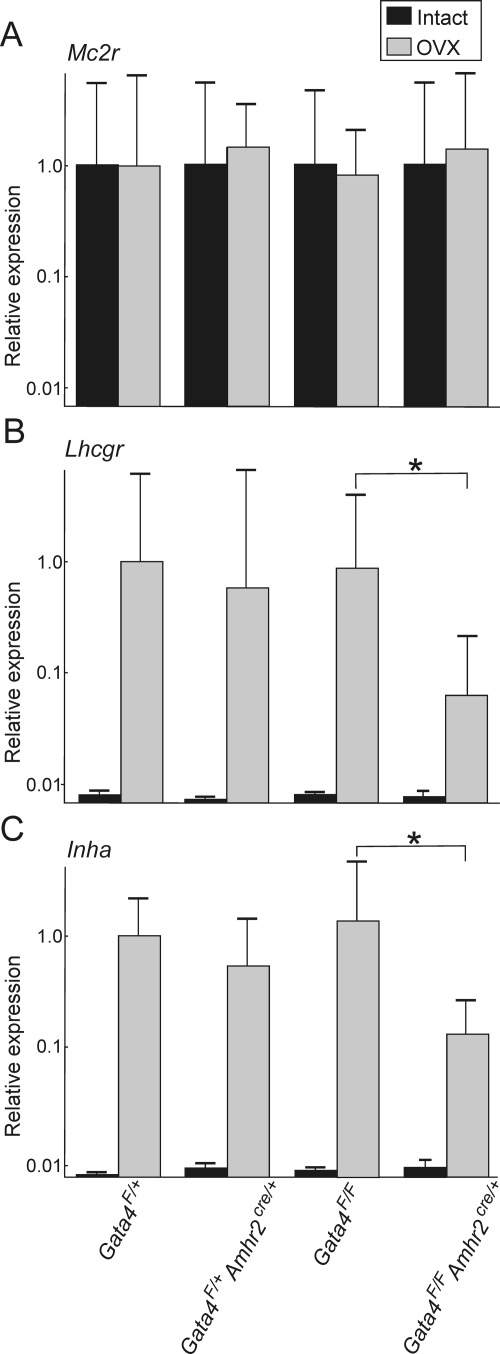
qRT-PCR was used to assess ovariectomy-induced changes in expression of steroidogenic markers in the adrenal glands of conditional knockout and control mice (Fig. 8). Results were normalized to β-actin; the outcome was similar when results were normalized to L19 or GAPDH. In general, ovariectomy-induced changes in gene expression were more variable in B6.129 (mixed genetic background) mice (Fig. 8) than in B6D2F1 or B6AF1 mice (Fig. 3). As expected, expression of the prototypical adrenocortical marker, melanocortin 2 receptor, was similar in conditional knockout and control mice, whether the mice were ovariectomized or intact (Fig. 8A). In contrast, expression of the gonadal markers Lhcgr (Fig. 8B) and inhibin-α (Fig. 8C) was reduced in the ovariectomized Gata4F/F;Amhr2cre/+ mice vs. control Gata4F/F mice (P < 0.05). Similarly, expression of Cyp17 was decreased in the ovariectomized Gata4F/F;Amhr2cre/+ mice, although this did not reach statistical significance (P = 0.08; data not shown). We conclude that Amhr2-cre-mediated deletion of Gata4F in nascent adrenocortical neoplasms of ovariectomized mice reduces expression of gonadal-like markers in a Gata4F dose-dependent manner.
Fig. 8.
Expression of steroidogenic differentiation markers in adrenal glands from ovariectomized Gata4 conditional knockout mice. Weanling mice of the specified genotypes were subjected to ovariectomy (OVX, gray bars) or left intact (black bars). Adrenal glands were harvested 3 months later and subjected to qRT-PCR analysis for Mc2r (A), Lhcgr (B), or Inha (C). The graphs show the relative expression (mean ± sd) of different transcripts in ovariectomized (n = 4–8) vs. intact mice (n = 7–14) of the indicated genotypes. Results were normalized to expression of β-actin. The y-axis is plotted on a logarithmic scale. For each marker, the average expression in ovariectomized Gata4F/+ mice was arbitrarily set at 1. Note that Lhcgr and Inha transcripts are less abundant in the adrenals of ovariectomized Gata4F/F;Amhr2cre/+ mice than Gata4F/F mice (*, P < 0.05).
Discussion
Gonadectomy-induced adrenocortical neoplasia has been attributed to continuous gonadotropin secretion by the pituitary gland (7). Adrenal transplantation and parabiosis experiments have established that the adrenal glands of susceptible mouse strains exhibit an inherent predisposition to sex steroidogenic tumor formation in response to persistently elevated serum gonadotropin levels (7, 9–11, 64). However, the molecular mechanisms underlying adrenal gland susceptibility have remained elusive. Our findings offer genetic proof that GATA4 is a key player insofar as loss-of-function mutations in Gata4 impair ovariectomy-induced adrenocortical neoplasia in multiple susceptible strain backgrounds (B6D2F1, B6AF1, and B6.129). The consistent GATA4 deficiency phenotype in germline haploinsufficient mice bearing a deletion in exon 2 and in conditional knockout mice with an acquired deletion in exons 3–5 of suggests that: 1) Gata4 is a dose-dependent modifier of postgonadectomy adrenocortical neoplasia, and 2) the Gata4 effect is not the result of a particularly engineered allele but rather a general property of loss-of-function mutations. We conclude that the hormonal changes that accompany gonadectomy cannot drive adrenocortical tumorigenesis in susceptible strains of mice without the proper dosage of Gata4.
The present results offer insights into the mechanism by which GATA4 deficiency attenuates postgonadectomy adrenocortical neoplasia. In theory, GATA4 could promote tumor initiation, sex steroidogenic differentiation, or tumor cell growth/survival. The conditional knockout model, in which recombination of the Gata4F gene occurs after up-regulation of Amhr2-cre in nascent tumors, dissociates the effects of GATA4 on tumor initiation from its effects on the subsequent stages of tumor development (gonadal-like differentiation and neoplastic cell proliferation/survival). That features of the haploinsufficiency phenotype are conserved in the conditional knockout model suggests that GATA4 deficiency has direct effects on sex steroidogenic differentiation or tumor proliferation/survival. In preliminary experiments, we found no evidence for decreased tumor proliferation or increased tumor apoptosis in the adrenal glands of gonadectomized GATA4-deficient mice (data not shown). These findings, together with the paucity of type B cells in the germline haploinsufficiency and conditional knockout mouse models and the absence of extragonadal estrogen production in the haploinsufficiency model, lead us to surmise that GATA4 deficiency disrupts postgonadectomy tumorigenesis principally via inhibition of sex steroidogenic differentiation in the neoplastic cells.
During normal gonadal development, GATA4 promotes sex steroidogenic cell differentiation by repression of genes that maintain stem/progenitor cells in an undifferentiated state, such as genes in the Wnt/β-catenin-signaling pathway (16, 65) and by subsequent activation of genes involved in sex steroid biosynthesis (e.g. Lhcgr, Cyp17, Cyp19) (14, 21, 66–68). Mice homozygous for the Gata4ki allele, a mutation that blocks the interaction between GATA4 and its cofactor FOG2, fail to properly repress Dkk1, a secreted inhibitor of the canonical Wnt signaling pathway, leading to impaired ovarian development (16, 65). Activation of β-catenin is common in human adrenocortical tumors (69), and constitutive activation of β-catenin in the adrenal cortex of transgenic mice promotes adrenocortical neoplasia (70). It is unclear whether disruption of the Wnt/β-catenin signaling pathway accounts for the lack of adrenocortical tumor formation in gonadectomized Gata4 haploinsufficient or conditional knockout mice. In preliminary studies we did not detect significant differences in expression of Dkk1 or other components of this signaling pathway (data not shown). Identification of the GATA4 target genes essential for postgonadectomy adrenocortical neoplasia awaits further study.
Like Gata4, the Sf1 (Nr5a1, Ad4BP) gene has been shown to be a dosage-sensitive modifier of steroidogenic cell differentiation and adrenocortical tumorigenesis. Sf1 null mouse embryos exhibit aberrant adrenal and gonadal development (71), and haploinsufficiency for this factor disrupts the organization, growth, and function of the adrenal gland (36, 55). Transgenic expression of SF1 in fetal adrenal progenitor cells of mice leads to ectopic adrenal formation (72), and increased Sf1 dosage in mice leads to subcapsular adrenocortical tumors that express GATA4 and other gonadal markers (73, 74). GATA4 and SF1 have been shown to cooperate in the expression of gonadal genes (14, 75), implying that synergistic interactions between these two transcription factors might contribute to gonadectomy-induced adrenocortical neoplasia. Arguing against a possible genetic interaction, combined haploinsufficiency of Sf1 and Gata4 does not cause abnormalities in mouse gonadal development beyond that of Sf1 haploinsufficiency alone (76).
Linkage analysis of crosses between susceptible DBA/2J and nonsusceptible C57Bl/6 mice has shown that postgonadectomy tumorigenesis is a complex trait and that a major locus for tumorigenesis resides on chromosome 8 (11). One of the candidate genes in the linkage region is secreted frizzled related protein 1 (Sfrp1), a tumor suppressor that inhibits Wnt/β-catenin signaling. Although Sfrp1 is an attractive candidate gene for tumorigenesis, no causal mutations have been identified in the coding or noncoding regions of the gene in DBA/2J mice (11). This same genome-wide scan found no evidence for linkage to Gata4 (chromosome 14) or Sf1 (chromosome 2), implying that any polymorphisms between DBA/2J and C57Bl/6 in the Gata4 and Sf1 genes are not functionally significant. Our haploinsufficiency and conditional knockout models provide a complementary candidate gene approach to genetic linkage analyses.
Our findings have implications for gonadotropin-dependent adrenocortical tumors in other species. The phenomenon of postgonadectomy adrenocortical neoplasia is not limited to mice; subcapsular sex steroidogenic tumors have been reported in the adrenal glands of gonadectomized ferrets, hamsters, cats, and goats (69, 77). GATA4, Lhcgr, inhibin-α, and other gonadal-like markers are hallmarks of postgonadectomy adrenocortical tumors in ferrets (7, 64, 78, 79). The human adrenal cortex constitutively expresses low levels of Lhcgr, and this receptor has been shown to be functionally active in the adrenal during pregnancy and other high gonadotropin states (9). Therefore, it has been proposed that adrenal responsiveness to LH, influenced by modifier genes, may contribute to adrenocortical tumorigenesis in humans (6, 9). Although rare, adrenocortical neoplasms with histological features resembling luteinized ovarian stroma (thecal metaplasia) have been reported in postmenopausal women (80, 81) and men with acquired testicular atrophy (82).
In addition to triggering adrenocortical tumorigenesis, ovariectomy elicits obesity in mice. Consequently, ovariectomized mice have been used to model the metabolic consequences of human menopause (53). In the mouse, postovariectomy weight gain is strain dependent (B6D2F1 > DBA/2J > C57Bl/6) (11), and ovariectomy-induced gains in visceral adiposity can be offset by estrogen repletion (53). Data presented here indicate that Gata4 is a genetic modifier of not only adrenocortical tumorigenesis but also postovariectomy weight gain in B6D2F1 mice. We propose that Gata4 haploinsufficiency exacerbates postovariectomy obesity in aged B6D2F1 mice by limiting adrenocortical estrogen production. Changes in adrenal glucocorticoid production can also impact obesity in mice, but studies suggest that postovariectomy adrenocortical neoplasia is not associated with alterations in the ACTH/glucocorticoid axis (7, 9, 10).
Although structurally and functionally distinct, the major steroid-producing organs, i.e. the adrenal cortex and gonads, arise from a common pool of progenitors in the adrenogonadal primordium (7, 9, 83–85). Despite extensive investigation, the factors that determine whether a steroidogenic cell precursor adopts an adrenocortical or gonadal phenotype are not fully understood. The phenotypic switch from production of corticoids to sex steroids makes postgonadectomy adrenocortical neoplasia an attractive model for the study of not only tumorigenesis but also steroidogenic lineage specification. Our results support the premise that GATA4 is a key regulator of a sex steroidogenic cell specification. We propose that GATA4, acting in concert with gonadotropins and other hormones, determines the functional identity of sex steroidogenic cells.
Supplementary Material
Acknowledgments
We thank William Pu (Children's Hospital, Boston) for providing mice and Simone Wagner (University of Applied Sciences, Mannheim) for performing pilot experiments.
This work was supported by National Institutes of Health grants DK075618 and DK52574 (to D.B.W.), and grants from the Academy of Finland (to M.H.) and the Sigrid Juselius Foundation (to M.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine
- Cyp17
- cytochrome P450 17α-hydroxylase/C17-C20 lyase
- Cyp19
- cytochrome P450 aromatase
- E1
- estrone
- E2
- estradiol
- ERα
- estrogen receptor α
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- H&E
- hematoxylin and eosin
- Lhcgr
- luteinizing hormone chorionic gonadotropin receptor
- qRT-PCR
- quantitative RT-PCR
- SF1
- steroidogenic factor-1
- WT
- wild type.
References
- 1. Thompson GB, Young WF., Jr 2003. Adrenal incidentaloma. Curr Opin Oncol 15:84–90 [DOI] [PubMed] [Google Scholar]
- 2. Bertherat J, Mosnier-Pudar H, Bertagna X. 2002. Adrenal incidentalomas. Curr Opin Oncol 14:58–63 [DOI] [PubMed] [Google Scholar]
- 3. Barlaskar FM, Hammer GD. 2007. The molecular genetics of adrenocortical carcinoma. Rev Endocr Metab Disord 8:343–348 [DOI] [PubMed] [Google Scholar]
- 4. Volante M, Buttigliero C, Greco E, Berruti A, Papotti M. 2008. Pathological and molecular features of adrenocortical carcinoma: an update. J Clin Pathol 61:787–793 [DOI] [PubMed] [Google Scholar]
- 5. Cavlan D, Bharwani N, Grossman A. 2010. Androgen- and estrogen-secreting adrenal cancers. Semin Oncol 37:638–648 [DOI] [PubMed] [Google Scholar]
- 6. Beuschlein F, Galac S, Wilson DB. 2012. Animal models of adrenocortical tumorigenesis. Mol Cell Endocrinol 351:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. 2006. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. Vet Pathol 43:97–117 [DOI] [PubMed] [Google Scholar]
- 8. Johnsen IK, Slawik M, Shapiro I, Hartmann MF, Wudy SA, Looyenga BD, Hammer GD, Reincke M, Beuschlein F. 2006. Gonadectomy in mice of the inbred strain CE/J induces proliferation of sub-capsular adrenal cells expressing gonadal marker genes. J Endocrinol 190:47–57 [DOI] [PubMed] [Google Scholar]
- 9. Bernichtein S, Alevizaki M, Huhtaniemi I. 2008. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol Metab 19:231–238 [DOI] [PubMed] [Google Scholar]
- 10. Bernichtein S, Peltoketo H, Huhtaniemi I. 2009. Adrenal hyperplasia and tumours in mice in connection with aberrant pituitary-gonadal function. Mol Cell Endocrinol 300:164–168 [DOI] [PubMed] [Google Scholar]
- 11. Bernichtein S, Petretto E, Jamieson S, Goel A, Aitman TJ, Mangion JM, Huhtaniemi IT. 2008. Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinology 149:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppäluoto J, Rahman N, Heikinheimo M, Wilson DB. 2005. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology 146:3975–3984 [DOI] [PubMed] [Google Scholar]
- 13. Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB. 2003. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology 144:4123–4133 [DOI] [PubMed] [Google Scholar]
- 14. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. 2008. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol 22:781–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. 2007. The regulation of Sox9 gene expression by the GATA4/FOG2 transcriptional complex in dominant XX sex reversal mouse models. Dev Biol 307:356–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manuylov NL, Smagulova FO, Leach L, Tevosian SG. 2008. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development 135:3731–3743 [DOI] [PubMed] [Google Scholar]
- 17. Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. 2002. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129:4627–4634 [DOI] [PubMed] [Google Scholar]
- 18. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. 2007. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn 236:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouma GJ, Washburn LL, Albrecht KH, Eicher EM. 2007. Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci USA 104:14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kyrönlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, Heikinheimo M, Wilson DB. 2011. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol 333:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kyrönlahti A, Vetter M, Euler R, Bielinska M, Jay PY, Anttonen M, Heikinheimo M, Wilson DB. 2011. GATA4 deficiency impairs ovarian function in adult mice. Biol Reprod 84:1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. 2011. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol 353:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parviainen H, Kiiveri S, Bielinska M, Rahman N, Huhtaniemi IT, Wilson DB, Heikinheimo M. 2007. GATA transcription factors in adrenal development and tumors. Mol Cell Endocrinol 265–266:17–22 [DOI] [PubMed] [Google Scholar]
- 24. Molkentin JD, Lin Q, Duncan SA, Olson EN. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev 11:1061–1072 [DOI] [PubMed] [Google Scholar]
- 25. Kuo CT, Morrisey EE, Anadappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. 1997. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev 11:1048–1060 [DOI] [PubMed] [Google Scholar]
- 26. Narita N, Bielinska M, Wilson D. 1997. Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development 122:3755–3764 [DOI] [PubMed] [Google Scholar]
- 27. Narita N, Bielinska M, Wilson DB. 1997. Wild type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol 189:270–274 [DOI] [PubMed] [Google Scholar]
- 28. Watt AJ, Battle MA, Li J, Duncan SA. 2004. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci USA 101:12573–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. 2004. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol 275:235–244 [DOI] [PubMed] [Google Scholar]
- 30. Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. 2005. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest 115:1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. 2006. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res 98:837–845 [DOI] [PubMed] [Google Scholar]
- 32. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. 2002. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- 33. Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. 2004. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18:1610–1619 [DOI] [PubMed] [Google Scholar]
- 34. Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- 35. Alexander BM, Van Kirk EA, Naughton LM, Murdoch WJ. 2007. Ovarian morphometrics in TP53-deficient mice. Anat Rec (Hoboken) 290:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD. 2002. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology 143:3122–3135 [DOI] [PubMed] [Google Scholar]
- 37. Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. 2003. FOG-2 and GATA-4 are coexpressed in the mouse ovary and can modulate Müllerian-inhibiting substance expression. Biol Reprod 68:1333–1340 [DOI] [PubMed] [Google Scholar]
- 38. Fahle M, Palm G. 1983. Calculation of surface areas from serial sections. J Neurosci Methods 9:75–85 [DOI] [PubMed] [Google Scholar]
- 39. Woolley GW. 1949. The adrenal cortex and its tumors. Ann NY Acad Sci 50:616–626 [DOI] [PubMed] [Google Scholar]
- 40. Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. 2007. Impaired mesenchymal cell funciton in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev Biol 301:602–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pangas SA, Jorgez CJ, Matzuk MM. 2004. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem 279:32281–32286 [DOI] [PubMed] [Google Scholar]
- 42. Ward JM, Anver MR, Mahler JF, Devor-Henneman DE, Maronpot RR, Sundberg JP, Frederickson RM. 2002. Pathology of mice commonly used in genetic engineering (C57BL/6; B6,129; and FVB/n). In: Pathology of genetically engineered mice. Ames, IA: Iowa State University Press; 161–179 [Google Scholar]
- 43. Frith CH, Dunn TB. 1994. Tumours of the adrenal gland. IARC Sci Publ 111:595–609 [PubMed] [Google Scholar]
- 44. Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. 2006. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol 20:1366–1377 [DOI] [PubMed] [Google Scholar]
- 45. Lo A, Zheng W, Gong Y, Crochet JR, Halvorson L. 2011. GATA transcription factors regulate luteinizing hormone β (LHβ) gene expression. J Mol Endocrinol 47:45–58 [DOI] [PubMed] [Google Scholar]
- 46. Kim JS, Kubota H, Kiuchi Y, Doi K, Saegusa J. 1997. Subcapsular cell hyperplasia and mast cell infiltration in the adrenal cortex of mice: comparative study in 7 inbred strains. Exp Anim 46:303–306 [DOI] [PubMed] [Google Scholar]
- 47. Teng CT. 2002. Lactoferrin gene expression and regulation: an overview. Biochem Cell Biol 80:7–16 [DOI] [PubMed] [Google Scholar]
- 48. Rossi JA, Bruschi LC. 1989. Alteration of the submandibular glands of female mice during pregnancy and lactation. A histological, histometric and histochemical study. Acta Morphol Hung 37:135–145 [PubMed] [Google Scholar]
- 49. Freeman ME, Kanyicska B, Lerant A, Nagy G. 2000. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- 50. Rulli SB, Kuorelahti A, Karaer O, Pelliniemi LJ, Poutanen M, Huhtaniemi I. 2002. Reproductive disturbances, pituitary lactotrope adenomas, and mammary gland tumors in transgenic female mice producing high levels of human chorionic gonadotropin. Endocrinology 143:4084–4095 [DOI] [PubMed] [Google Scholar]
- 51. Kero J, Poutanen M, Zhang FP, Rahman N, McNicol AM, Nilson JH, Keri RA, Huhtaniemi IT. 2000. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J Clin Invest 105:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kiss R, de Launoit Y, L'Hermite-Balériaux M, L'Hermite M, Paridaens RJ, Danguy AJ, Pasteels JL. 1987. Effect of prolactin and estradiol on cell proliferation in the uterus and the MXT mouse mammary neoplasm. J Natl Cancer Inst 78:993–998 [PubMed] [Google Scholar]
- 53. Rogers NH, Perfield JW, II, Strissel KJ, Obin MS, Greenberg AS. 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, Heikinheimo M. 2002. Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology 143:3136–3143 [DOI] [PubMed] [Google Scholar]
- 55. Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. 2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, Chiang HS, Yeh S, Chang C. 2007. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 32:96–106 [DOI] [PubMed] [Google Scholar]
- 57. Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. 2008. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D. 2008. Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in Sertoli cells. Biol Reprod 79:475–485 [DOI] [PubMed] [Google Scholar]
- 59. Archambeault DR, Yao HH. 2010. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci USA 107:10526–10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. 2010. Constitutive WNT/β-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod 82:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ren Y, Cowan RG, Harman RM, Quirk SM. 2009. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol 23:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharawy MM, Liebelt AG, Dirksen TR, Penney DP. 1980. Fine structural study of postcastrational adrenocortical carcinomas in female CE-mice. Anat Rec 198:125–133 [DOI] [PubMed] [Google Scholar]
- 63. Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH. 2009. Conditional deletion of β-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod 80:1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schoemaker NJ, Teerds KJ, Mol JA, Lumeij JT, Thijssen JH, Rijnberk A. 2002. The role of luteinizing hormone in the pathogenesis of hyperadrenocorticism in neutered ferrets. Mol Cell Endocrinol 197:117–125 [DOI] [PubMed] [Google Scholar]
- 65. Tevosian SG, Manuylov NL. 2008. To β or not to β: Canonical β-catenin signaling pathway and ovarian development. Dev Dyn 237:3672–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. LaVoie HA, Singh D, Hui YY. 2004. Concerted regulation of the porcine steroidogenic acute regulatory protein gene promoter activity by follicle-stimulating hormone and insulin-like growth factor I in granulosa cells involves GATA-4 and CCAAT/enhancer binding protein β. Endocrinology 145:3122–3134 [DOI] [PubMed] [Google Scholar]
- 67. Kwintkiewicz J, Cai Z, Stocco C. 2007. Follicle-stimulating hormone-induced activation of Gata4 contributes in the up-regulation of Cyp19 expression in rat granulosa cells. Mol Endocrinol 21:933–947 [DOI] [PubMed] [Google Scholar]
- 68. Rahman NA, Kiiveri S, Rivero-Müller A, Levallet J, Vierre S, Kero J, Wilson DB, Heikinheimo M, Huhtaniemi I. 2004. Adrenocortical tumorigenesis in transgenic mice expressing the inhibin α-subunit promoter/SV40 virus T-antigen transgene: relationship between ectopic expression of luteinizing hormone receptor and transcription factor GATA-4. Mol Endocrinol 18:2553–2569 [DOI] [PubMed] [Google Scholar]
- 69. Bielinska M, Parviainen H, Kiiveri S, Heikinheimo M, Wilson DB. 2009. Origin and molecular pathology of adrenocortical neoplasms. Vet Pathol 46:194–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrançois-Martinez AM, Martinez A, Val P. 2010. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19:1561–1576 [DOI] [PubMed] [Google Scholar]
- 71. Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. 2004. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol 215:89–94 [DOI] [PubMed] [Google Scholar]
- 72. Zubair M, Oka S, Parker KL, Morohashi K. 2009. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol 23:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Doghman M, Cazareth J, Lalli E. 2008. The Tcf/β-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab 93:3222–3225 [DOI] [PubMed] [Google Scholar]
- 74. Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E. 2007. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- 75. Tremblay JJ, Viger RS. 2003. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J Biol Chem 278:42637–42642 [DOI] [PubMed] [Google Scholar]
- 76. Pelusi C, Zhao L, Stallings NR, Parker KL. 2007. Combined haploinsufficiency of SF-1 and GATA4 does not reveal a genetic interaction in mouse gonadal development. Sex Dev 1:152–160 [DOI] [PubMed] [Google Scholar]
- 77. Meler EN, Scott-Moncrieff JC, Peter AT, Bennett S, Ramos-Vara J, Salisbury SK, Naughton JF. 2011. Cyclic estrous-like behavior in a spayed cat associated with excessive sex-hormone production by an adrenocortical carcinoma. J Feline Med Surg 13:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wagner S, Kiupel M, Peterson RA, II, Heikinheimo M, Wilson DB. 2008. Cytochrome b5 expression in gonadectomy-induced adrenocortical neoplasms of the domestic ferret (Mustela putorius furo). Vet Pathol 45:439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peterson RA, II, Kiupel M, Bielinska M, Kiiveri S, Heikinheimo M, Capen CC, Wilson DB. 2004. Transcription factor GATA-4 is a marker of anaplasia in adrenocortical neoplasms of the domestic ferret (Mustela putorius furo). Vet Pathol 41:446–449 [DOI] [PubMed] [Google Scholar]
- 80. Fidler WJ. 1977. Ovarian thecal metaplasia in adrenal glands. Am J Clin Pathol 67:318–323 [DOI] [PubMed] [Google Scholar]
- 81. Wont TW, Warner NE. 1971. Ovarian thecal metaplasia in the adrenal gland. Arch Pathol 92:319–328 [PubMed] [Google Scholar]
- 82. Romberger CF, Wong TW. 1989. Thecal metaplasia in the adrenal gland of a man with acquired bilateral testicular atrophy. Arch Pathol Lab Med 113:1071–1075 [PubMed] [Google Scholar]
- 83. Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. 2009. In search of adrenocortical stem and progenitor cells. Endocr Rev 30:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zubair M, Parker KL, Morohashi K. 2008. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Val P, Jeays-Ward K, Swain A. 2006. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol 299:250–256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.