“Resilience is critical in all things. Grit and zest are qualities most predictive of success.”
— Wylie W. Vale
The structure and full chemical and biological characterization of ovine corticotropin-releasing factor (oCRF) was reported in 1981 by Wylie W. Vale and colleagues working at the Salk Institute (La Jolla, CA) in temporary barracks located west of the main building of the Salk Institute with a full view of the Pacific (1). It was an extraordinary time in the field of endocrinology, and the competition for discovery was fierce. The climate at the Salk, where the superstars of today (including George Koob, Catherine and Jean Rivier, Paul Sawchenko, and Wylie Vale) were being trained by remarkable leaders in science (Larry Swanson, Floyd Bloom, and Roger Guillemin), was no different. One trainee in particular, Wylie Vale, stood out as a resilient and strong-willed young scientist, passionate about isolating CRF. Having come to Salk initially as a postdoctoral fellow of Roger Guillemin's in 1970, Wylie was a Texan thrust into Southern California at a time when surf boards and palm trees still outnumbered residents. “It was like a scientific playground,” described George Koob, “the Salk Institute was full of passion and energy. We'd get together and play intensely competitive racquetball or tennis in the morning before heading into the lab — where that competition to make the next big breakthrough continued.” The search for CRF, over the next 30 yr, took Wylie and his colleagues on an intriguing journey that none of them could have predicted. From the race to elucidate its unpredicted sequence to identifying its unimaginable central effects on behavior, CRF (similar to the man who led the search for its identification) turned out to be a pretty remarkable story.
The Isolation of CRF. A Story of Perseverance and Patience (and a Whole Lot of Sheep)
In 1969, Roger Guillemin's lab at the Baylor School of Medicine (Houston, TX) was searching for hypothalamic-releasing hormones. At that time, Wylie Vale was a postdoc working on a project searching for TSH-releasing factor (TRF) and other hypothalamic releasing factors (2, 3). Also present in Houston was a young graduate student, Catherine Rivier, who had moved from her native Switzerland with her husband, Jean Rivier, to do her graduate work in Guillemin's lab, bringing with her a requested allotment of ACTH antibody, with which she would later develop the first bioassay for CRF in rodents. “Wylie helped us to adjust and introduced us to the Texan way of life by taking us to rodeos and teaching us the local vernacular. He was always so thoughtful,” commented Catherine. Endocrine physiology research in the late 1960s was far different than it is today. To determine what effect TRF had on the body, Wylie himself was injected with it (under medical supervision). Certainly not something we would think about doing today. “It felt like you were going to die — you wet your pants, your heart raced, you become very light-sensitive!” Catherine recalls Wylie's description. “It was an incredibly powerful, but short-lived experience.” This would turn out to be some of the first major evidence that these hypothalamic hormones had central nervous system effects. Shortly thereafter, Guillemin accepted an offer and moved his lab to the Salk Institute in 1970.
Wylie's focus turned to CRF after arriving at the Salk, declaring to his young mentee, Catherine, “Now, we are working on isolating CRF.” And that is exactly what they did. Working night and day, and even working through the night on many occasions, Catherine, Jean, and Wylie were obsessed with finding this peptide. The three scientists had separated from Guillemin by this point and were working in the temporary space out near the parking lot. It was less than a glamorous location, but one that they had been given as their own lab space by the Salk president, Frederic de Hoffman. Although Guillemin retained the aqueous extracts from the half million sheep hypothalami, Wylie had been given access to the original hydrophobic fraction from the petroleum ether extracts where everyone presumed it would be much less likely CRF would be found. Less likely. But not impossible. This became a labor of love, and a story of incredible perseverance and elbow grease. “After working night and day for hours and hours on these extracts, we smelled so bad that people in the supermarket would run away,” recalled Catherine. To determine whether they had the correct CRF-containing fraction, Catherine would infuse the sample into anesthetized rats and then measure plasma ACTH 10–15 min later. Nothing worked. Little did they know that CRF was a much larger and stickier peptide hormone than known peptides, such as TRF, GnRH, somatostatin, arginine vasopressin (AVP), or oxytocin, “It stuck to everything!” explained Catherine, “the glassware, the tubes, the chromatographic supports, everything.” Because they were already working with the ether extract samples containing lower concentrations of the hormone, frustration was around every corner. Throughout these struggling times, Catherine would often skulk into Wylie's office deflated but not defeated, looking for wisdom from Wylie. “I tried, I tried, I tried,” she would whine. He would look at her and say with that classic Wylie smile, “Effort only counts through the 3rd grade, Catherine. After that, only results matter.” At some point in 1978, thanks to Jean Rivier's expert HPLC techniques, they felt they finally had a fraction that contained pure CRF. The group originally tried to get Merck (Darmstadt, Germany) to conduct the peptide sequencing, but their system was not sensitive enough at that time, because this was before solid or gas phase sequencing. Wylie then convinced the Salk to invest in a Beckman spinning cup sequencer (Brea, CA), and after two long years of sequencing by Joachim Spiess, oCRF was officially characterized (1) (Fig. 1). The team's relief was enormous after so many years of hard work. Wanting to provide some humorous relief in the then extraordinarily tense atmosphere, on April 1 Ron Kaiser, Jean Rivier's technician, had doctored the table of contents from an issue of Science to include a fake paper entitled “Discovery of Corticoliberin” by Andrew Schally's team (thus effectively scooping them on the isolation of oCRF). This document was taped to Wylie's office door, and “while he immediately knew it was a joke, Joachim Spiess read it and nearly passed out,” recalls Koob. The first author on that fake publication was “A. Prilful”. Crafty.
Fig. 1.
The oCRF isolation team. Standing in front of the Beckman spinning cup sequencer at the Salk Institute, Joachim Spiess, Catherine Rivier, Jean Rivier, and Wylie Vale in 1980 after the confirmation that they had finally isolated oCRF.
During the race to isolate oCRF, there were many who argued that in fact AVP was the CRF. Wylie and Catherine worked closely together on establishing a bioassay that could distinguish between the physiological effects of these two hormones, AVP and CRF, because both produced an increase in ACTH release. The assay for AVP action at the time was a pressure assessment where iv AVP administration in rats produced an increase in blood pressure due to increased water reabsorption and peripheral vascular resistance. Wylie insisted on developing a pituitary bioassay where screening for oCRF would require less material for testing than an in vivo assessment. Every week, rat pituitaries were harvested and digested into single-cell cultures. Three days later, column fractions could be tested on them for stimulation of ACTH release. This assay no doubt contributed to a much more rapid characterization and purification of CRF. Although CRF was a potent stimulator of ACTH release, it was not until antibodies were generated by Wylie's group that it became clear that CRF was the driver of the HPA stress response. Antisera against oCRF markedly reduced the CRF-induced rise in plasma ACTH in nonstressed rats, and more impressively, these antibodies blocked an ether stress ACTH response by more than 75% (4). This was the first functional evidence demonstrating an endogenous role for CRF in stimulating pituitary corticotropes.
Second only to the isolation of oCRF, the development of the CRF antibody proved to be one of the biggest advancements in our understanding of stress neurocircuitry. Paul Sawchenko came to the Salk Institute in 1980 to work with Larry Swanson to learn neuroanatomy techniques. At this time, no one had determined where CRF was in the hypothalamus. “We expected the CRF neurons to be in the arcuate or maybe ventromedial nucleus,” said Paul. “What we didn't expect was to find it in the PVN.” It turns out that the median eminence had been included in those original sheep hypothalamus dissections and likely was the source of most of the oCRF isolated from the extracts. In 1982, Floyd Bloom's group first used the oCRF antisera to map CRF neurons and fibers in the rat hypothalamus (5). Wylie also gave Sawchenko and Swanson an aliquot of the oCRF antibody, and they went to town mapping CRF cell bodies and projections in the rat brain (6). With Larry focusing on the forebrain and Paul on the brain stem, the duo found that CRF in fact had vast projections throughout the brain, extending from the hypothalamus to many midbrain structures and brain stem autonomic nuclei, as well as from limbic forebrain CRF neurons. From these results, Laurel Fisher, working with Marvin Brown, went on to demonstrate that intracerebroventricular infusion of CRF stimulated sympathetic outflow, increasing oxygen consumption and arterial pressure, establishing what we know today as the critical role CRF plays in orchestrating stress responsivity and maintaining organismal homeostasis (7).
Although the anatomy and physiology of CRF was being worked out, Wylie realized that he needed to pull in a behavioral neuroscientist to ascertain the potential effects of central CRF administration. During one of their many walks around the Salk courtyard, in which they would dynamically debate science, Wylie convinced George Koob that they needed a unifying hypothesis for their National Institute of Diabetes and Digestive and Kidney Diseases Center grant on CRF, what if CRF controls behavioral responses to stress? Koob remembers thinking, “Yeah, right. How could this peptide do everything? Now this is getting farfetched.” Yet he trusted Wylie's instincts, so off he went with CRF that Jean had synthesized to determine what possible behavioral changes he could tease apart. Not knowing the receptor pharmacology yet or what dose to use, they infused 10 μg of CRF intracerebroventricularly to rats and stood back to watch their response. George recalls that the rats had the most bizarre response, “They were climbing the walls, walking on their tiptoes, standing on their hind legs, and shadow boxing.” He remembers calling Wylie on the phone, “you've got to come over and see this — the rats are levitating!” They would later determine that at this high dose, CRF induced preseizure activity. The well-known effects of CRF on locomotion, home cage activity, and the exaggerated response to stress were completed in these first studies and published less than a year after CRF had been isolated (8). Jean Rivier had also designed a peptide antagonist, α-helical CRF(9–41), which was used to demonstrate CRF's endogenous involvement in stress-mediated locomotion and anxiogenesis (9) and stress-induced fighting in rats (10), results that sparked great translational potential for understanding the involvement of stress circuitry in neuropsychiatric disease etiology. In fact, it was during this time that clinical studies investigating stress dysregulation in mental health disorders discovered elevated CRF levels in the CSF of depressed patients, launching a new era focusing drug targets in pharmaceutical treatment of affective disorders and other stress-related diseases (11, 12).
The Isolation of CRF Receptors and the Related Urocortin (Ucn) Peptides, It Is All About Balance
It is important to remember that after the isolation of CRF, nothing was known about CRF receptors (CRFR), where they were or what they did in the brain. In collaboration with Errol DeSouza and Tom Insel, they had begun to define the breadth of CRF binding sites in the brain using radiolabeled ligand and autoradiography (13, 14). In Wylie's lab, Marilyn Perrin was working on describing the pharmacology of this newly isolated peptide, whereas Louise Bilezikjian was characterizing the intracellular signaling in pituitary cell cultures (15, 16). But it was not until 1993, with the advancement of better molecular biology tools, that Wylie and his team isolated the cDNA encoding the first CRFR, CRFR1, from a human Cushing's corticotropic adenoma expression library (17, 18). Cloning by sequence homology then led to the discovery of a second CRFR, CRFR2, with high expression in the heart, gastrointenstinal tract, and brain, suggesting additional functions of CRF (19). The molecular biology era reignited intense competition in the field for identifying new receptors and ligands. “Several groups were racing to find and publish new sequences, even within San Diego itself there was a fierce race to find this second receptor. It was an extremely exciting time,” recalled Marilyn Perrin. Although CRFR1 was established as the receptor critical for CRF action on pituitary corticotropes, CRFR2 became the focus of studies on peripheral functions, such as cardiac inotropy, vasodilation, and colonic motility, due to its high expression in cardiac myocytes, blood vessels, and gastrointestinal tract, respectively (20–23). In the brain, CRFR2 was found to have a unique stress-feedback role in brain regions, including the lateral septum and the serotonergic dorsal raphe, and to show stress-mediated processing (24–26). Interestingly, although sharing a high degree of sequence homology, these two receptors are encoded by two separate genes. However, they have a vastly different central and peripheral distribution, supporting diverse stress-related physiological functions. Over the next decade, transgenic mouse technology, in addition to pharmacological approaches, was used to determine more specific functions of the CRFR (27). These approaches have established experimental support for exciting and complementary roles for these two receptors, where CRFR1 seems to be the main stress driver and CRFR2 the feedback, maintenance, and brake.
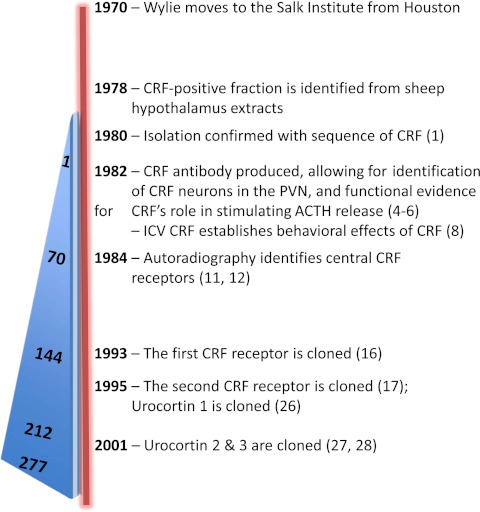
Once again connecting dots that others may have missed, Wylie noted that fish and amphibians, in addition to the CRF ortholog (peptides that retain the same CRF function over the course of evolution), also expressed a paralog (peptides that evolve new functions, even if these are related to the original one) and thus decided to pursue the identification of additional CRF-related ligand family members in the mammalian brain. Based on the similarities between fish urotensin-I and amphibian sauvagine, his group identified a new member of the mammalian CRF family, which they named Ucn (Ucn1), to reflect its relationship to fish urotensin (28). “Urotensin I was considered the fish CRF, but with the identification of authentic fish CRF, together with a mismatch between CRF and receptors in brain sites, there was a push to find additional mammalian molecules. We made a huge antisera library to every fish urotensin, frog sauvagine, and mammalian CRFs, while Paul Sawchenko's group used the antisera to locate a region of the brain with immunoreactivity that was not CRF,” recalled Joan Vaughan, a research technician in Wylie's lab. This is where the pharmacology of these family members really started to get interesting. Ucn1 was indeed more potent than CRF at binding and activating CRFR2, suggesting it was perhaps the endogenous ligand for this second CRFR. However, when Sawchenko and others began lining up the brain distribution of the two receptors and the known ligands (CRF and Ucn1), they quickly realized that there had to be additional ligands that were projecting to CRFR2 sites in the brain. And indeed after the completion of the genome project, Wylie and his team found two more ligands, Ucn2 and Ucn3 (29, 30). Since the identification of these additional family members, more then 1000 studies have been published regarding the important central and peripheral functions of these conserved peptides (31–33; Fig. 2).
Fig. 2.
Timeline of major CRF findings from the Vale lab. Starting with Wylie's move to the Salk Institute in 1970, the timeline includes the identification of novel CRFR and ligand family members. The blue triangle on the left indicates the impressive number of CRF publications with Wylie's authorship over this same timeframe.
As further testimony to the importance and impact Wylie Vale and the story of the isolation of CRF have had on science and medicine, Wylie cofounded Neurocrine Biosciences in 1992, where one of the two major technologies that were the starting blocks for this small start-up was CRF and the search for small molecule antagonists to use in stress-related diseases, because studies of the CRF system have suggested molecular targets for new drug development, biological risk factors, and predictors of treatment response (34). “We opened the doors to a lab in early spring of 1993 in an industrial unit in Sorrento Valley with one tissue culture hood, one centrifuge, a liquid handler, one −80 freezer and one 6 inch lizard that lived in our sink!” remarked Dimitri Grigoriadis. Their first CRF small molecule receptor antagonist collaboration was with Janssen Pharmaceuticals in Belgium, and that deal helped Neurocrine to go public in 1996. This program has been a huge part of Neurocrine's translational efforts with multiple compounds going into clinical trials, with the last of these molecules currently in a phase 2 trial for efficacy in posttraumatic stress disorder. “Wylie's guidance throughout this project was invaluable,” commented Dimitri. “With many compounds being developed he would keep us on track by reminding us to “go back and look at what the peptides are doing,” ensuring that the small molecules work like the peptide antagonists that Jean Rivier had developed. That drive kept us invested in not just finding another high affinity molecule but forced us to look closely at the discrete interactions of these small molecules with this peptide receptor and try to understand the system.”
Presenting the scientific community with the discoveries, isolation, and characterization of the CRF family of peptides, receptors, and binding protein, Wylie Vale, with his amazing biological insight and incredible team of colleagues, collaborators, and trainees, provided us with the building blocks for a better understanding of how the brain and body respond to stressful stimuli and the underlying mechanisms contributing to stress-related diseases. Wylie was a force of nature, a larger-than-life Texan with grit and zest and a whole lot of charisma. He had an incredibly generous spirit, a quick wit, passionate motivation, and a deep wisdom. In his presence, you always walked away with more knowledge than you came with. Wylie left us far too early, but his gifts to science and medicine remain a legacy for generations to come (Fig. 3).
Fig. 3.
Wylie W. Vale. October, 2009. Photography by Joe Belcovson.
Acknowledgments
This review is dedicated in loving memory of Dr. Wylie W. Vale who passed away on January 3, 2012.
To our fearless leader, who always maintained an incredible passion and love for science, and inspired generations of his trainees to ask the difficult questions and push harder to find the answers, your spirit and determination live forever in our hearts. You will always be the wind beneath our wings. —Alon Chen and Tracy Bale
Wylie approached science like a chess master, thinking many moves ahead, and viewing the possibilities from angles no one else thought to consider. He often attributed his success to being lucky. Luck had nothing to do with it. —Paul Sawchenko
Wylie was incredibly smart, but never acted arrogant. Most PIs have off days, but in 41 yr working with him, I never remember Wylie having one. —George Koob
Wylie had what the Greeks call “kefi”: a spirit of joy, love of life, passion and enthusiasm that he brought to all aspects of his life and the ability to spread that kefi to everyone around him. —Dimitri Grigoriadis
Wylie had an unbelievably quick mind, and he could recognize connections between dots that no one else saw. —Catherine Rivier
Wylie gave his collaborators both the full freedom and the tools to explore. In other words, Wylie had the ability to recognize talent and empower people. —Jean Rivier
For thirty-five years, Wylie was a patient mentor, tireless supporter, respected adviser, trusted colleague, inspirational leader and treasured friend who graced our lives and gilded our days —Marilyn Perrin
This work was supported in part by National Institutes of Health Grants MH073030, MH087597, and MH091258 (to T.LB.) and by the European Research Council FP7 Grant 260463 and a Research Grant from the Israel Science Foundation (A.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVP
- Arginine vasopressin
- CRFR
- CRF receptor
- oCRF
- ovine corticotropin-releasing factor
- TRF
- TSH-releasing factor
- Ucn
- urocortin.
References
- 1. Vale W, Spiess J, Rivier C, Rivier J. 1981. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- 2. Vale W, Blackwell R, Grant G, Guillemin R. 1973. TRF and thyroid hormones on prolactin secretion by rat anterior pituitary cells in vitro. Endocrinology 93:26–33 [DOI] [PubMed] [Google Scholar]
- 3. Vale W, Grant G, Rivier J, Monahan M, Amoss M, Blackwell R, Burgus R, Guillemin R. 1972. Synthetic polypeptide antagonists of the hypothalamic luteinizing hormone releasing factor. Science 176:933–934 [DOI] [PubMed] [Google Scholar]
- 4. Rivier C, Rivier J, Vale W. 1982. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science 218:377–379 [DOI] [PubMed] [Google Scholar]
- 5. Bloom FE, Battenberg EL, Rivier J, Vale W. 1982. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept 4:43–48 [DOI] [PubMed] [Google Scholar]
- 6. Swanson LW, Sawchenko PE, Rivier J, Vale WW. 1983. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186 [DOI] [PubMed] [Google Scholar]
- 7. Fisher LA, Rivier J, Rivier C, Spiess J, Vale W, Brown MR. 1982. Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology 110:2222–2224 [DOI] [PubMed] [Google Scholar]
- 8. Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. 1982. Corticotropin releasing factor produces behavioural activation in rats. Nature 297:331–333 [DOI] [PubMed] [Google Scholar]
- 9. Britton KT, Lee G, Vale W, Rivier J, Koob GF. 1986. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and 'anxiogenic' actions of CRF in the rat. Brain Res 369:303–306 [DOI] [PubMed] [Google Scholar]
- 10. Tazi A, Dantzer R, Le Moal M, Rivier J, Vale W, Koob GF. 1987. Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Regul Pept 18:37–42 [DOI] [PubMed] [Google Scholar]
- 11. Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. 1984. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344 [DOI] [PubMed] [Google Scholar]
- 12. Nemeroff CB. 1998. Psychopharmacology of affective disorders in the 21st century. Biol Psychiatry 44:517–525 [DOI] [PubMed] [Google Scholar]
- 13. De Souza EB, Perrin MH, Insel TR, Rivier J, Vale WW, Kuhar MJ. 1984. Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification. Science 224:1449–1451 [DOI] [PubMed] [Google Scholar]
- 14. De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. 1985. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci 5:3189–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. 1986. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res 381:49–57 [DOI] [PubMed] [Google Scholar]
- 16. Perrin MH, Haas Y, Rivier JE, Vale WW. 1986. Corticotropin-releasing factor binding to the anterior pituitary receptor is modulated by divalent cations and guanyl nucleotides. Endocrinology 118:1171–1179 [DOI] [PubMed] [Google Scholar]
- 17. Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. 1993. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 133:3058–3061 [DOI] [PubMed] [Google Scholar]
- 18. Chen R, Lewis KA, Perrin MH, Vale WW. 1993. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA 90:8967–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. 1995. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 92:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taché Y, Maeda-Hagiwara M, Turkelson CM. 1987. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol 253:G241–G245 [DOI] [PubMed] [Google Scholar]
- 21. Taché Y, Goto Y, Gunion MW, Vale W, River J, Brown M. 1983. Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science 222:935–937 [DOI] [PubMed] [Google Scholar]
- 22. Bale TL, Giordano FJ, Hickey RP, Huang Y, Nath AK, Peterson KL, Vale WW, Lee KF. 2002. Corticotropin-releasing factor receptor 2 is a tonic suppressor of vascularization. Proc Natl Acad Sci USA 99:7734–7739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL. 2004. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101:3697–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henry B, Vale W, Markou A. 2006. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci 26:9142–9152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. 2004. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci 24:1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. 2005. A soluble mouse brain splice variant of type 2α corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci USA 102:2620–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bale TL, Vale WW. 2004. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557 [DOI] [PubMed] [Google Scholar]
- 28. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. 1995. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378:287–292 [DOI] [PubMed] [Google Scholar]
- 29. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. 2001. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98:2843–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. 2001. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98:7570–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. 2010. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci USA 107:19020–19025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, Li C, Nagy TR, Shulman GI, Lee KF, Vale W. 2006. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 103:16580–16585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen A, Vaughan J, Vale WW. 2003. Glucocorticoids regulate the expression of the mouse urocortin II gene: a putative connection between the corticotropin-releasing factor receptor pathways. Mol Endocrinol 17:1622–1639 [DOI] [PubMed] [Google Scholar]
- 34. Nemeroff CB, Vale WW. 2005. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry 66(Suppl 7):5–13 [PubMed] [Google Scholar]