Abstract
We previously showed that advancing the increase in estradiol levels from the second to the first third of baboon pregnancy suppressed placental extravillous trophoblast (EVT) invasion and remodeling of the uterine spiral arteries. Cell culture studies show that vascular endothelial cell growth factor (VEGF) plays a central role in regulating EVT migration and remodeling of the uterine spiral arteries by increasing the expression/action of certain integrins that control extracellular matrix remodeling. To test the hypothesis that the estradiol-induced reduction in vessel remodeling in baboons is associated with an alteration in VEGF and integrin expression, extravillous placental VEGF and integrin expression was determined on d 60 of gestation (term is 184 d) in baboons in which uterine artery transformation was suppressed by maternal estradiol administration on d 25–59. EVT uterine spiral artery invasion was 5-fold lower (P < 0.01), and VEGF protein expression, quantified by in situ proximity ligation assay, was 50% lower (P < 0.05) in the placenta anchoring villi of estradiol-treated than in untreated baboons. α1β1 and α5β1 mRNA levels in cells isolated by laser capture microdissection from the anchoring villi and cytotrophoblastic shell of estradiol-treated baboons were over 2-fold (P < 0.01) and 40% (P < 0.05) lower, respectively, than in untreated animals. In contrast, placental extravillous αvβ3 mRNA expression was unaltered by estradiol treatment. In summary, extravillous placental expression of VEGF and α1β1 and α5β1 integrins was decreased in a cell- and integrin-specific manner in baboons in which EVT invasion and remodeling of the uterine spiral arteries were suppressed by prematurely elevating estradiol levels in early pregnancy. We propose that estrogen normally controls the extent to which the uterine arteries are transformed by placental EVT in primate pregnancy by regulating expression of VEGF and particular integrin extracellular remodeling molecules that mediate this process.
Placental extravillous trophoblast (EVT) migration to and invasion and remodeling of the uterine spiral arteries during the first trimester of human and nonhuman primate pregnancy are fundamentally important processes thought to be essential in promoting blood flow to and development of the fetus. We have recently shown that advancing the increase in maternal estradiol levels from the second to the first third of baboon pregnancy suppressed EVT remodeling of the uterine spiral arteries (1, 2). We have proposed, therefore, that the low levels of estradiol during early pregnancy promote EVT invasion of the uterine arteries, whereas the rise in estradiol thereafter suppresses and thus controls the extent to which the uterine spiral arteries are remodeled by EVT. The mechanism(s) by which estrogen regulates uterine vessel transformation, however, are not understood.
Based on cell culture studies, it has been proposed that vascular endothelial cell growth factor (VEGF) plays a central role in regulating EVT migration and remodeling of the uterine vessels (3–6). Consistent with the latter postulate, we have shown that VEGF mRNA levels in the placental anchoring villi and cytotrophoblastic shell were decreased in baboons in which uterine vessel transformation was suppressed by estradiol administration early in pregnancy (2). The human, baboon, and rhesus monkey extravillous placenta also expresses α1β1, α5β1, and αvβ3 integrins and their respective laminin, collagen IV, and fibronectin extracellular matrix binding proteins (7–10). Binding of integrins to extracellular matrix proteins initiates signals that promote cell migration and differentiation. As EVT differentiate and become capable of migration and invasion, they undergo an epithelial to endothelial phenotype transformation (11–14), in which they gain expression of α1β1 (8, 15). Moreover, interaction of α1β1 with collagen (16) and α5β1 with fibronectin (17) promoted, while inhibition of α1β1 and α5β1 decreased, human trophoblast migration and invasion as assessed in vitro (11, 13, 18). α1β1 expression by and invasion of EVT were also suppressed in culture by blocking binding of VEGF-A to its receptor, consistent with the concept that VEGF promotes trophoblast α1β1 expression and invasion (14, 19, 20). In clinical problems of pregnancy associated with defects in vessel remodeling, e.g. preeclampsia, there is misexpression of VEGF and α1β1 and up-regulation of the soluble truncated VEGF sflt-1 receptor (sflt-1), which binds to and interferes with the bioavailability of VEGF (14, 21–24).
Considering the link between VEGF, integrins, and trophoblast invasion, the present study was conducted to test the hypothesis that the subnormal expression of placental extravillous VEGF mRNA exhibited in baboons, in which uterine artery transformation is suppressed in early pregnancy by prematurely elevating estradiol, is associated with an alteration in VEGF protein and integrin expression. Therefore, proximity ligation assay (PLA), a PCR-based immunofluorescence method, was employed to quantify in situ VEGF protein expression in, while α1β1, α5β1, and αvβ3 integrin mRNA levels were quantified in cells isolated from, the placenta of baboons in which EVT invasion and remodeling of the uterine spiral arteries had been suppressed by prematurely elevating the levels of estradiol in early pregnancy.
Materials and Methods
Animals
Female baboons (Papio anubis) were procured from the Southwest National Primate Research Center (San Antonio, TX), housed individually in large primate cages, and received standard primate chow (Harlan Primate Diet, Madison, WI) and fresh fruit twice daily, a multivitamin daily, and water ad libitum. Females were paired with male baboons for 5 d at the anticipated time of ovulation as estimated by menstrual cycle history and the pattern of external sex skin turgescence. Day 1 of pregnancy was designated as the day preceding perineal deturgescence. Baboons were cared for and used strictly in accordance with the United States Department of Agriculture regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academy Press, 7th ed., 1996). The present experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Maternal baboons were untreated (n = 8) or treated daily on d 25–59 with estradiol benzoate (25 μg/kg body weight/d sc in 1.0 ml of sesame oil, n = 7). Placentas were removed by cesarean section after isoflurane anesthetization on d 60 of gestation (length of gestation is 184 d). At 1- to 2-d intervals throughout the study period, baboons were sedated briefly with ketamine HCl (10 mg/kg body weight, im) and a 2- to 4-ml blood sample obtained from a maternal peripheral saphenous vein. At cesarean section, blood samples (2 ml) were obtained from the uterine veins. Serum estradiol levels were determined by RIA using an automated chemiluminescent immunoassay system (Immulite; Diagnostic Products Corp., Los Angeles, CA) as described previously (25).
Placental sampling and uterine artery invasion
A minimum of five randomly selected areas (5 mm3) of placental basal plate were collected from each baboon. Placental samples were fixed in 10% formalin, embedded in paraffin, sectioned (5 μm), and processed for hematoxylin/eosin histology and cytotrophoblast/epithelial cell-specific cytokeratin immunocytochemistry. Light microscopy (Nikon Eclipse E 1000 M; Nikon, Tokyo, Japan) and an image analysis system (IP Lab, version 3.63; Scanalytics, Inc., Fairfax, VA) were used to analyze all arteries more than 25 μm in diameter in each tissue sample. Vessel diameter was assessed via a micrometer as the smallest distance across the center of the lumen from the inside edge of the surrounding smooth muscle (not invaded) or cytotrophoblast (invaded) layers. Each observed artery/arteriole was considered a separate vessel, although any single vessel may have appeared more than once as it traversed a given tissue section. The number of arterioles/arteries exhibiting invasion was quantified by identifying spiral arterioles/arteries, in which the vessel wall was occupied by cytokeratin-positive cytotrophoblasts.
Laser capture microdissection (LCM) of placental cells
Placental cells were isolated from specific compartments of the basal plate by LCM as described previously (26). Briefly, serial sections (8 μm) of placental basal plate were cut longitudinally (to include floating villi, anchoring villi, cytotrophoblastic shell, and decidua basalis) via a Jung Frigocut 2800E cryostat at −20 C (Leica Corp., Deerfield, IL) and mounted onto glass slides. Sections were immediately fixed in 70% ethanol, lightly stained with hematoxylin, and dehydrated in 100% ethanol then xylene. Slides were air dried and transferred to a desiccator at room temperature and an Arcturus PixCell II LCM system equipped with an Olympus Corp. microscope (Arcturus Engineering, Inc., Mountain View, CA) used to capture cells from the anchoring villi, cytotrophoblastic shell, and the floating villi. A laser power of 40–60 mW, duration of 1.5–2.5 msec, and spot size of 15–30 μm was used for cell isolation. Isolated cells were mixed with lysis buffer (RNeasy; QIAGEN, Valencia, CA), microcentrifuged, stored in lysate buffer overnight at −80 C, and RNA extracted within 72 h.
RT-PCR of α1β1, α5β1, and αvβ3 mRNA
Oligonucleotide primers were designed using LightCycler probe design software (Roche Diagnostics Corp., Penzberg, Germany).
α1 primers
Oligonucleotide primers were based on the α1 integrin human gene sequence (NCBI database accession no. NM_181501) and supplied by Integrated DNA Technologies (IDT) (Coralville, IA): 5′-GGGAGAACTTCGGAGTGAAA-3 (3370–3389) and 5′-CCAATCTTCCACAGTGCTAA-3′ (3552–3533).
α5 primers
Oligonucleotide primers were based on the α5 integrin human gene sequence (NCBI database accession no. BC008786) and supplied by IDT: 5′-GCCGTGTGGTTTTAGG-3′ (position 712–727) and 5′-AGCCGTAAGTGAGGTT-3′ (position 966–951).
αv primers
Oligonucleotide primers were based on the αv integrin human gene sequence (NCBI database accession no. NM_002210) and supplied by IDT: 5′-TAGCGTATCTGCGGGATG-3′ (position 1972–1989) and 5′-GTCTTCACCACAGTCAAG-3′ (position 2153–2136).
18S rRNA primers
Oligonucleotide 18S rRNA primers were based on the human gene sequence (NCBI database, accession no. M10098): 5′-TCAAGAACGAAAGTCGGAGG-3′ (positions 1126–1145) and 5′-GGACATCTAAGGGCATCACA-3′ (positions 1614–1595).
RT and real-time PCR
RT of total RNA from LCM isolates was performed according to manufacturer's directions (Invitrogen, Carlsbad, CA). A 13-μl reaction volume containing 1 mm each of deoxy (d)ATP, dCTP, dGTP, and dTTP, 250 ng of random primers, water, and total RNA was incubated at 65 C for 5 min and put on ice for 1 min. Supplied reaction buffer, 0.1 m dithiothreitol, 200 U of Superscript III RT (Invitrogen), and 40 U of RNAguard (Amersham Pharmacia Biotech, Piscataway, NJ) were then added, and the entire mixture was incubated at 25 C for 5 min and 50 C for 60 min. The RT reaction was terminated by heat inactivation at 70 C for 15 min and cooled to 4 C.
mRNA levels were quantified via a LightCycler real-time PCR unit (Roche Diagnostics Corp.) using Fast Start DNA Master SYBR Green I kit for PCR. A 19-μl reaction mix containing either target mRNA- or 18S rRNA-specific primers was combined with 1-μl aliquot of RT reaction for a final volume of 20 μl. The reaction profile consisted of denaturation at 95 C for 8 min, 40 cycles of amplification (95 C for 5 sec, 52 C for 5 sec, and 72 C for 16 sec), and product formation measured and displayed in real time. Amplification cycle annealing temperatures and extension times were adjusted for each set of primers (52–60 C) and products to achieve maximum amplification efficiency. Efficiency-corrected calibrator-normalized relative quantification was performed with LightCycler version 4 software using premade standard curves specific for each product to correct for differences in the efficiencies of target and reference genes and an in-run calibrator to normalize quantification. Specificity of the products was confirmed by melting curve analysis, agarose gel electrophoresis, and inclusion of negative controls with no template or no RT in the reaction.
Immunocytochemistry of α1β1, α5β1, and αvβ3
For antigen retrieval and optimization of specificity, placental tissue sections were boiled in 0.01 m Na citrate for 5 min and cooled at room temperature for 20 min, incubated in H2O2 to inhibit endogenous peroxidase, and blocked with serum-free protein block (Dako Corp., Carpenteria, CA). Tissues were incubated overnight at 4 C with mouse monoclonal antibodies to human α1β1 (1:50 dilution; Upstate Biotechnology, Lake Placid, NY), human α5β1 (1:50 dilution; Chemicon, Temecula, CA), αvβ3 (1:50 dilution; Chemicon), or polyclonal goat cytokeratin (1:1000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Sections were incubated 1 h at room temperature with biotinylated antimouse immunoglobulins (Vector Laboratories, Inc., Burlingame, CA) and 1 h with an avidin-biotin-peroxidase complex (ABC Elite; Vector Laboratories), developed using diaminobenzidine (Sigma, St. Louis, MO) and lightly counterstained with hematoxylin. Negative controls included replacement of the primary antibody with nonimmune IgG or substitution with species inappropriate secondary immunoglobulin (Dako Corp.).
PLA of VEGF
PLA was performed using reagents and directions supplied by Olink Bioscience (Uppsala, Sweden). Paraffin-embedded placental tissue sections (5 μm thick) were subjected to antigen retrieval and protein block and incubated overnight at 4 C with rabbit polyclonal primary VEGF antibody (Zymed/Invitrogen, Carlsbad, CA). Tissue was then incubated for 2 h at 37 C in a humidity chamber with PLA probes consisting of two secondary antirabbit antibodies each tagged with an oligonucleotide (diluted 1:5 in antibody diluent supplied with PLA kit). A hybridization solution consisting of two oligonucleotide linkers, complementary to each PLA probe, was added to tissue for 15 min at 37 C in a humidity chamber. Tissue sections were washed and incubated for 15 min at 37 C with a Duolink Ligation stock (supplied in the PLA kit and diluted 1:5 in water) containing ligase (diluted 1:40 in buffer) and for 90 min with Duolink polymerase (diluted 1:80 in amplification buffer). The ligation step connects the hybridization linkers to the PLA probe oligonucleotides, forming a complete circle connecting both antibodies, and the polymerization step uses rolling circle DNA amplification to generate a concatameric oligonucleotide product linked to the antibody complex. After washing, tissue was incubated for 60 min with a detection solution (diluted 1:5 in water) consisting of fluorescently labeled oligonucleotides which hybridize to the rolling circle amplification product and Hoechst 33342 stain for detection of the nuclei. The tissues were then washed in decreasing concentrations of sodium citrate buffer containing 0.05% Tween 20, washed in 70% ethanol, dried, and coverslipped with mounting media. VEGF protein red PLA signals were visualized by fluorescence microscopy (Nikon Eclipse E 1000) and image-analysis software (IP Lab). PLA signals were quantified and expressed per nuclear area using ImageJ software (version 1.41o, National Institutes of Health open software) in a minimum of five randomly selected areas (32,350 μm2) of the proximal and distal regions of the anchoring villi of the placental basal plate and the floating villi. Negative controls for PLA included omission of the primary antibody or the PLA probe.
Statistical analysis
Data were expressed as the mean ± se. Serum estradiol levels, placental VEGF protein expression by PLA, and placental integrin mRNA expression were analyzed by ANOVA with post hoc comparisons of the means by Newman-Keuls multiple comparison test. Spiral artery invasion and tissue weight data were analyzed by Student's t test.
Results
Serum estradiol levels
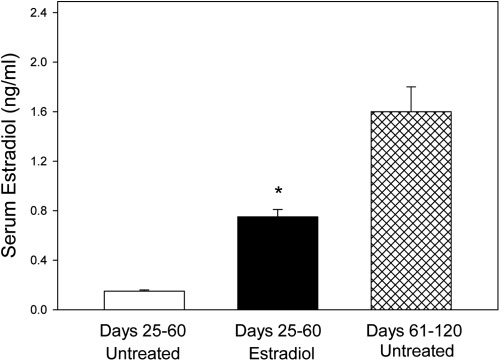
Overall mean (±se) level of maternal serum estradiol in baboons treated with estradiol on d 25–59 of gestation (0.75 ± 0.06 ng/ml) was 5-fold greater (P < 0.01) than in untreated animals on d 25–60 (0.15 ± 0.01 ng/ml) but 50% lower (P < 0.01) than in a contemporaneous cohort of untreated baboons in our primate colony on d 61–120 of the second trimester of normal pregnancy (1.6 ± 0.2 ng/ml) (Fig. 1). Maternal saphenous vein and uterine vein serum estradiol levels on d 60 were approximately 5- and 2-fold greater (P < 0.01), respectively, in estradiol-treated than in untreated baboons, whereas placental and fetal body weights were unaltered (Table 1).
Fig. 1.
Maternal peripheral serum estradiol levels (mean ± se) on d 25–60 of gestation in baboons untreated (n = 8) or treated with estradiol benzoate (25 μg/kg body weight, sc) daily on d 25–59 of gestation (n = 7). *, Different (P < 0.01) from respective value in animals untreated on d 25–60 and in a contemporaneous cohort of untreated baboons on d 61–120 of gestation from our primate colony (ANOVA and Newman-Keuls multiple comparison statistic).
Table 1.
Maternal serum estradiol levels and placental and fetal body weights in baboons
| Treatment | N | Estradiol (ng/ml) |
Placental weight (g) | Fetal body weight (g) | |
|---|---|---|---|---|---|
| Saphenous vein | Uterine vein | ||||
| Untreated | 8 | 0.17 ± 0.02 | 0.60 ± 0.06 | 31.5 ± 1.6 | 12.0 ± 0.4 |
| Estradiol | 7 | 0.77 ± 0.06a | 1.10 ± 0.18a | 28.9 ± 1.2 | 11.6 ± 1.1 |
Mean ± se maternal saphenous and uterine vein serum estradiol levels and placental and fetal body weights on d 60 of gestation in baboons untreated or treated daily on d 25–59 of gestation (term, 184 d) with estradiol benzoate (25 μg/kg body weight/d, sc).
Values different (P < 0.01) than in untreated baboons (unpaired t test).
Histology of placental basal plate
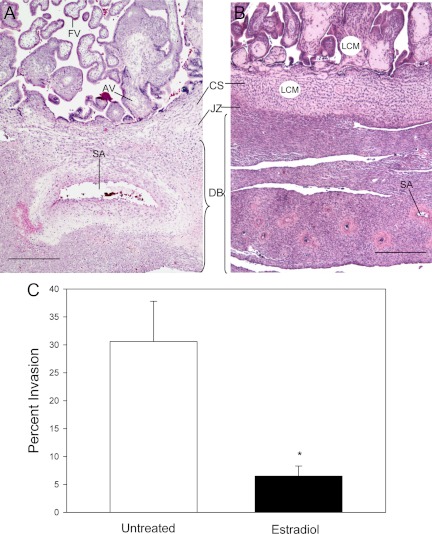
Figure 2 illustrates the histology of the placental basal plate in representative untreated and estradiol-treated baboons. The striking structural modification of a uterine spiral artery into a low-resistance high-capacity vessel in the untreated baboon (Fig. 2A) contrasts with the intact coiled high-resistance low-capacity spiral arteries retained in the decidua basalis of the estradiol-treated animal (Fig. 2B).
Fig. 2.
Histology of placental basal plate on d 60 of gestation in baboons untreated (A) or treated on d 25–59 of gestation with estradiol (B). SA, Spiral artery; FV, floating villi; AV, anchoring villi; CS, cytotrophoblastic shell; JZ, junctional zone; DB, decidua basalis. Representative areas of cells isolated by LCM from the AV and CS are illustrated by white circles. C, Percent invasion of uterine spiral arteries (i.e. number of vessels exhibiting trophoblast invasion divided by total number of vessels counted) of greater than 25 μm in diameter on d 60 of gestation in baboons untreated (n = 8) or treated with estradiol daily on d 25–59 (n = 7). *, Different (P < 0.01) from value in untreated animals (unpaired Student's t test). Magnification scale bars, 400 μm.
The level of EVT uterine spiral artery invasion on d 60 of gestation for all vessels of greater than 25 μm in diameter, expressed as the mean (±se) percentage of the total number of vessels counted, was approximately 5-fold lower (P < 0.01) in estradiol-treated (6.5 ± 1.8) than in untreated (30.6 ± 7.2) baboons (Fig. 2C).
VEGF protein expression by PLA
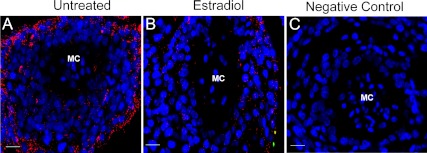
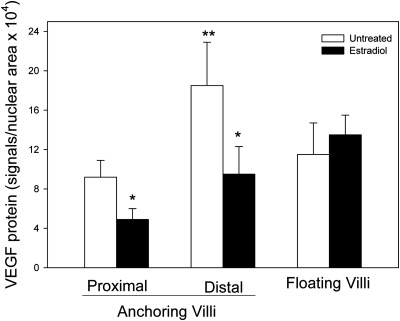
VEGF protein, detected by small red immunofluorescent PLA signals, was abundant on d 60 of gestation in the trophoblast, but not mesenchymal core, of the anchoring villi of untreated baboons (Fig. 3A) and markedly reduced in estradiol-treated animals (Fig. 3B). VEGF protein expression, quantified as the number of PLA signals per nuclear area × 104, was 2-fold greater (P < 0.03) in cells of the distal (18.5 ± 4.4) compared with the proximal (9.2 ± 1.7) region of the anchoring villi of untreated animals (Fig. 4). However, VEGF protein levels were 50% lower (P < 0.05) in both the proximal (4.9 ± 1.1) and distal (9.5 ± 2.8) regions of the anchoring villi of the placenta of estradiol-treated baboons than in untreated animals. In contrast, VEGF protein expression in the trophoblast layer covering the floating villi of the villous placenta was not significantly different in estradiol-treated and untreated animals (Fig. 4).
Fig. 3.
VEGF protein assessed by PLA in anchoring villi of the placenta on d 60 of gestation in representative baboons untreated (A) or treated with estradiol on d 25–59 of gestation (B). Each red PLA signal represents a single molecule of VEGF protein detected by primary VEGF antibody tagged with secondary antibody conjugated to fluorescently labeled oligonucleotide. Nuclei are labeled blue. MC, Mesenchymal core. C, Negative control with omission of primary VEGF antibody. Magnification scale bars, 20 μm.
Fig. 4.
VEGF protein expression quantified by PLA in the placental anchoring and floating villi on d 60 of gestation in baboons untreated (n = 8) or treated with estradiol daily on d 25–59 (n = 7). *, Different (P < 0.05) from respective values in untreated animals (ANOVA and Newman-Keuls multiple comparison statistic); **, different (P < 0.03) from value in proximal anchoring villi of untreated baboons.
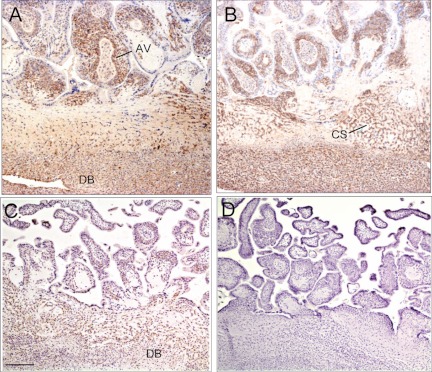
Integrin protein localization by immunocytochemistry
α1β1 (Fig. 5A), α5β1 (Fig. 5B), and αvβ3 (Fig. 5C) proteins were localized by immunocytochemistry in abundant level in cells within the anchoring villi, cytotrophoblastic shell, and decidua basalis of untreated baboons on d 60 of gestation (Fig. 5). Based on this pattern of integrin immunolocalization, cells were isolated by LCM from the anchoring villi and cytotrophoblastic shell for mRNA quantification by RT-PCR.
Fig. 5.
Immunocytochemical localization of α1β1 (A), α5β1 (B), and αvβ3 (C) in the placenta on d 60 of gestation in representative untreated baboons. D, Negative control in which primary α1β1 antibody was omitted from the reaction. Magnification scale bar, 200 μm. AV, Anchoring villi; CS, cytotrophoblastic shell; DB, decidua basalis.
Integrin mRNA expression by RT-PCR
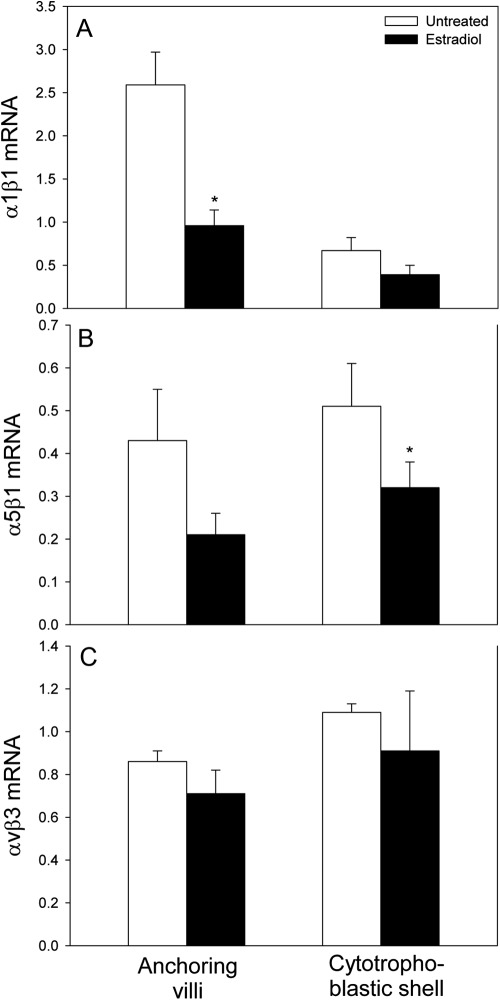
α1β1 mRNA level, expressed as a ratio of 18S rRNA, in cells isolated by LCM from the anchoring villi (0.96 ± 0.18) of estradiol-treated baboons was approximately 2.5-fold (P < 0.01) lower than in untreated animals (2.59 ± 0.38) (Fig. 6A). α1β1 mRNA levels were 2-fold, but not significantly, lower in the cytotrophoblastic shell of estradiol-treated animals. In contrast, α1β1 mRNA expression in trophoblasts captured from the villous placenta/floating choronic villi was almost 3-fold greater (P < 0.01) after estradiol administration (1.41 ± 0.26) than in untreated baboons (0.48 ± 0.04). α5β1 mRNA levels in cells isolated from the anchoring villi (0.21 ± 0.05) and cytotrophoblastic shell (0.32 ± 0.06) of estradiol-treated animals were 2-fold (P = 0.08) and 40% (P < 0.05) lower, respectively, than in cells captured from the anchoring villi (0.43 ± 0.12) and cytotrophoblastic shell (0.51 ± 0.10) of untreated baboons (Fig. 6B). In contrast to the decline in α1β1 and α5β1 mRNA expression, αvβ3 mRNA levels in cells obtained from the anchoring villi and cytotrophoblastic shell were not significantly different in untreated and estradiol-treated baboons (Fig. 6C).
Fig. 6.
α1β1 (A), α5β1 (B), and αvβ3 (C) mRNA/18S rRNA, quantified by real-time RT-PCR, in cells isolated by LCM from the placental anchoring villi (proximal and distal regions collectively) and cytotrophoblastic shell of untreated (n = 8) and estradiol-treated (n = 7) baboons. *, Different (P < 0.01) from respective values in untreated animals (ANOVA and Newman-Keuls multiple comparison test).
Discussion
The present study shows that extravillous placental expressions of VEGF and the α1β1 and α5β1 integrins were decreased in baboons in which EVT invasion and remodeling of the uterine spiral arteries were suppressed by prematurely elevating estradiol levels in the first trimester of pregnancy. We have previously shown that EVT are the primary cells that comprise the anchoring villi and cytotrophoblastic shell of the extravillous placenta in early baboon pregnancy (2). Thus, estrogen appears to down-regulate VEGF and α1β1/α5β1 expression by placental EVT in early primate pregnancy. Moreover, this effect of estradiol appears to be specific, because extravillous placental αvβ3 expression was unaltered in estradiol-treated baboons. Based on in vitro studies, VEGF has been proposed to play a pivotal role in regulating EVT migration to and remodeling of the uterine arteries (4–6). The up-regulation of VEGF expression in the distal compared with proximal region of the anchoring villi of untreated baboons of the current study is consistent with enhanced ability of EVT to express VEGF and invade uterine arteries as these cells migrate down the extravillous pathway. Also, consistent with the role of VEGF in uterine artery invasion and transformation are reports showing that placental expression and maternal serum levels of VEGF are reduced in those problems of pregnancy, notably preeclampsia (22), which are associated with defective uterine spiral artery remodeling. VEGF appears to modulate the expression of α1β1 (19, 20), and the migration of EVT during the process of vessel invasion involves binding of the cell surface α1β1 and α5β1 integrins to proteins of the extracellular matrix (11, 16, 17). Interestingly, α5β1 binds to and promotes signaling of the VEGF flt-1 receptor, thereby regulating endothelial cell migration, adhesion, and chemotaxis (27–29). VEGF also augmented fibronectin-induced human placental multipotent mesenchymal cell migration and adhesion through α5β1 but not αvβ3 (30). Collectively, on the basis of the latter findings and results of the present study, we propose that the estradiol-induced control of EVT migration and invasion of the uterine spiral arteries in early primate pregnancy is mediated by VEGF, α1β1, and α5β1. Additional studies, e.g. restoration of VEGF in baboons treated with estrogen early in pregnancy, are required to definitively establish the regulatory role of VEGF under these experimental conditions. Moreover, whether VEGF induces the expression of, or the VEGF flt-1 receptor interacts with/binds to, α5β1 and/or α1β1 during the control of EVT migration and vessel transformation by estrogen in baboon pregnancy remains to be determined.
In contrast to the estradiol-induced decline in the expression of VEGF and α1β1 by the extravillous placenta, VEGF (31, 32) and α1β1 (present study) mRNA expression by placental villous trophoblasts was increased by prematurely elevating estradiol levels in early baboon pregnancy. The divergent effects of estradiol on VEGF expression by extravillous and villous placental trophoblasts are also exhibited in other estrogen responsive systems. For example, estradiol increased VEGF transcription in estrogen receptor (ER)α-positive ERβ-positive human MCF-7 breast cancer cells and decreased VEGF expression in ERα-negative ERβ-positive human MDA breast cancer cells (33, 34). Interestingly, human and baboon villous placental trophoblasts are ERα-positive and ERβ-positive, whereas EVT are ERα-negative ERβ-positive (2, 35). Therefore, the differential regulation of VEGF and α1β1 by estradiol in the extravillous and villous placenta may reflect cell-specific presence/absence of the ER subtypes, as well as functional enhancers, coactivators, and/or corepressors that modulate estrogen-regulated target gene transcription (36–38).
We have recently shown that uterine and umbilical artery downstream flow impedance was increased and volume flow decreased and fetal bradycardia induced after serotonin vasochallenge late in gestation in baboons in which trophoblast remodeling of the uterine spiral arteries was repressed by elevating estradiol levels early in gestation (39). It appears, therefore, that suppressing uterine vessel transformation by prematurely increasing estradiol in the first trimester of baboon pregnancy disrupts uteroplacental blood flow dynamics and fetal homeostasis after vasochallenge in late gestation. Improper uterine vessel remodeling, also referred to as failure of physiologic transformation of the spiral arteries, underlies several complications of human pregnancy, including preeclampsia, intrauterine growth restriction, spontaneous miscarriage, and preterm birth (40–44), and is associated with abnormal uteroplacental blood flow (45, 46). We suggest, therefore, that the results obtained in baboons of the present study translate to the human.
We propose that the low levels of estradiol typical of early human and nonhuman pregnancy permit aggressive EVT migration and remodeling of the uterine spiral arteries, whereas the elevation in endogenous estradiol with advancing normal pregnancy is a physiologically important event which represses and thus controls the extent of uterine vessel transformation. We further suggest that suppressing EVT vessel transformation by shifting the rise in estradiol to early in baboon pregnancy is not a pharmacologic effect, because the levels of estradiol achieved by estradiol administration in the first third of gestation were substantially lower than the endogenous level exhibited in the second third of normal baboon pregnancy. This aspect of the physiology of pregnancy takes on added biomedical significance considering circumstances in which there would be an elevation in estradiol or enhanced ER signaling in early human pregnancy. For example, multiple follicle induction during in vitro fertilization regiments is associated with elevated levels of estradiol in the first trimester (47, 48). The latter situation, as well as exposure to environmental endocrine disruptors that enhance ER-mediated estrogen action (49, 50) early in pregnancy, would be expected to suppress uterine vessel transformation. In contrast, excessive remodeling of the uterine arteries, a condition which occurs in placental acreta (51), conceivably would disrupt vasoregulatory mechanisms and lead to uncontrolled bleeding within the uterine vessel bed after birth.
In our previous and current studies, we have shown that elevating estradiol in early baboon pregnancy markedly suppressed EVT uterine spiral artery invasion and remodeling and that this was associated with a decline in extravillous placental VEGF and integrin expression, increase in uterine vein serum sflt-1 levels (2), elevation in maternal blood pressure, and vasochallenge-induced disruption of uteroplacental blood flow and fetal bradycardia (39). These changes in maternal, placental, and fetal physiology are hallmark features of preeclampsia in human pregnancy. Many other experimental animal models (for review, see Ref. 52), including uterine artery ligation, have been employed in an attempt to experimentally replicate these key features of human preeclampsia. In contrast to the results obtained in baboons of the present study, however, these other experimental approaches have not induced a defect in uterine spiral artery remodeling, a process which appears to have a pivotal role in the etiology of preeclampsia.
In summary, the present study shows that extravillous placental expressions of VEGF and α1β1 and α5β1 integrins were decreased in a cell- and integrin-specific manner in baboons in which EVT invasion and remodeling of the uterine spiral arteries were suppressed by prematurely elevating estradiol levels in early pregnancy. We propose that estrogen normally controls the extent to which the uterine arteries are transformed by placental EVT in primate pregnancy and that VEGF and particular integrin extracellular remodeling molecules mediate this process.
Acknowledgments
We thank Wanda H. James for secretarial assistance with the manuscript.
This work was supported by National Institutes of Health Research Grant R01 HD13294.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- d
- Deoxy
- ER
- estrogen receptor
- EVT
- placental extravillous trophoblast
- IDT
- Integrated DNA Technologies
- LCM
- laser capture microdissection
- PLA
- proximity ligation assay
- sflt-1
- soluble truncated VEGF sflt-1 receptor
- VEGF
- vascular endothelial cell growth factor.
References
- 1. Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. 2006. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 27:483–490 [DOI] [PubMed] [Google Scholar]
- 2. Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. 2008. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 149:5078–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cross JC, Werb Z, Fisher SJ. 1994. Implantation and the placenta: key pieces of the development puzzle. Science 266:1508–1518 [DOI] [PubMed] [Google Scholar]
- 4. Aplin JD, Haigh T, Lacey H, Chen CP, Jones CJ. 2000. Tissue interactions in the control of trophoblast invasion. J Reprod Fertil Suppl 55:57–64 [PubMed] [Google Scholar]
- 5. Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. 2003. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang 2, PIGF, and VEGF-C. Dev Biol 263:114–125 [DOI] [PubMed] [Google Scholar]
- 6. Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S. 2004. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod 10:229–235 [DOI] [PubMed] [Google Scholar]
- 7. Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, Gotsch F, Than NG, Papp Z, Jai Kim C. 2006. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med 34:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aplin JD. 1993. Expression of integrin α6β4 in human trophoblast and its loss from extravillous cells. Placenta 14:203–215 [DOI] [PubMed] [Google Scholar]
- 9. Blankenship TN, Enders AC, King BF. 1993. Trophoblastic invasion and the development of uteroplacental arteries in the macaque: immunohistochemical localization of cytokeratins, desmin, type IV collagen, laminin, and fibronectin. Cell Tissue Res 272:227–236 [DOI] [PubMed] [Google Scholar]
- 10. Qin L, Wang YL, Bai SX, Ji SH, Qiu W, Tang S, Piao YS. 2003. Temporal and spatial expression of integrins and their extracellular matrix ligands at the maternal-fetal interface in the rhesus monkey during pregnancy. Biol Reprod 69:563–571 [DOI] [PubMed] [Google Scholar]
- 11. Aplin JD, Haigh T, Jones CJ, Church HJ, Viovac L. 1999. Development of cytotrophoblast columns from explanted first trimester human placental villi: role of fibronectin and integrin α5β1. Biol Reprod 60:828–838 [DOI] [PubMed] [Google Scholar]
- 12. Burrows TD, King A, Loke YW. 1994. Expression of adhesion molecules by endovascular trophoblast and decidual endothelial cells: implications for vascular invasion during implantation. Placenta 15:21–33 [DOI] [PubMed] [Google Scholar]
- 13. Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. 1997. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest 99:2139–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. 2002. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 160:1405–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damsky CH, Fitzgerald ML, Fisher SJ. 1992. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 89:210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. 1994. Integrin switching regulates normal trophoblast invasion. Development 120:3657–3666 [DOI] [PubMed] [Google Scholar]
- 17. Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Iwashita M. 2004. The role of α5β1-integrin in the IGF-I-induced migration of extravillous trophoblast cells during the process of implantation. Mol Hum Reprod 10:91–97 [DOI] [PubMed] [Google Scholar]
- 18. Irving JA, Lala PK. 1995. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGFβ, IGF-II, and IGFBP-1. Exp Cell Res 217:419–427 [DOI] [PubMed] [Google Scholar]
- 19. Witmer AN, van Blijswijk BC, van Noorden CJ, Vrensen GF, Schlingemann RO. 2004. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-A. J Histochem Cytochem 52:39–52 [DOI] [PubMed] [Google Scholar]
- 20. Fukushima K, Miyamoto S, Tsukimori K, Kobayashi H, Seki H, Takeda S, Kensuke E, Ohtani K, Shibuya M, Nakano H. 2005. Tumor necrosis factor and vascular endothelial growth factor induce endothelial integrin repertories, regulating endovascular differentiation and apoptosis in a human extravillous trophoblast cell line. Biol Reprod 73:172–179 [DOI] [PubMed] [Google Scholar]
- 21. Zhou Y, Genbacev O, Fisher SJ. 2003. The human placenta remodels the uterus by using a combination of molecules that govern vasculogenesis or leukocyte extravasation. Ann NY Acad Sci 995:73–83 [DOI] [PubMed] [Google Scholar]
- 22. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. 2003. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. 2004. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350:672–683 [DOI] [PubMed] [Google Scholar]
- 24. Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. 2004. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 89:770–775 [DOI] [PubMed] [Google Scholar]
- 25. Albrecht ED, Aberdeen GW, Pepe GJ. 2000. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 182:432–438 [DOI] [PubMed] [Google Scholar]
- 26. Niklaus AL, Aberdeen GW, Babischkin JS, Pepe GJ, Albrecht ED. 2003. Effect of estrogen on vascular endothelial growth/permeability factor expression by glandular epithelial and stromal cells in the baboon endometrium. Biol Reprod 68:1977–2004 [DOI] [PubMed] [Google Scholar]
- 27. Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K, Aziz S, Cardona C, Hammond WP, Savidge GF, Rafii S, Sobel M. 2002. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res 91:25–31 [DOI] [PubMed] [Google Scholar]
- 28. Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. 2006. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res 99:853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orecchia A, Lacal PM, Schietroma C, Morea V, Zambruno G, Failla CM. 2003. Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the α5β1 integrin. J Cell Science 116:3479–3489 [DOI] [PubMed] [Google Scholar]
- 30. Lee MY, Huang JP, Chen YY, Aplin JD, Wu YH, Chen CY, Chen PC, Chen CP. 2009. Angiogenesis in differentiated placental multipotent mesenchymal stromal cells is dependent on integrin α5β1. PLoS ONE 4:e6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albrecht ED, Robb VA, Pepe GJ. 2004. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab 89:5803–5809 [DOI] [PubMed] [Google Scholar]
- 32. Robb VA, Pepe GJ, Albrecht ED. 2004. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod 71:1694–1698 [DOI] [PubMed] [Google Scholar]
- 33. Coradini D, Pellizzaro C, Speranza A, Daidone MG. 2004. Hypoxia and estrogen receptor profile influence the responsiveness of human breast cancer cells to estradiol and antiestrogens. Cell Mol Life Sci 61:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JE, Chung KW, Han W, Kim SW, Kim SW, Shin HJ, Bae JY, Noh DY. 2004. Effect of estrogen, tamoxifen and epidermal growth factor on the transcriptional regulation of vascular endothelial growth factor in breast cancer cells. Anticancer Res 24:3961–3964 [PubMed] [Google Scholar]
- 35. Bukovsky A, Caudle MR, Cekanova M, Fernando RI, Wimalasena J, Foster JS, Henley DC, Elder RF. 2003. Placental expression of estrogen receptor β and its hormone binding variant-comparison with estrogen receptor α and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod Biol Endocrinol 1:36–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bi S, Gavrilova O, Gong DW, Mason MM, Reitman M. 1997. Identification of a placental enhancer for the human leptin gene. J Biol Chem 272:30583–30588 [DOI] [PubMed] [Google Scholar]
- 37. Hu X, Lazar MA. 2000. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11:6–10 [DOI] [PubMed] [Google Scholar]
- 38. Yoon HG, Wong J. 2006. The corepressors silencing mediator or retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist and antagonist-regulated transcription by androgen receptor. Mol Endocrinol 20:1048–1060 [DOI] [PubMed] [Google Scholar]
- 39. Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. March 16, 2012. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. Am J Physiol Heart Circ 10.1152/ajpheart.00590.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khong TY, De Wolf F, Robertson WB, Brosens I. 1986. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93:1049–1059 [DOI] [PubMed] [Google Scholar]
- 41. Khong TY, Liddell HS, Robertson WB. 1987. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol 94:649–655 [DOI] [PubMed] [Google Scholar]
- 42. Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. 2002. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 187:1137–1142 [DOI] [PubMed] [Google Scholar]
- 43. Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. 2011. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruption placentae. Best Pract Res Clin Obstet Gynaecol 25:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brosens I, Pijnenborg R, Vercruysse L, Romero R. 2011. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 204:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brosens JJ, Pijnenborg R, Brosens IA. 2002. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187:1416–1423 [DOI] [PubMed] [Google Scholar]
- 46. Pijnenborg R, Vercruysse L, Hanssens M. 2006. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27:939–958 [DOI] [PubMed] [Google Scholar]
- 47. Paulson RJ, Sauer MV, Lobo RA. 1990. Embryo implantation after human in vitro fertilization: importance of endometrial receptivity. Fertil Steril 53:870–874 [DOI] [PubMed] [Google Scholar]
- 48. Simon C, Domínguez F, Valbuena D, Pellicer A. 2003. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab 14:197–199 [DOI] [PubMed] [Google Scholar]
- 49. McLachlan JA. 2001. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22:319–341 [DOI] [PubMed] [Google Scholar]
- 50. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. 2003. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 189:1063–1069 [DOI] [PubMed] [Google Scholar]
- 52. McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. 2011. Animal models of preeclampsia; uses and limitations. Placenta 32:413–419 [DOI] [PubMed] [Google Scholar]