Abstract
G protein signaling through human somatostatin receptor subtype 2 (SSTR2) is well known, but the amino acids involved in stimulation of intracellular responses upon ligand binding have not been characterized. We constructed a series of point mutants in SSTR2 at amino acid positions 89, 139, and 140 in attempts to disrupt G protein signaling upon ligand binding. The aspartic acid changes at position 89 to either Ala, Leu, or Arg generated mutant receptors with varying expression profiles and a complete inability to bind somatostatin-14 (SST). Mutations to Asp 139 and Arg 140 also led to varying expression profiles with some mutants maintaining their affinity for SST. Mutation of Arg 140 to Ala resulted in a mutated receptor that had a Bmax and dissociation constant (Kd) similar to wild-type receptor but was still coupled to the G protein as determined in both a cAMP assay and a calcium-release assay. In contrast, mutation of Asp 139 to Asn resulted in a mutated receptor with Bmax and Kd values that were similar to wild type but was uncoupled from G protein-mediated cAMP signaling, but not calcium release. Thus, we identified mutations in SSTR2 that result in either receptor expression levels that are similar to wild type but is completely ablated for ligand binding or a receptor that maintains affinity for SST and is uncoupled from G protein-mediated cAMP signaling.
The human somatostatin receptors (SSTR) are members of the G protein-coupled receptor family and are responsible for antiproliferation of cells expressing the receptor(s) when bound by the agonist somatostatin-14 (SST). Somatostatin is a GH release inhibiting factor that was originally isolated from extracts of ovine hypothalamus (1). There are five subtypes of SSTR (SSTR1–5), each having a different affinity for the natural ligand SST. They are widely expressed in a variety of tissues including the brain, kidneys, thyroid, pancreas, gut, pituitary, adrenals, and in immune cells (2–6). Additionally, SSTR have been found to be expressed in a number of human tumor types including breast cancer, lung carcinomas, pancreatic lesions, neuroendocrine tumors, thyroid carcinomas, leukemia, lymphomas, and myelomas (2–5, 7, 8). Due to the wide expression of the somatostatin receptors, they have been targeted as a means of therapy for a variety of conditions, such as cancer, acromegaly, and Alzheimer's (9, 10). Further, the confirmation that SSTR subtype 2 (SSTR2) is predominantly expressed in human tumors has led to both the clinical implementation of SST-analog therapy (10–14) as well as the ability to image SSTR2-positive tumors using radiolabeled somatostatin analogs (15–17).
In this regard, our laboratory has used SSTR2 as a means of imaging gene transfer in several human tumor types (18–20). However, an ideal reporter gene for the molecular imaging of gene transfer should have only a minimal effect on cellular signaling or more ideally, no effect at all. Binding of somatostatin or an analog to SSTR2 (or the other SSTR) initiates a cascade of intracellular pathways leading to various effects on the cells, and the majority of these pathways are mediated either by inhibitory G protein coupling to adenylate cyclase, modulation of calcium and potassium channels, or the activation of phosphotyrosine phosphatases (PTP) or MAPK (10). The downstream results of these pathways include growth cessation, hormone secretion and/or apoptosis, with the induction and mediation of apoptosis varying from receptor to receptor (21). Both the inhibition of cAMP production via adenylate cyclase and the phosphorylation of proteins leading to apoptosis in SSTR2-expressing cells are due to the coupling of either G protein or PTP [short heterodimer partner (SHP)-1, SHP-2] to the receptor (22, 23).
Even though there is considerable information on the signaling cascades of the SSTR, the amino acids involved in the interactions of the receptors with either G protein or SHP-1 are not well understood, especially in the case of SSTR2. Studies in mouse and rat SSTR2 have shown that alterations to amino acids within transmembrane region 2 can affect SST-mediated signaling or binding, respectively (24, 25). It has also been shown that the C-terminal tail of SSTR2 is involved with G protein signaling through adenylate cyclase (26), whereas others have shown that truncations of the C-terminal tail cause constitutive activation of the receptor (27) or a receptor that is more effectively coupled to adenylate cyclase (28). It is clear from various results that the signal transduction pathways of SSTR2 are complex and warrant further investigation at the level of potential amino acid residues involved in signaling and/or binding of ligand.
In this study, we systematically changed residues within human SSTR2 using site-directed mutagenesis to determine the effects of these changes on cell surface expression, ligand binding, and G protein signaling of the receptor. We describe the effects of changing the charge of both transmembrane and intracellular loop amino acids on the function of SSTR2 using a variety of assays and describe how these residues are critical for normal function of SSTR2.
Materials and Methods
Cell lines
The human non-small-cell lung A-427 cell line was obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in DMEM (Invitrogen, Carlsbad, CA) plus 10% (vol/vol) of heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, MO). The cells were cultured in a humidified incubator at 37 C with 5% CO2.
Construction of mutant receptors
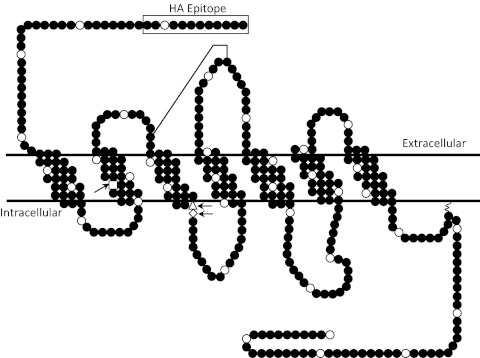
For the construction of the SSTR2 point mutants, the wild-type receptor sequence, with the addition of a hemagglutinin (HA) epitope sequence on the 5′-end, was first amplified from pα+HASSTR2 (29) using primers containing either a BglII (5′) or a HindIII (3′) restriction site. The resulting product was then restriction digested, purified, and ligated into pShuttle-CMV (Stratagene, La Jolla, CA) to create pS-HASSTR2. The insert was sequenced to verify the absence of mutations. Plasmid pS-HASSTR2 was then used as the template for the construction of the point mutants, and this was performed using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The locations of the point mutations in the protein sequence are shown in Fig. 1. The mutations, with respective sequence modifications, were chosen based on the human codon bias. The mutations were verified by sequencing of the insert via the Protein and Nucleic Acids Core Laboratory at Washington University School of Medicine.
Fig. 1.
Schematic representation of the human SSTR2. The white circles indicate every tenth amino acid. The point mutations are indicated by the arrows and are D89 (□), D139 (Δ), and R140 (♢). A disulfide bridge is shown by the line connecting two cysteines from extracellular loop 1 to extracellular loop 2.
mRNA Analysis
A-427 cells were transiently transfected with either wild-type receptor or one of the mutant receptors using Lipofectamine LTX (Invitrogen) according to the manufacturer's instructions. Cells transfected with an empty pShuttle-CMV vector were used as a negative control for sst2 expression. Total RNA from the cells was extracted 24 h after transfection using the RNEasy Extraction Kit (QIAGEN, Valencia, CA) as directed. Semiquantitative measurement of the amount of both gapd and sst2 mRNA was performed using the QIAGEN OneStep RT-PCR Kit. Briefly, 500 ng of total RNA for each sample were used as template for the reverse transcriptase reaction, after which 0.3 μm primers for either gapd or sst2 were included for the PCR amplification of the desired product, with gapd being 132 bp and sst2 being 103 bp.
ELISA
To determine the cell surface expression of the SSTR2 mutant receptors, an ELISA was performed as described by Kundra et al. (30) with some modifications. A-427 cells were plated in triplicate in a 96-well plate at 2 × 104 cells per well. The following day, the cells were transiently transfected with either wild-type receptor or one of the mutant receptors. At 24 h after transfection, the cells were washed, fixed, blocked with 5% nonfat milk, and incubated with 50 mU/ml of horseradish peroxidase-rat-anti-HA antibody clone 3F10 (Roche, Indianapolis, IN). Upon addition of Single Component TMB Peroxidase Enzyme Immunoassay Substrate (Bio-Rad Laboratories, Inc., Hercules, CA), color was developed for 30 min, upon which the peroxidase reaction was stopped with 0.18 m H2SO4, and the resulting absorbance was read at 450 nm. The data are plotted as the percent HA expression compared with the wild-type receptor.
Immunofluorescence of SSTR2
A-427 cells were either mock transfected or were transfected with wild-type receptor or one of the mutants. The day after transfection, the cells were seeded into eight-chamber culture slides at 5000 cells per chamber and were incubated at 37 C overnight in culture medium. The cells were then washed with PBS and fixed in methanol for 5 min. The fixed cells were washed and incubated for 1 h at room temperature in 1% BSA, 10% normal goat serum, 0.3 m glycine, and 0.1% Tween in PBS to permeabilize the cells and block nonspecific protein-protein interactions. SSTR2 was detected using a mouse antihuman monoclonal SSTR2 antibody (R&D Systems, Minneapolis, MN) at 5 μg/ml, and the endoplasmic reticulum (ER) was detected with a rabbit antihuman polyclonal antibody to GRP78 (Abcam, Cambridge, MA) at 1 μg/ml. The antibodies were diluted in PBS plus 2% BSA and were incubated overnight at 4 C. The following day, the cells were washed and were incubated with secondary antibodies diluted 1:200 in PBS plus 2% BSA. The secondary for SSTR2 was conjugated with fluorochrome NL557 and fluoresces red, and the secondary for GRP78 was fluorescein isothiocyanate conjugated. The cells were counterstained with 4′,6-diamidino-2-phenylindole to visualize the nucleus. Cell images were acquired using a Zeiss Axioplan 2 (Carl Zeiss, Inc., Thornwood, NY).
Radioligand-binding assays
A-427 cells were transiently transfected as before, and 24 h after transfection, the cells were harvested for membrane preparations as previously described (31). Protein concentrations were determined using the Pierce Non-Reducing Agent Compatible Kit. Briefly, 25 μg of membrane protein in a volume of 100 μl of binding buffer [50 mm Tris-Cl, (pH 7.4), 5 mm MgCl2, 0.1% (wt/vol) BSA, 0.5 mg/ml aprotinin, 200 mg/ml bacitracin, 10 mg/ml leupeptin, 10 mg/ml pepstatin] were applied to 0.1% polyethyleneimine-pretreated wells of a 96-well Multiscreen Durapore filtration plate (Millipore Corp., Bedford, MA) via vacuum manifold aspiration. This was performed in triplicate for each sample. The wells were then washed three times with wash buffer (10 mm HEPES, 1 mm EDTA, 5 mm MgCl2, 0.1% BSA). After the addition of 25 μg of membrane protein to each well, various concentrations of Tyr11-SST (Bachem California, Inc., Torrance, CA) ranging from 0.01 to 55 nm were added in a volume of 10 μl to triplicate wells. To each well, approximately 0.04 nm of 125I-Tyr11-SST-14 (PerkinElmer, Boston, MA) in a volume of 100 μl of binding buffer was added. The plate was incubated at room temperature for 1 h, after which the wells were washed twice. The membranes were allowed to dry, removed, and placed in separate tubes for determination of bound radioactivity. The radioactivity was counted using a Packard II γ counter (PerkinElmer), and the data were plotted in GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA) for the generation of homologous competitive binding curves using the one-site homologous competition with depletion equation.
Intracellular cAMP assay
The intracellular levels of induced cAMP were measured using the cAMP Biotrak Enzyme Immunoassay System (GE Healthcare Bio-Sciences, Piscataway, NJ) nonacetylation procedure. Briefly, A-427 cells were transiently transfected with either wild-type, D139A, D139N, R140A, or R140L receptor. The next day, the cells were collected and reseeded into six wells of a 96-well plate at a concentration of 5 × 104 cells per well. On the following day, the media from each well was aspirated and replaced with 180 μl of DMEM containing either 10 μm forskolin or 10 μm forskolin + 5 nm SST. Isobutylmethylxanthine was added to each well at 1 mm to act as nonspecific inhibitor of cAMP phosphodiesterase. The cells were stimulated for 30 min, after which 20 μl of lysis reagent were added to each well with mechanical manipulation to facilitate cell lysis. An aliquot of each lysate was diluted 100-fold in sample buffer, and 100 μl of each diluted lysate were analyzed for cAMP content following the manufacturer's instructions. The data were plotted as the percent of intracellular cAMP produced upon forskolin-induced SST treatment normalized to the amount produced with no SST.
Calcium-release assay
Intracellular calcium efflux was measured in transfected cells using the Fluo-4 NW Calcium Assay Kit (Invitrogen) according to the manufacturer's instructions. Briefly, A-427 cells were transfected with either wild-type receptor, D89R, D139A, D139N, R140A, or R140L. On the following day, the cells were reseeded into poly-d-lysine-coated wells of a 96-well plate, at 25,000 cells per well. Untransfected cells served as a negative. The cells were incubated overnight in culture medium. On the day of the assay, the cells were washed with Assay buffer (1 × Hanks balanced salt solution, 20 mm HEPES), after which fluo-4 in Assay buffer plus 2.5 mm probenecid was added. The cells were incubated for 30 min at 37 C, followed by 30 min at room temperature. The cells were then stimulated with 5 nm SST, with water being used in a set of wells to determine the baseline, and the calcium release was immediately measured kinetically for 90 sec using a fluorescence plate reader (485 nm Excitation, 520 nm Emission). At the completion of the measurement, maximum fluorescence was measured after the addition of 25 μm ionomycin. The data are shown as the percentage of maximum fluorescence, with each data point consisting of triplicate measurements.
Statistical analysis
All data are presented as the mean ± sem, and Student's two-tailed t test was used to calculate significance at the 95% confidence level, with P ≤ 0.05 being considered significantly different.
Results
Transcript analysis
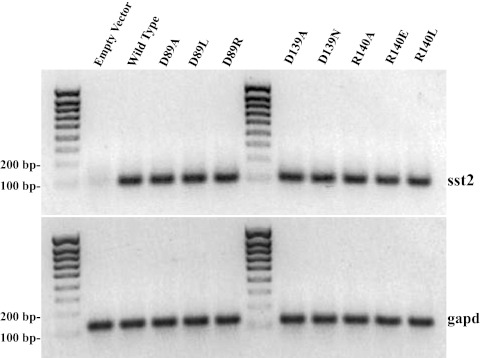
A-427 non-small-cell lung cancer cells were transiently transfected as described, and total RNA was extracted from the transfected cells and was analyzed for the mRNA levels for the receptors. Primers specific for sst2 were used to amplify a 103-bp fragment (Fig. 2A), and primers specific for gapd were used to amplify a 132-bp fragment (Fig. 2B). mRNA levels for the sst2 receptor sequences were similar in all the samples, and the cells transfected with empty vector showed essentially no amplification of sst2, indicating specificity of amplification in the samples that contained sst2. The levels in the control (gapd) were similar, showing that the amount of starting material was the same for all the transfectants.
Fig. 2.
mRNA analysis of A-427 cells transiently transfected with empty vector, wild-type SSTR2, or one of the mutated receptors. In panel A, the levels of sst2 transcript are shown, and in panel B, the levels of internal control gapd transcript are shown. The ladder increments are 100–800, and 1000 bases.
Whole-cell ELISA
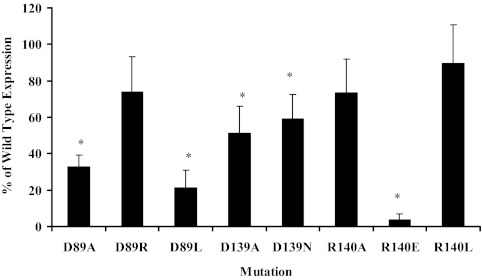
A-427 cells were again transiently transfected with either pS-HASSTR2 or one of the mutant receptor plasmids. The cells were then analyzed for the expression and localization of the receptor to the cell surface via an ELISA against the N-terminal HA epitope expressed in all of the receptors (Fig. 3). These results showed that the receptors were expressed at various levels. The expression of D89A, D89L, D139A, D139N, and R140E were significantly lower (P < 0.03) than the wild-type receptor. Only D89R, R140A, and R140L were expressed to levels not significantly different than the wild-type receptor at 74.0 ± 19.3%, 73.3 ± 18.4%, and 89.3 ± 21.4% of wild-type, respectively.
Fig. 3.
Anti-HA ELISA on A-427 cells transiently transfected with the mutated receptors. The data are shown as the percent expression compared with the wild-type receptor for each mutant. Each bar represents the mean ± sem of three to six experiments, with each experiment consisting of triplicate data points. *, Significant difference (P ≤ 0.024) compared with the wild type.
Immunofluorescent imaging of SSTR2
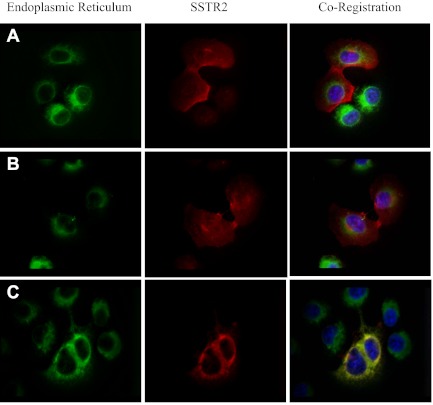
Based on the results of the membrane localization of the mutant receptors, transfected A-427 cells were used to determine the cellular localization of the wild-type, D89R, and R140E receptors. There is clear membrane localization with the wild-type receptor and with the D89R mutant receptor (Fig. 4, A and B, respectively). However, mutant receptor R140E colocalized with the ER, as shown in Fig. 4C. These findings are in agreement with the ELISA results, which showed significantly lower membrane localization for this receptor.
Fig. 4.
Immunofluorescent imaging of SSTR2 and the ER in A-427 cells transiently transfected with either wild-type SSTR2 (A), D89R (B), or R140E (C). The first column are ER images, with the second column being SSTR2 images, and the third column shows the overlay images.
Radioligand-binding analysis
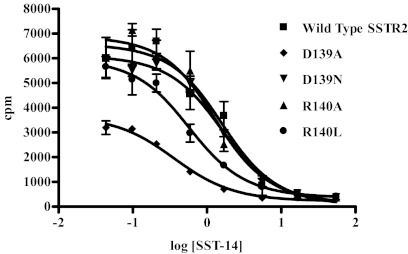
The mutants showing at least 50% expression compared with the wild type were analyzed in a homologous competitive binding assay to quantitate the number of active binding sites (Bmax) and affinity [dissociation constant (Kd)] for SST compared with the wild-type receptor (Table 1). Although the D89R mutant was expressed at the membrane to a level similar to wild type in the ELISA, its ability to bind ligand was completely ablated (data not shown). Both the D139A and R140L mutants showed significantly less (P ≤ 0.012) ligand-binding receptor than the wild-type, with 311 ± 29 and 957 ± 10 fmol/mg, respectively, compared with 3034 ± 295 fmol/mg for the wild type. However, both the D139N and R140A mutants had levels of ligand-binding receptor not significantly different than the wild type, at 1739 ± 440 and 2181 ± 110 fmol/mg, respectively. Figure 5 shows a representative binding curve for each receptor, with each point generated from triplicate measurements.
Table 1.
Bmax values ± sem and dissociation constants ± sem (n = 2–3) from competitive binding assays on transfected A-427 cells using 125I-Tyr11-SST-14
| Receptor | Bmax (fmol/mg) | Kd (nm) |
|---|---|---|
| Wild type | 3034 ± 295 | 0.59 ± 0.03 |
| D139A | 311 ± 29 | 0.28 ± 0.07 |
| D139N | 1739 ± 440 | 0.47 ± 0.01 |
| R140A | 2181 ± 110 | 0.45 ± 0.01 |
| R140 L | 957 ± 10 | 0.25 ± 0.00 |
Fig. 5.
Representative homologous competitive binding curves using 125I-Tyr11-SST-14 on A-427 cells transiently transfected with wild-type SSTR2, D139A, D139N, R140A, or R140L mutant receptor. The data represent the counts per minute (cpm) of radioligand bound in the presence of various concentrations of unlabeled SST for triplicate data points ± sem.
G protein signaling analysis via intracellular cAMP measurements and calcium release
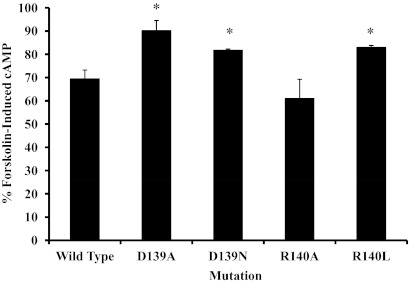
A-427 cells were transiently transfected with either wild-type, D139A, D139N, R140A, or R140L receptor and were analyzed via an intracellular cAMP assay (Fig. 6). Forskolin-induced cAMP production was inhibited by 30% after addition of SST in cells transfected with the wild-type receptor. The percent of forskolin-induced cAMP production was significantly greater (P < 0.05) in the D139A, D139N, and R140L mutants at 90.1 ± 4.4%, 81.7 ± 0.6%, and 83.0 ± 0.9%, respectively. The R140A mutant exhibited cAMP production that was not significantly different than the wild-type receptor, or 61.0 ± 8.3%.
Fig. 6.
cAMP inhibition assay on A-427 cells transiently transfected with wild-type SSTR2, a D139, or an R140 mutant receptor. The data represent the percent of forskolin-induced cAMP production in the presence of SST normalized to the amount produced when SST was not present. Each bar represents the mean ± sem of three to four experiments, with each experiment consisting of duplicate points. The wild-type receptor data are the average of 10 experiments. *, Significant difference (P ≤ 0.02) compared with the wild type.
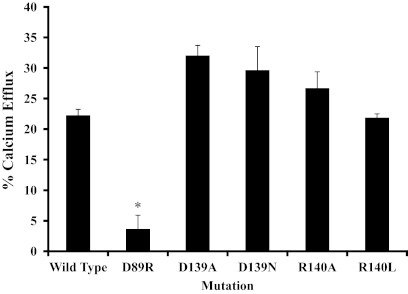
A-427 cells were transfected as above, with the addition of D89R, and were tested for calcium release via a fluorescent probe for Ca2+ (Fig. 7). The percent calcium release in the wild-type transfected cells was 22.3 ± 1.0% after stimulation with SST. The percent release was significantly less (P < 0.02) in the D89R mutant, which showed ablation of ligand binding, at 3.6 ± 2.3%. There was not a decrease in the calcium release in the other mutants tested.
Fig. 7.
Calcium release assay on A-427 cells transiently transfected with wild-type SSTR2, D89R, a D139, or an R140 mutant receptor. The data represent the percent of maximum calcium efflux after stimulation with SST normalized to maximum fluorescence after addition of ionomycin. Each bar represents the mean of n = 2–3 measurements ± sem. *, indicates a significant difference (P < 0.02) as compared to the wild type.
Discussion
The intracellular signaling pathways of human SSTR2 are well understood. Further, a number of protein-protein interactions with SSTR2 have been identified. It is well known that SSTR2 directly interacts with the PTP SHP-1, which upon agonist binding stimulates a cascade of intracellular processes (32, 33). It has also been shown that not only does SSTR2 exist at the cell surface as a homodimer (34, 35), but the receptor also interacts with receptors 3 and 5 of the somatostatin family (36, 37). In addition, SSTR2 is coupled to inhibitory G protein, which modulates cAMP-mediated signaling and ionic transport, as well as other intracellular signals. However, the amino acid residues involved in these protein-protein interactions of SSTR2 have not been characterized. In terms of using SSTR2 for the molecular imaging of gene transfer, these interactions are important in that expression of the receptor should not have a biological effect on the cell, given that it is exogenous to the cell's normal function and should only act as a marker of gene transfer. We have previously analyzed SSTR2 as a molecular imaging target in stable cell lines (19) and for the imaging of gene transfer using nonreplicative adenovirus (18, 20, 29). In the current study, we sought to determine residues involved in SSTR2-G protein coupling within the receptor in an attempt to uncouple the receptor from G protein signaling.
Human SSTR2 is a member of the seven-transmembrane G protein-coupled receptor superfamily, and there are a number of highly conserved residues within this family. The aspartic acid within transmembrane region 2 of SSTR2 at position 89 (D89) was chosen due to its homology within other seven-transmembrane receptors that are also coupled to G protein (38) with which this residue has shown an effect on the receptor. In the dopamine 2 receptor, an aspartic acid corresponding to D89 of SSTR2 was found to completely ablate the receptor's coupling to G protein (39). It has also been shown that mutation of D89 in rat SSTR2 to either alanine or glutamic acid does not affect the affinity of the receptor for SST (25) but does affect the number of active binding sites. These results provided a basis for the mutation of D89 in SSTR2.
Additionally, there is a tripeptide at the N-terminal edge of intracellular loop 2 of SSTR2 consisting of the residues aspartic acid, arginine, and tyrosine (DRY) at positions 139–141, and this motif is highly conserved in members of the seven-transmembrane G protein-coupled receptor A (rhodopsin-like) family. It was shown that the DRY loop in human CC chemokine receptor 5 was involved in G protein activation and binding of β-arrestin (40), which is a key molecule involved in receptor internalization. Mutations in the aspartic acid of the motif in β- and α2-adrenergic receptors caused a significant decrease or ablation of G protein coupling but did not affect ligand binding (41, 42). The D139 homolog in the human angiotensin II type-2 receptor, or aspartic acid 141, was found to be involved in both ligand binding affinity and G protein signaling, but the mutation did not affect functional expression (43). Thus, D139 and R140 as potential residues involved in G protein coupling were targeted for mutation and subsequent receptor analysis. There is little data as to the effects of the Tyr in the DRY motif of G protein-coupled receptor with regard to intracellular signaling. However, in the μ-opioid receptor, the SSTR2 Y141 homolog at position 166 was changed to Phe, and there were no changes in internalization, EC50 values, or G protein signaling compared with the wild-type receptor (44). Therefore, we chose to not mutate tyrosine at position 141 in SSTR2.
Mutations to D89, D139, and R140 were constructed and were transiently transfected into the human non-small-cell lung carcinoma line A-427. The mRNA levels appeared to be similar for all of the mutations made compared with the wild-type receptor (Fig. 2). The receptors were then evaluated for protein expression and membrane localization via an ELISA (Fig. 3), and the levels of receptor at the cell surface compared with the wild-type varied with each amino acid mutation, although the total protein levels of the mutated receptors appeared to be similar by Western blot (data not shown). The D89R, R140A, and R140L mutations resulted in expression levels that were not significantly different from wild-type receptor, although the D139A and D139N receptors expressed at levels greater than 50%. Conversely, the R140E mutated receptor was essentially not present in the membrane, which was verified with a microscopic evaluation of R140E cellular localization in which the receptor is halted within the ER (Fig. 4). This was also seen with the D89A and D89L mutants (data not shown). Other studies have shown that mutation of the conserved D89 to asparagine in mouse SSTR2 did not affect receptor localization (24), and mutation of the homologous aspartic acid to Asn in the porcine α2-adrenergic receptor produced a receptor that underwent normal processing and membrane insertion (45). However, both of these mutations were to the polar, uncharged side chain of Asn, whereas the mutations presented in this study were to either a hydrophobic or a basic amino acid side chain. Interestingly, replacement of the negative charge of the Asp at position 89 in human SSTR2 with that of the positive charge from Arg resulted in a mutant receptor that was localized to the membrane in amounts statistically equal to that of the wild type, thereby implying the importance of the charge in the proper insertion of the receptor into the membrane. This point was seen in the rat SSTR2 in which D89 was mutated to E89, resulting in a receptor with equivalent numbers of receptors as opposed to a change to A89 in which there was a 97.5% reduction in receptors (25). Another study showed that angiotensin II type-2 receptor's structural stability was compromised by changes to either Asp or Arg of the DRY loop (43), and thus adding another negatively charged amino acid in the DRY to DEY SSTR2 mutant caused the structural stability of the mutant receptor to likely be destabilized.
We then looked at the binding characteristics of the D139 and R140 (A and L) receptors that showed greater than 50% expression compared with the wild type. The D89R mutation was also evaluated for ligand binding, and it was found to be completely ablated (data not shown). This is a significant finding, because the receptor is expressed and localized to the membrane to levels similar to that of the wild type. This mutant receptor represents a potential target for identifying new somatostatin analogs or even receptor antagonists, because the charge differential presented in the mutant receptor could be an alternate thermodynamic state for antagonists. Elling et al. (46) hypothesized such interconverting receptor states in all G protein-coupled receptors for agonist and antagonists. Additionally, this finding indicates that the D89 residue in SSTR2 is important for active site conformation given that the D89R receptor is expressed in similar amounts to that of the wild-type receptor but is completely unable to bind the native SST ligand.
As seen in Table 1 and Fig. 5, the mutations had varying effects on the ability of receptor to bind ligand. There was a significant (P < 0.006) decrease in the Bmax of the D139A mutant compared with the wild type, but there was a 2-fold increase in the affinity of the mutant receptor for the SST ligand. When the same D to A mutation was performed in the human angiotensin II type-2 receptor, there was a 1.5-fold increase in Bmax compared with wild type but a 40% decrease in ligand-binding affinity (43). The D139N mutation resulted in a receptor with Bmax values and ligand-binding affinity not significantly different than wild type. This is in contrast to a report in which the corresponding Asp of the conserved DRY motif was mutated to Asn in the rat m1 muscarinic acetylcholine receptor, and the Bmax values were drastically reduced (47). Additionally, mutation of the Asp in the DRY motif of the human β-adrenergic receptor to Asn also produced a mutated receptor with increased affinity for ligand (48). The R140A mutant receptor displayed Bmax values and ligand-binding affinity not statistically different than that of wild-type SSTR2. This is in agreement with the same Arg to Ala mutation in the DRY loop of angiotensin II type 2 receptor, in which there was no effect on Bmax or Kd (45). In the case of R140L, there was a significant decrease (P = 0.012) in Bmax, but there was an increase in ligand-binding affinity. Thus, although the levels of the mutant receptors that localized to the cell surface were similar to wild type (Fig. 3), these studies showed that the functionality of the mutant receptors with regard to their ability to bind ligand varied greatly.
The D139 and R140 (A and L) mutants were analyzed for G protein coupling to adenylate cyclase, and only R140A was not significantly uncoupled from G protein (Fig. 6). In contrast, when the Arg in the ERY motif of rhodopsin was mutated to Ala, the receptor had a higher affinity for rhodopsin but was unable to interact with G protein (49). However, compared with the wild type, D139A, D139N, and R140L were partially uncoupled from G protein signaling. The D139A mutant receptor localized less to the membrane than wild type and had a significantly lower Bmax value. It has been shown in mouse AtT-20 cells that knockdown of expression of SSTR2 has a direct impact on cAMP inhibition, indicating that the amount of functional receptor is critical for intracellular signaling through G protein (50). Thus, the uncoupling effects seen in the D139A mutant are probably due to decreased functional receptor expression and not an actual uncoupling of the receptor from G protein. This is also the likely explanation for the observed uncoupling of the R140L mutant. The D139N mutation shows partial uncoupling from G protein. It has been stated that activation of receptor upon G protein binding requires protonation of the Asp in the DRY motif (51), and this would account for the uncoupling results found in the SSTR2 D139N mutant.
The D139 and R140 (A and L) mutants were analyzed for G protein-mediated calcium release (Fig. 7). The results indicate that neither the D139 nor R140 mutations are involved in G protein signaling of calcium release. It is known that calcium channels in cells expressing SSTR2 are regulated by Gαi/o, which also inhibits adenylate cyclase. However, the regulation of these channels may also be regulated by the Gβγ heterodimer (52) or by direct effects on high-voltage-dependent calcium channels via Gαo2 (53). These G protein subunits are not involved in adenylate cyclase signaling. Therefore, although the D139N mutation appears to be partially uncoupled from G protein signaling via adenylate cyclase, it does not appear to be uncoupled via calcium signaling, most likely due to the different subunits involved. These data indicate that more complicated mutations are needed to further elucidate G protein-signaling pathways in cells expressing SSTR2.
In the present study, we constructed various mutants of human SSTR2 by changing the D89, D139, or R140 amino acids. These changes did not affect the levels of mRNA for each mutant receptor but resulted in different levels of receptor localization at the cell surface compared with wild-type receptor. Although the D89R mutant had levels of receptor at the cell surface similar to wild type, it was unable to bind SST. Similarly, the D139 and R140 mutants that localized to the cell surface demonstrated various levels of SST binding. Of the two mutants, D139N and R140A, that had cell surface expression and Bmax values similar to those of wild-type receptor, only the D139N mutant was uncoupled from G protein-mediated cAMP signaling but not from G protein-mediated calcium release. Thus, this particular mutation will be evaluated for further characterization of amino acids involved in ligand-activated SSTR2 intracellular signaling with the hope to generate a mutant SSTR2 encoded in adenoviral vectors for use in the noninvasive imaging of gene transfer.
Acknowledgments
We thank Thomas Kelly, Kristina Joy, Lujia Zhang, Stephanie Krieger, and Dr. Girdhar Sharma (all from the Department of Radiation Oncology, Washington University School of Medicine) for their technical assistance in the preparation of experiments. Fluorescent plate reading was performed at the Molecular Imaging High Throughput Core Facility at the Washington University School of Medicine.
This work was supported by National Institutes of Health Grant R01 EB04533.
Disclosure Summary: J.J.P., R.C., R.A., K.A.L., and B.E.R. have nothing to declare.
Footnotes
- DRY
- Tripeptide consisting of aspartic acid, arginine, and tyrosine
- ER
- endoplasmic reticulum
- HA
- hemagglutinin
- PTP
- phosphotyrosine phosphatases
- SSTR2
- somatostatin receptor subtype 2
- SST
- somatostatin-14.
References
- 1. Krulich L, Dhariwal AP, McCann SM. 1968. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology 83:783–790 [DOI] [PubMed] [Google Scholar]
- 2. Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB. 1995. The somatostatin receptor family. Life Sci 57:1249–1265 [DOI] [PubMed] [Google Scholar]
- 3. Reisine T, Bell GI. 1995. Molecular biology of somatostatin receptors. Endocr Rev 16:427–442 [DOI] [PubMed] [Google Scholar]
- 4. Patel YC. 1997. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest 20:348–367 [DOI] [PubMed] [Google Scholar]
- 5. Patel YC. 1999. Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198 [DOI] [PubMed] [Google Scholar]
- 6. Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. 2004. Regulation and function of somatostatin receptors. J Neurochem 89:1057–1091 [DOI] [PubMed] [Google Scholar]
- 7. Lamberts SW, de Herder WW, Hofland LJ. 2002. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol Metab 13:451–457 [DOI] [PubMed] [Google Scholar]
- 8. Hofland LJ, Lamberts SW. 2003. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24:28–47 [DOI] [PubMed] [Google Scholar]
- 9. Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. 1996. Octreotide. N Engl J Med 334:246–254 [DOI] [PubMed] [Google Scholar]
- 10. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. 2003. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2:999–1017 [DOI] [PubMed] [Google Scholar]
- 11. Lamberts SW, Krenning EP, Reubi JC. 1991. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 12:450–482 [DOI] [PubMed] [Google Scholar]
- 12. Patch D, Burroughs A. 2002. Vapreotide in variceal bleeding. J Hepatol 37:167–168 [DOI] [PubMed] [Google Scholar]
- 13. Goldenberg DM. 2002. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med 43:693–713 [PubMed] [Google Scholar]
- 14. Labeur M, Theodoropoulou M, Sievers C, Paez-Pereda M, Castillo V, Arzt E, Stalla GK. 2006. New aspects in the diagnosis and treatment of Cushing disease. Front Horm Res 35:169–178 [DOI] [PubMed] [Google Scholar]
- 15. Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L, Schumacher J, Henze M, Heppeler A, Meyer J, Knapp H. 2001. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med 28:1751–1757 [DOI] [PubMed] [Google Scholar]
- 16. Virgolini I, Traub T, Novotny C, Leimer M, Füger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M, Andreae F, Kurtaran A, Dudczak R. 2001. New trends in peptide receptor radioligands. Q J Nucl Med 45:153–159 [PubMed] [Google Scholar]
- 17. de Herder WW, Kwekkeboom DJ, Feelders RA, van Aken MO, Lamberts SW, van der Lely AJ, Krenning EP. 2006. Somatostatin receptor imaging for neuroendocrine tumors. Pituitary 9:243–248 [DOI] [PubMed] [Google Scholar]
- 18. Rogers BE, Parry JJ, Andrews R, Cordopatis P, Nock BA, Maina T. 2005. MicroPET imaging of gene transfer with a somatostatin receptor-based reporter gene and 94mTc-Demotate 1. J Nucl Med 46:1889–1897 [PubMed] [Google Scholar]
- 19. Parry JJ, Eiblmaier M, Andrews R, Meyer LA, Higashikubo R, Anderson CJ, Rogers BE. 2007. Characterization of somatostatin receptor subtype 2 expression in stably transfected A-427 human cancer cells. Mol Imaging 6:56–67 [PubMed] [Google Scholar]
- 20. Chen R, Parry JJ, Akers WJ, Berezin MY, El Naqa IM, Achilefu S, Edwards WB, Rogers BE. 2010. Multimodality imaging of gene transfer with a receptor-based reporter gene. J Nucl Med 51:1456–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar U, Grant M. 2010. Somatostatin and somatostatin receptors. Results Probl Cell Differ 50:137–184 [DOI] [PubMed] [Google Scholar]
- 22. Meyerhof W. 1998. The elucidation of somatostatin receptor functions: a current view. Rev Physiol Biochem Pharmacol 133:55–108 [DOI] [PubMed] [Google Scholar]
- 23. Florio T. 2008. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol 286:40–48 [DOI] [PubMed] [Google Scholar]
- 24. Kong H, Raynor K, Yasuda K, Bell GI, Reisine T. 1993. Mutation of an aspartate at residue 89 in somatostatin receptor subtype 2 prevents Na+ regulation of agonist binding but does not alter receptor-G protein association. Mol Pharmacol 44:380–384 [PubMed] [Google Scholar]
- 25. Strnad J, Hadcock JR. 1995. Identification of a critical aspartate residue in transmembrane domain three necessary for the binding of somatostatin to the somatostatin receptor SSTR2. Biochem Biophys Res Commun 216:913–921 [DOI] [PubMed] [Google Scholar]
- 26. Han L, Yang D, Kundra V. 2007. Signaling can be uncoupled from imaging of the somatostatin receptor type 2. Mol Imaging 6:427–437 [PubMed] [Google Scholar]
- 27. Schwartkop CP, Kreienkamp HJ, Richter D. 1999. Agonist-independent internalization and activity of a c-terminally truncated somatostatin receptor subtype 2 (δ349). J Neurochem 72:1275–1282 [DOI] [PubMed] [Google Scholar]
- 28. Vanetti M, Vogt G, Höllt V. 1993. The two isoforms of the mouse somatostatin receptor (mSSTR2A and mSSTR2B) differ in coupling efficiency to adenylate cyclase and in agonist-induced receptor desensitization. FEBS Lett 331:260–266 [DOI] [PubMed] [Google Scholar]
- 29. Rogers BE, Chaudhuri TR, Reynolds PN, Della Manna D, Zinn KR. 2003. Non-invasive γ camera imaging of gene transfer using an adenoviral vector encoding an epitope tagged receptor as a reporter. Gene Ther 10:105–114 [DOI] [PubMed] [Google Scholar]
- 30. Kundra V, Mannting F, Jones AG, Kassis AI. 2002. Noninvasive monitoring of somatostatin receptor subtype 2 chimeric gene transfer. J Nucl Med 43:406–412 [PubMed] [Google Scholar]
- 31. Rogers BE, McLean SF, Kirkman RL, Della Manna D, Bright SJ, Olsen CC, Myracle AD, Mayo MS, Curiel DT, Buchsbaum DJ. 1999. In vivo localization of [111In]-DTPA-D-Phe1-octreotide to human ovarian tumor xenografts induced to express the somatostatin receptor subtype 2 using an adenoviral vector. Clin Cancer Res 5:383–393 [PubMed] [Google Scholar]
- 32. Lopez F, Estève JP, Buscail L, Delesque N, Saint-Laurent N, Théveniau M, Nahmias C, Vaysse N, Susini C. 1997. The tyrosine phosphatase SHP-1 associates with the sst2 somatostatin receptor and is an essential component of sst2-mediated inhibitory growth signaling. J Biol Chem 272:24448–24454 [DOI] [PubMed] [Google Scholar]
- 33. Hortala M, Ferjoux G, Estival A, Bertrand C, Schulz S, Pradayrol L, Susini C, Clemente F. 2003. Inhibitory role of the somatostatin receptor SST2 on the intracrine-regulated cell proliferation induced by the 210-amino acid fibroblast growth factor-2 isoform. J Biol Chem 278:20574–20581 [DOI] [PubMed] [Google Scholar]
- 34. Grant M, Collier B, Kumar U. 2004. Agonist-dependent dissociation of human somatostatin receptor 2 dimers. J Biol Chem 279:36179–36183 [DOI] [PubMed] [Google Scholar]
- 35. Durán-Prado M, Bucharles C, Gonzalez BJ, Vázquez-Martínez R, Martínez-Fuentes AJ, García-Navarro S, Rhodes SJ, Vaudry H, Malagón MM, Castaño JP. 2007. Porcine somatostatin receptor 2 displays typical pharmacological sst2 features but unique dynamics of homodimerization and internalization. Endocrinology 148:411–421 [DOI] [PubMed] [Google Scholar]
- 36. Pfeiffer M, Koch T, Schröder H, Klutzny M, Kirscht S, Kreienkamp HJ, Höllt V, Schulz S. 2001. Homo- and heterodimerization of somatostatin receptor subtypes. J Biol Chem 276:14027–14036 [DOI] [PubMed] [Google Scholar]
- 37. Grant M, Alturaihi H, Jaquet P, Collier B, Kumar U. 2008. cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol Endocrinol 22:2278–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. 1992. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol 11:1–20 [DOI] [PubMed] [Google Scholar]
- 39. Liang Q, Satyamurthy N, Barrio JR, Toyokuni T, Phelps MP, Gambhir SS, Herschman HR. 2001. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther 8:1490–1498 [DOI] [PubMed] [Google Scholar]
- 40. Huttenrauch F, Nitzki A, Lin FT, Höning S, Oppermann M. 2002. β-Arrestin binding to CC chemokine receptor 5 requires multiple c-terminal receptor phosphorylation sites and involves a conserved asp-arg-tyr sequence motif. J Biol Chem 277:30769–30777 [DOI] [PubMed] [Google Scholar]
- 41. Dixon RA, Sigal IS, Strader CD. 1988. Structure-function analysis of the β-adrenergic receptor. Cold Spring Harb Symp Quant Biol 53:487–497 [DOI] [PubMed] [Google Scholar]
- 42. Fraser CM, Chung FZ, Wang CD, Venter JC. 1988. Site-directed mutagenesis of human β-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci USA 85:5478–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moore SA, Patel AS, Huang N, Lavin BC, Grammatopoulos TN, Andres RD, Weyhenmeyer JA. 2002. Effects of mutations in the highly conserved DRY motif on binding affinity, expression, and G-protein recruitment of the human angiotensin II type-2 receptor. Mol Brain Res 109:161–167 [DOI] [PubMed] [Google Scholar]
- 44. Clayton CC, Bruchas MR, Lee ML, Chavkin C. 2010. Phosphorylation of the μ-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol 77:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang CD, Buck MA, Fraser CM. 1991. Site-directed mutagenesis of α 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol 40:168–179 [PubMed] [Google Scholar]
- 46. Elling CE, Nielsen SM, Schwartz TW. 1995. Conversion of antagonist-binding site to metal-ion site in the tachykinin NK-1 receptor. Nature 374:74–77 [DOI] [PubMed] [Google Scholar]
- 47. Fraser CM, Wang CD, Robinson DA, Gocayne JD, Venter JC. 1989. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol 36:840–847 [PubMed] [Google Scholar]
- 48. Arora KK, Sakai A, Catt KJ. 1995. Effects of second intracellular loop mutations on signal transduction and internalization of the gonadotropin-releasing hormone receptor. J Biol Chem 270:22820–22826 [DOI] [PubMed] [Google Scholar]
- 49. Shi W, Sports CD, Raman D, Shirakawa S, Osawa S, Weiss ER. 1998. Rhodopsin arginine-135 mutants are phosphorylated by rhodopsin kinase and bind arrestin in the absence of 11-cis-retinal. Biochemistry 37:4869–4874 [DOI] [PubMed] [Google Scholar]
- 50. Ben-Shlomo A, Pichurin O, Barshop NJ, Wawrowsky KA, Taylor J, Culler MD, Chesnokova V, Liu NA, Melmed S. 2007. Selective regulation of somatostatin receptor subtype signaling: evidence for constitutive receptor activation. Mol Endocrinol 21:2565–2578 [DOI] [PubMed] [Google Scholar]
- 51. Fahmy K, Sakmar TP, Siebert F. 2000. Transducin-dependent protonation of glutamic acid 134 in rhodopsin. Biochemistry 39:10607–10612 [DOI] [PubMed] [Google Scholar]
- 52. Takano K, Yasufuku-Takano J, Teramoto A, Fujita T. 1997. Gi3 mediates somatostatin-induced activation of an inwardly rectifying K+ current in human growth hormone-secreting adenoma cells. Endocrinology 138:2405–2409 [DOI] [PubMed] [Google Scholar]
- 53. Ikeda SR, Schofield GG. 1989. Somatostatin blocks a calcium current in rat sympathetic ganglion neurons. J Physiol 409:221–240 [DOI] [PMC free article] [PubMed] [Google Scholar]