Abstract
Although several lines of evidence have indicated that menopause is associated with increased susceptibility to neurological disorders, the mechanisms involved in this phenomenon remain to be elucidated. Because neuroinflammation is a common feature of a number of brain diseases, we hypothesized that the cessation of ovarian functions and the consequent decrease in estrogen receptor (ER)-mediated antiinflammatory activity may represent a trigger for postmenopausal brain dysfunctions. The aim of the present study was to investigate the effects of aging and surgical menopause on the activity of ER in neuroinflammation. The present study shows that ER genes are expressed in the hippocampus, but ER transcriptional activity decreases significantly beginning at 12 months of age in intact and ovariectomized mice. With ovariectomy, we observe an age-dependent accumulation of mRNA encoding inflammatory mediators (e.g. TNFα, IL1β, and macrophage inflammatory protein-2) and changes in the morphology of astroglia and microglia. In addition, we show that aging itself is coupled with an exaggerated response to acute inflammatory stimuli with a major accumulation of TNFα, IL1β, macrophage inflammatory protein-2, and macrophage chemoattractant protein-1 mRNA in response to lipopolysaccharide administration. The response to acute inflammatory stimuli appears to be differentially modulated by the duration of hormone deprivation in 12-month-old mice. Taken together, the present results show that aging is associated with decreased ER activity, despite continuous ER synthesis, and that age-dependent neuroinflammation is strongly influenced by hormone deprivation.
Aging is characterized by a systemic low-grade inflammatory status (1). In women, menopause contributes to a dysregulated production of inflammatory molecules and an increase in circulating cytokines, which amplify the basal state of inflammation (2). These changes may extend to the brain and confer a higher susceptibility to brain-related inflammatory disorders and neurodegeneration. Scientists widely accept that estrogen receptors (ER) act as natural antiinflammatory agents in immune cells of the monocyte lineage (3, 4). Thus, a decline in circulating estrogens could further exacerbate the state of inflammation connected with aging and precipitate the onset of neurological dysfunctions that are associated with menopause. Several lines of evidence have shown that estrogens are neuroprotective (5, 6), and an involvement of sex hormones may explain the sexual dimorphism in neurological disorders in which neuroinflammation plays a significant role, such as amyotrophic lateral sclerosis and Alzheimer's disease (7, 8). The cellular and molecular mechanisms of the protective effects of estrogen are not fully understood. Studies have established that the two receptors for the hormone, ERα and ERβ, are expressed in glia and neurons (9), where they regulate cell functions through either direct control of the expression of specific target genes or by the modulation of intracellular transduction pathways via interactions with plasma or nuclear proteins (10). Several groups have demonstrated that 17β-estradiol (E2) and ER in astrocytes and microglia repress the release of proinflammatory compounds (11–14) and nuclear factor (NF)-κB transcriptional activity in response to inflammatory stimuli (15, 16). In neurons, the neuroprotective actions of ERα and ERβ have been associated with their ability to control several genes in the apoptotic cascade (17–20). Thus, a better comprehension of the extent to which ER antiinflammatory activity is responsible for the neuroprotective actions of ER may help to improve the neurological effects of current hormone replacement therapies, which are still controversial (21–24). Indeed, the beneficial effects of hormone therapies on cognitive functions and neurodegeneration were initially suggested by a series of observational studies; however, the results were not supported by the first results of the Women's Health Initiative (25–29). More recently, a meta-analysis of the Women's Health Initiative study in women of different ages showed that the benefits of estrogen administration were associated with treatments initiated near the onset of menopause, but not in women treated years after the cessation of menses (24, 30). Whether the reduced beneficial effects of hormone replacement therapy are caused by compensatory mechanisms that are set in motion and accumulate with time after menopause or by a reduced stimulation, activity, or expression of ER is not known.
The present study was devised to clarify the effects of aging and the cessation of ovarian functions on brain ER transcriptional activity and the ability of ER to control the inflammatory response. We used the estrogen response element (ERE)-Luc reporter mouse as a well-characterized indicator of the state of ER activation. Furthermore we focused on the hippocampus because it was previously shown as a relevant target for ER antiinflammatory activity (31) and because in this brain region ER expression is modulated by aging (32, 33). Neuroinflammation increases with age, and the present results reveal that ER activity in the brain decreased with aging to the same extent in intact and ovariectomized (OVX) mice. OVX amplifies the activation of glial cells in aged mice but not in young mice, which may indicate that the lack of ovarian hormones per se is not sufficient to significantly increase basal neuroinflammation. A lack of ovarian hormones, however, causes changes in the immune response that may synergize with the aging process and offset the balance between inflammatory and antiinflammatory mechanisms.
Materials and Methods
The animals used in this study were heterozygous female (and males for Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) C57BL/6 repTOP-ERE-Luc mice (Transgenic Operative Products srl; Lodi, Italy) that were bred in our facilities. Each of the three aging experiments shown here was planned in advance, and all of the mice that were used in each study were generated during the same period of time by a large number of breeding pairs. We ensured that littermates were distributed among the different experimental groups to avoid confounding issues related to genetic or environmental variability. The mice were aged in our facilities, housed in ventilated plastic cages with hardwood chip bedding, and provided filtered water ad libitum. The animal room was maintained within a temperature range of 22–25 C under an automatic 12-h light, 12-h dark cycle (lights on at 0700 h).
The mice were anesthetized with an sc injection of 50 μl of ketamine (93.6 mg/kg, Ketavet 100; Intervet, Milan, Italy) and xylazine (7.2 mg/kg, Rompun; Bayer, Milan, Italy) solution and then OVX or sham operated at 5 months of age for long-term OVX (LT-OVX) or 1 month before euthanasia for short-term OVX (ST-OVX). The phase of the reproductive cycle of the sham mice was assessed by vaginal smears, which were air dried and stained using the May-Grünwald-Giemsa method (MGG Quick Stain Kit; Bio-Optica, Milan, Italy) following the protocol provided by the manufacturer. ERE-Luc mice were maintained on the Mucedola 4RF21 diet. The mice were shifted 1 wk before the experiment to an estrogen-free diet (AIN93M, Mucedola, Settimo Milanese, Italy). At the required time point, the mice were anesthetized [ketamine 93.6 mg/kg (Intervet) and xylazine 7.2 mg/kg (Bayer)], the photon emission was quantified, and the animals were euthanized by cervical dislocation.
All of the mouse experiments were carried out according to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institute of Health and in accordance with the European Guidelines for Animal Care and Use of Experimental Animals. In addition, the experiments were approved by the Italian Ministry of Research and University and controlled by a panel of experts at the Department of Pharmacological Sciences (University of Milan, Milan, Italy).
Intracerebroventricular (icv) injections of lipopolysaccharide (LPS)
To avoid infections from the icv injection of LPS, the mice were treated sc with the antibiotic Baytril (0.1%, 5 μl/g/d; Bayer) for 3 d before the experiment.
Escherichia coli LPS [serotype 0.111:B4 (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany), 1 μg of LPS in 1 μl of saline] was injected into the third cerebral ventricle according to specific stereotaxic coordinates (bregma, −0.25 mm; lateral, 1 mm; depth, 2.25 mm) while the mice were under deep anesthesia [ketamine, 93.6 mg/kg (Intervet), and xylazine, 7.2 mg/kg (Bayer)] (3). The stereotaxic coordinates that we used were initially determined using a stereotaxic atlas (34; Figs. 33–34 between 3.58 and 3.34 mm from the interaural line) and experimentally validated. The mice were killed 3 h after the icv injection by cervical dislocation. The tissues were collected for gene expression analyses and immediately frozen on dry ice and stored at −80 C until subsequent analysis.
All of the experiments were repeated three times, and each experimental group consisted of at least four mice.
Ex vivo bioluminescence imaging
Bioluminescence imaging of freshly dissected brain slices was carried out in ERE-Luc mice as previously described (35).
Immunohistochemistry
The mice were killed by cervical dislocation under deep anesthesia [ketamine 93.6 mg/kg (Intervet) and xylazine 7.2 mg/kg (Bayer)]. The brains were removed and postfixed in 4% paraformaldehyde, cryoprotected, snap frozen in liquid nitrogen, and stored at −80 C until they were analyzed. Using a cryostat (Microm; Walldorf, Germany), coronal sections (30-μm-thick) were collected. Three free-floating sections were analyzed by immunohistochemistry. Before the immunological assay, the sections were washed three times with Tris-buffered saline, incubated for 10 min in 3% H2O2 in H2O at room temperature to inhibit endogenous peroxidases, and permeabilized (0.1% Triton X-100) and washed again. The sections were then incubated for 2 h at room temperature with primary antibodies against glial fibrillary acidic protein (GFAP, 2.9 μg/ml; Dako Italia S.p.A, Milan, Italy) or macrophage antigen complex-1 (Mac-1, 2 μg/ml; Serotec, Oxford, UK) diluted in Tris-buffered saline containing 10% normal goat serum for GFAP and normal rabbit serum for the Mac-1 antibody. Biotinylated goat antirabbit and rabbit antirat secondary antibodies (Vector Laboratories, Inc., Burlingame, CA) were used for GFAP and Mac-1 detection, respectively. Immunoreactivity amplification was performed with the avidin-biotin-peroxidase (Vectastain ABC kit, Vector Laboratories) and developed with 3,3′-diaminobenzidine substrate (0.7 mg/ml; Sigma-Aldrich).
The staining was captured with a ×4000 (Fig. 5, uppercase letters) or ×6300 (Fig. 5, lowercase letters) magnification using a Zeiss Axioscope microscope equipped with a digital camera (Carl Zeiss, Thornwood, NY).
Fig. 5.
Aging and OVX modify microglia and astrocyte immunoreactivity and morphology in the mouse hippocampus. Representative photomicrographs were taken from the hippocampi of sham (Aa, Cc, E, and G) or OVX (Bb, Dd, F, and H) mice at 6 months (Aa, Bb, E, and F) or 22 months of age (Cc, Dd, G, and H) and analyzed by immunohistochemistry for Mac-1 (A–D, a–d) or GFAP (E–H) expression. The scale bar equals 10 μm.
The area of the cell body of microglia cells and the thickness of the processes of astrocytes were measured on brain sections stained for Mac-1 and GFAP. We used Photoshop to measure the cell body area of microglia and the ImageJ software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) to measure the thickness of astrocyte processes.
Real-time PCR gene expression analysis
The hippocampi were homogenized with a TissueLyser, and the RNA was purified with the RNeasy MiniKit (QIAGEN, Milan, Italy) according to the manufacturer's instructions.
For the preparation of cDNA, 1 μg of RNA was denatured at 75 C for 5 min in the presence of 1.5 μg of random primers in a 15 μl final volume. Deoxynucleotide triphosphate and Moloney murine leukemia virus reverse transcriptase (RT) were added at final concentrations of 0.5 mm and 8 U/μl, respectively, in a final volume of 25 μl. The RT reaction was performed at 37 C for 1 h, and the enzyme was inactivated at 75 C for 5 min. Control reactions without the addition of the RT enzyme were performed for 20% of the samples.
The RT-PCR experiments were performed using TaqMan technology and the TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) to analyze mouse ERα (Mm00433149_m1), ERβ (Mm01281854_m1), progesterone receptor (PgR; Mm00435625_m1), prothymosin α (PTMA; Mm02342432_g1), TNFα (Mm00443258_m1), IL1β (Mm00434228_m1), macrophage chemoattractant protein-1 (MCP1; Mm00441242_m1), macrophage inflammatory protein-2 (MIP2; Mm00436450_m1), and IGF1 (Mm00439561_m1). We used the 18S rRNA VIC-MGB-PDAR (Applied Biosystems) as the reference for all of the assays except ERβ and IGF1 expression analysis. We used 36b4 (Ribosomal protein, large, P0) [forward: 5′-GGCGACCTGGAAGTCCAACT-3′; reverse: 5′-CCATCAGCACCACAGCCTTC-3′; Probe: 5′-ATCTGCTGCATCTGCTTGGAGCCCA-3′), which has a lower expression than the 18S rRNA, as the reference gene for the ERβ and IGF1 expression analyses because of the low expression of the two targets (i.e. to avoid interference between the amplification of the target and the reference gene). The reactions were carried out according to the manufacturer's protocol using a 7900HT fast real-time PCR system (Applied Biosystems, Inc.), and the data were analyzed by using the 2-ΔΔCt method (36).
Statistical analysis
Unless otherwise stated, statistical significance was assessed by a two-way ANOVA with Bonferroni's multiple comparison post hoc test using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results
Brain ER activity, but not its expression, decreases with aging
The effects of aging on the expression of ERα and ERβ in the brain were initially investigated in the mouse hippocampus by measuring ERα and ERβ mRNA in tissues that were dissected from mice at 6, 12, 18, and 22 months of age (Fig. 1). Vaginal smears indicated that female mice were cycling up to about 18 months of age and were generally in permanent diestrus by 22 months. In the present study, cycling mice were all euthanized at metestrus. Figure 1 shows that the ERα and ERβ mRNA levels in the hippocampus did not change with age and were unaffected by the natural blockade of the estrous cycle at 22 months of age. Parallel analysis of ERα and ERβ mRNA levels in the male hippocampus did not show any changes with aging in males (Supplemental Fig. 1).
Fig. 1.
The effects of aging on ERα and ERβ mRNA content in mouse hippocampus. Cycling mice were euthanized at metestrus, and the ERα (panel A) and ERβ (panel B) mRNA contents were measured using real-time PCR of tissue extracts from the hippocampi of sham and OVX female ERE-Luc mice. Data were analyzed with a two-way ANOVA followed by Bonferroni's post hoc test. *, P < 0.05; ** and °°, P < 0.01; and *** and °°°, P < 0.001; data are expressed as mean ± sem (n = 4–9).
To study the effects of surgical menopause on ER expression, mice were OVX at 5 months of age and then euthanized at 6, 12, 18, and 22 months. The ERα and ERβ mRNA content in the hippocampus did not change after the surgery in the younger mice (6 and 12 months), but the mRNA content of both receptors was significantly increased in 18- and 22-month-old mice (+213% and +529%, respectively, for ERα and +265% and +616%, respectively, for ERβ compared with intact mice) (Fig. 1).
To assess whether the OVX-related changes of ERα and ERβ mRNA content reflected the state of ER transcriptional activity, we used the ERE-Luc reporter mouse. In this model, the transgene luciferase is driven by a synthetic promoter that contains multimerized ERE, and the expression of luciferase has been demonstrated to be proportional to the ER transcriptional capacity (37). Despite the lack of an effect of aging on ERα and ERβ mRNA levels, the measurement of photon emission in the hippocampus showed that the transcriptional activity of the ER was significantly decreased with age in intact and OVX mice (Fig. 2A). This age-dependent decrease of ER activity was not found to be a peculiarity of the hippocampus because this effect was observed in all of the other regions of the brain that are known to express ER (Fig. 2B). In particular, we observed that the ER activity at 18 months of age was decreased by approximately 60% in most brain areas compared with the ER activity at 6 months of age. This effect was less pronounced in the hypothalamus and parietal cortex, where the decrease was 42% and 49%, respectively, and stronger in the endopyriform cortex, where the decrease was 80%. The dichotomy between mRNA expression and ER transcriptional activity was found to be a peculiarity of ER because a similar study carried out in the brains of PPAR response element-Luc mice showed a correlation between peroxisome proliferator-activated receptor (PPAR) activity and the mRNA levels of the PPAR most expressed in the brain (PPARβ/δ) (38) (Supplemental Fig. 2).
Fig. 2.
ER activity in the brain decreases with aging. A (left panel), A gray-scale image of the section where the area of the hippocampus used for the measurement of photon emission is identified (white grid); central panel, pseudocolor images associated with photon emission, which were generated by a Night Owl LB981 image processor after 15 min of exposure to a charge-coupled device camera; right panel, semiquantitative analysis of the photon emission in the hippocampus. B, ER activity was measured in selected brain areas of ERE-Luc female mice by optical imaging. Data were analyzed with a two-way ANOVA followed by Bonferroni's post hoc test. + and *, P < 0.05; ** and ++, P < 0.01; and +++, P < 0.001; data are expressed as mean ± sem (n = 4–6).
We proceeded to study the age-dependent reduction in ER transcriptional activity with the analysis of the expression of the endogenous genes targets of ERs, such as PgR and PTMA (39–41), or of genes that are unresponsive to hormonal treatments, such as 36b4 (42). Consistent with the imaging data, the PgR mRNA levels in the OVX mice were significantly lower in the aged mice compared with the younger mice (Fig. 3A). No effect of age or OVX was observed when we measured the mRNA of the proliferation marker PTMA (Fig. 3B) or 36b4 (Fig. 3C). The finding that 36b4 gene expression was not affected by age suggested that the overall transcriptional activity of hippocampal cells is preserved with aging. The differential response that was observed for the two ER targets, PgR and PTMA, however, indicated a selective action of aging and OVX on ER brain targets.
Fig. 3.
The effects of aging on the expression of endogenous ER target genes. PgR (A), PTMA (B), and the 36b4 negative control (C) mRNA were measured in the hippocampi of female ERE-Luc mice using real-time PCR. Data were analyzed with a two-way ANOVA followed by Bonferroni's post hoc test. *, P < 0.05; data are expressed as mean ± sem (n = 6–8).
LT-OVX leads to the accumulation of mRNA for inflammatory molecules in the hippocampi of aged mice
To examine the extent to which the decreased ER activity influenced the expression of inflammatory genes, we measured the mRNA levels of genes that encoded inflammatory proteins that had previously been reported to be regulated by estrogens: two cytokines (i.e. TNFα and IL1β) associated with inflammation, and two chemokines (i.e. MIP2 and MCP1) that are markers of microglial and astroglial activation (31, 43–45). The study was carried out by measuring the expression of inflammatory proteins and their mRNA levels in unstimulated mice at 6, 12, and 22 months of age. Because we observed higher variability when we measured the protein levels, which may have been due to protein diffusion through capillaries and brain parenchyma, we focused on the measurement of mRNA levels. Aging per se did not increase the expression of the selected inflammatory genes, with the exception of TNFα. Indeed, the TNFα mRNA level was found to be significantly augmented in mice at 22 months of age (Fig. 4). The expression of TNFα, IL1β, and MIP2 mRNA was significantly increased in 22-month-old OVX mice (Fig. 4). An age-dependent increased state of inflammation was shown using a morphological analysis of microglia and astrocytes. The staining of the microglia in the hippocampal sections was carried out using an antibody against Mac-1, which reacts with the microglia integrin receptor CD11b. The staining of astroglia was performed with an antibody against GFAP, which is an intermediate filament protein that is used as a specific marker for astrocytes. In agreement with prior biochemical results, Mac-1 staining showed an effect of aging. Indeed, the size of the microglia was visibly reduced in 22-month-old mice. In addition, morphometric analysis (Supplemental Fig. 3A) showed that the cell bodies were significantly smaller (−42%) whereas the processes, secondary branches, and lamellipodia appeared to be longer and more slender (Fig. 5, compare panel A, a and panel C, c). In the OVX mice, the ramified processes appeared to be retracted, and they became much thicker compared with the sham-operated mice in both young and aged mice (Fig. 5, panel B, b and panel D, d). Furthermore, the size of the cell body was increased up to 187% in 22-month-old OVX mice compared with sham mice of the same age (Supplemental Fig. 3A). We did not observe major age-related changes in astrocyte morphology (Fig. 5, E and G, and Supplemental Fig. 3B), but OVX resulted in visibly thicker cellular processes typical of reactive astrocytes (Fig. 5, F and H), as demonstrated by subsequent morphometric analysis (+63% in 6-month-old OVX mice and +55% in 22-month-old OVX mice compared with sham mice of the same age) (Supplemental Fig. 3B).
Fig. 4.
The expression of proinflammatory compounds in the hippocampi of female mice before and after OVX. TNFα, IL1β, MIP2, and MCP1 mRNA levels were measured using real-time PCR in the hippocampal tissue extracts of female ERE-Luc mice. Data were analyzed with a two-way ANOVA followed by Bonferroni's post hoc test *, °, and +, P < 0.05; **, P < 0.01; *** and °°°, P < 0.001; data are expressed as mean ± sem (n = 4–6). The experiment was repeated twice with two separate colonies of animals, and the results that are shown are the average of the two experiments.
These observations led us to conclude that the combined effect of aging plus OVX increased the glial cell activation, which raised the basal level of inflammatory molecules.
Effects of aging and OVX on brain susceptibility to acute inflammatory stimulation
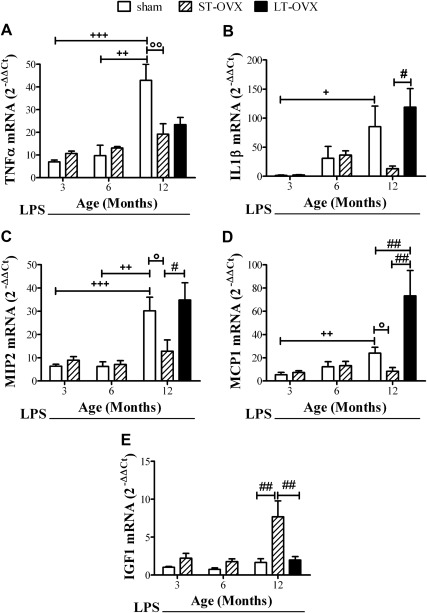
We also examined whether aging plus OVX had any influence on the ability of the hippocampus to respond to acute inflammatory stimuli. To test this, we injected LPS into the third ventricle to induce a neuroinflammatory response (3) (Fig. 6). In sham-operated animals, the accumulation of all of the inflammatory molecules measured in response to LPS was significantly affected by age. In 12-month-old mice, we observed an increased response to LPS that was significantly higher than the responses in 3-month-old mice (fold increase 12 months over 3 months: TNFα, 6.2; IL1β, 56.8; MIP2, 4.8; MCP1, 4.5) (Fig. 6). LT-OVX further increased the effect of LPS on MCP1 gene expression but not on the other cytokines [compare the white (sham) and black (LT-OVX) bars]; indeed in 12-month-old mice, also the basal expression of cytokines was only subtly modified by OVX (Fig. 4). Because OVX did not change the inflammatory response to LPS in 3- and 6-month-old mice (Fig. 6) when the ovariectomy was performed 1 month before the experiment, we investigated whether ST-OVX could further modify the inflammatory response in adult mice (12 months of age). Unexpectedly, the response to LPS was reduced when compared with the sham mice (Fig. 6). To explain this result, we hypothesized the existence of molecules other than the ovarian hormones that are able to activate the unliganded ER in the hippocampus. On the basis of previous reports that have shown that IGF1 is a potent modulator of ER in the nervous system (46), we investigated the effect of ST-OVX on IGF1 mRNA content. In line with our hypothesis, we observed a significant increase in IGF1 mRNA after ST-OVX in 12-month-old mice. In 3- and 6-month-old mice, however, this effect was not significant (Fig. 6).
Fig. 6.
The effects of age and OVX on the response to an acute inflammatory stimulus. TNFα, IL1β, MIP2, MCP1, and IGF1 mRNA levels were measured using real-time PCR in the hippocampi of female ERE-Luc mice treated with LPS. Data are represented as the fold induction with respect to 3-month-old sham mice treated with vehicle (data not shown). Data were analyzed with a two-way ANOVA followed by Bonferroni's post hoc test unless otherwise stated. + and °, P < 0.05; ++ and °°, P < 0.01; +++, P < 0.001; #, P < 0.05; and ##, P < 0.01 (unpaired t test); data are expressed as mean ± sem (n = 4–8).
Discussion
The aim of the present study was to evaluate the ER activity in aging and determine the involvement of ER in the progression and manifestation of age-dependent neuroinflammation in intact and OVX mice.
The present study focused on the hippocampus because it is one of the brain areas that is most relevant for the onset of neurodegenerative diseases, and we uncovered several novel findings: 1) ER activity decreases with aging in the brains of intact and OVX mice; OVX increases the accumulation of mRNAs encoding TNFα, IL1β, and MIP2 in the central nervous system of 22-month-old mice; 2) In 22-month-old mice there is a significant increased response to inflammatory stimuli such as LPS; 3) the length of OVX plays a role in the response to acute inflammation at 12 months of age.
Brain ER activity, but not its expression, decreases with aging
The effects of aging on ER expression and activity in the mammalian brain have long been debated. ERα expression has been shown to be differentially regulated in diverse regions of the hippocampus during aging (32, 33), and ERβ expression has been shown to decrease in the cortex of aging female mice (47). Epigenetic effects were suggested as a potential mechanism regulating ER expression (48–50) with age in different tissues (51, 52), including the brain (53, 54).
The expression of ERα mRNA or protein, however, is not a demonstration of the transcriptional efficiency of the sex hormone receptor on its target genes. The use of the ERE-Luc reporter mouse in the present study was instrumental for the demonstration that age affects ER activity. The imaging methodology here used was previously validated by demonstrating that, in the brain areas analyzed, estrogen treatment increased bioluminescence in parallel with the reporter luciferase in a dose-dependent manner; this effect was blocked by pretreatment with the full antagonist ICI 182,780 (35). We also studied the distribution of bioluminescence in the different phases of the cycle demonstrating that at proestrus ER activity was augmented only in specific brain regions (e.g. hypothalamus); in others (e.g. caudate putamen) the cycle had no effect possibly due to a strong basal activation by nonestrogenic factors known to influence ER (35). This phenomenon may explain why we found a relatively low bioluminescence in regions rich in ER (e.g. hypothalamus) vs. others in which these receptors are less present (e.g. caudate putamen). In fact, the mice were euthanized at metestrus, a phase of the cycle in which circulating estrogens are low and, because of that, we believed is more appropriate to highlight the effect of aging characterized by a decreased ovarian activity also in mice. At the present time, we cannot exclude the presence in the brain of factors other than ER, that are able to activate the reporter promoter.
The ERE-Luc model indicated that, with age, ER activity decreases significantly in the whole hippocampus of intact and OVX animals. This effect was also observed in other brain areas, but not in peripheral organs such as vagina, breast, and liver (data not shown). The localization to the brain suggested that the decreased transcriptional activity of ER is specific to brain tissue and cannot simply be explained by lower hormone levels.
We previously demonstrated that the transcriptional activity of ER in the mouse brain is very high and is hormone dependent (35). Here, we showed that OVX did not translate into a decreased activity of ER in young or aged mice. This observation supports prior findings in the central nervous system that demonstrate that ER are regulated by factors other than ovarian estrogen, such as the local synthesis of steroids or neurotransmitters and growth factors that are able to regulate the unliganded receptor. Indeed, E2 is synthesized in the hippocampus at concentrations that are sufficient for receptor activity (55, 56), and this local synthesis may compensate, in part, for the loss of ovarian functions. In addition to neurosteroids, growth factors (e.g. IGF-1) (57, 58) and neurotransmitters (e.g. dopamine) (59) have been shown to regulate ER activity in neural cells.
Unfortunately, we cannot provide a definitive answer on the effect of aging on each receptor subtype because the reporter system is modulated by both ER (i.e. the luciferase distribution is the result of both ER activities). Because we observed a strong decrease in bioluminescence in brain areas where both ERα and ERβ are present, we concluded that the observed aging effect involved both receptor subtypes. It should be emphasized that the mouse reporter model was conceived to highlight the ER availability for transcription. Thus, the reporter transgene was placed under the control of a simplified synthetic promoter that is highly responsive to activated ER because of the presence of multimerized ERE and insulators that prevent any interference from the chromatin at the transgene integration site. The ERE-Luc reporter thus represents a direct measurement of the state of ER activity (35, 60). In the case of endogenous genes that are regulated by complex promoters, a number of transcription factors may intervene in addition to ER. Thus, it is conceivable that, in a given physiological setting, the association of the activated receptor with its ERE may not be measurable because it is blunted by other prevailing transcription factors. This may explain the lack of a perfect match between the measure of endogenous and surrogate genes.
LT-OVX leads to the accumulation of mRNA for inflammatory molecules in the hippocampi of aged mice
In the present study, we showed that aging per se increased significantly TNFα mRNA; however, the production of inflammatory molecules (TNFα, IL-1β, and MIP2 mRNAs) was very pronounced only in LT-OVX mice 22 months of age. This indicates that the lack of estradiol is not sufficient to increase brain inflammation. A lack of estradiol, however, constitutes an element of dysregulation that synergizes with aging. Our morphological studies showed that microglia acquire a proinflammatory phenotype with aging and OVX, which indicates a role for brain cells in the reported neuroinflammation. We cannot rule out the possible role of peripheral monocytes because of the decreased ability of ER and estrogens to regulate the tight junction proteins (61–63) and blood-brain barrier (BBB) permeability (4), which facilitates the brain penetration of cells involved in systemic age-related inflammation.
The present study does not directly address the molecular mechanisms by which the loss of ovarian function translates into increased neuroinflammation; however, it demonstrates that OVX in aged mice correlates with increased accumulation of inflammatory molecules. The reporter mouse model provides evidence that ER activity decreases with age. Previous reports have shown that both ER may exert antiinflammatory actions via genomic (64–66) and nongenomic mechanisms (13, 15, 16). The exact mechanism by which the age-dependent reduction in ER activity influences neuroinflammation, however, remains to be elucidated.
Effects of aging and OVX on brain susceptibility to acute inflammatory stimulation
To limit the confounding effects of peripheral inflammation and age-related changes in BBB permeability (67), we tested the brain response to acute inflammation in a model system in which LPS was injected into the third ventricle. Using this previously validated model (31), we favored the measurement of inflammatory products that were primarily generated by microglia and astrocytes and limited the effects of peripheral inflammatory cells and their passage through the BBB.
Our results showed that the hippocampal inflammatory response to an acute insult increased with aging and led to the exaggerated accumulation of mRNA encoding TNFα, IL1β, MIP2, and MCP1, which are all relevant factors in the neuroinflammation process and targets of E2/ER-mediated antiinflammatory activity (31, 43–45). The present results are in agreement with several previous studies that have shown that aging leads to an increased response to LPS (68); however, other authors did not observe a similar effect of aging on the response to inflammatory stimuli (69). It is likely that the experimental setting (e.g. the route of LPS administration, the target genes analyzed, and genetics) has a major influence on the inflammatory response, which limits the value of the comparative analyses of certain studies in the literature.
The lack of effect of LT-OVX was unexpected, and this may have resulted because the inflammatory response in the aged animals is much higher than in young mice. Thus, a further increase may not be feasible.
ST-OVX decreased the inflammatory response in aged mice (12 months old) whereas LT-OVX did not. This led us to hypothesize that the sudden disappearance of ovarian functions may trigger a physiological compensatory response with the synthesis of an array of molecules that are able to temporarily buffer the lack of ER stimulation. Over time, however, the compensatory response fades, and ER are not able to overcome inflammation. To test this hypothesis, we investigated the effects of ST-OVX and LT-OVX in the local accumulation of a well-known activator of unliganded ER, IGF1, in 12-month-old mice (46). As shown in Fig, 6E, ST-OVX, but not LT-OVX, was associated with a significant increase in IGF1 mRNA in the 12-month-old mice.
These results provide an explanation for the epidemiological data that show that a long period of hormone deprivation in women increases the risk of neurodegenerative disease (22). In addition, the present results may explain the timing hypothesis, which claims that hormone replacement therapy only exerts beneficial effects when it is initiated near the onset of menopause (in what is currently defined as the “window of opportunity of estrogen therapy”) (24).
Conclusions
The present study fills a gap in our knowledge of estrogen effects by demonstrating that brain ER activity decreases with age. In addition, we show that the presence of peripheral hormones limits the age-related increase of basal neuroinflammation and that the length of hormone deprivation is a determinant for the antiinflammatory actions of ER.
Studies of the effects of long-term hormone replacement therapies on basal neuroinflammation and acute inflammatory responses may lead to the development of more efficacious therapies for postmenopausal neurological disorders.
Supplementary Material
Acknowledgments
We thank L. Brambilla (Salvatore Maugeri Foundation, Pavia, Italy) for her immunohistochemical skills and E. Faggiani and P. Sparaciari (Department of Pharmacological Sciences, Milan, Italy) for technical and veterinary assistance.
This work was supported by the National Institute of Health Grant RO1AG027713 and by grants from the European community [STREP EWA LSHM-CT-2005-518245, IP CRESCENDO LSHM-CT-2005-018652, NoE DIMI LSHB-CT-2005-512146] and by a grant of the Italian Ministry of Health (Ricerca Corrente 2009 in collaboration with the Istituto di Ricovero e Cura a Carattere Scientifico Mondino Foundation).
Disclosure Summary: the authors have nothing to disclose.
Footnotes
- BBB
- Blood-brain barrier
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- GFAP
- glial fibrillary acidic protein
- icv
- intracerebroventricular
- LPS
- lipopolysaccharide
- LT-OVX
- long-term OVX
- MCP1
- macrophage chemoattractant protein-1
- MIP2
- macrophage inflammatory protein-2
- OVX
- ovariectomy/ovariectomized
- PgR
- progesterone receptor
- PPAR
- peroxisome proliferator-activated receptor
- PTMA
- prothymosin α
- RT
- reverse transcriptase
- ST-OVX
- short-term OVX.
References
- 1. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908:244–254 [DOI] [PubMed] [Google Scholar]
- 2. Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ. 2009. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183:1393–1402 [DOI] [PubMed] [Google Scholar]
- 3. Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. 2003. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100:9614–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CM, Mulcahey TA, Filipek NC, Wise PM. 2010. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors α and β. Endocrinology 151:4916–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- 6. Brann D, Raz L, Wang R, Vadlamudi R, Zhang Q. 2012. Oestrogen signalling and neuroprotection in cerebral ischemia. J Neuroendocrinol 24:34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCombe PA, Henderson RD. 2010. Effects of gender in amyotrophic lateral sclerosis. Gend Med 7:557–570 [DOI] [PubMed] [Google Scholar]
- 8. Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. 1999. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology 53:1992–1997 [DOI] [PubMed] [Google Scholar]
- 9. Santagati S, Melcangi RC, Celotti F, Martini L, Maggi A. 1994. Estrogen receptor is expressed in different types of glial cells in culture. J Neurochem 63:2058–2064 [DOI] [PubMed] [Google Scholar]
- 10. Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. 2006. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781 [DOI] [PubMed] [Google Scholar]
- 11. Tenenbaum M, Azab AN, Kaplanski J. 2007. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res 13:158–166 [DOI] [PubMed] [Google Scholar]
- 12. Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. 2010. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-γ-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 58:93–102 [DOI] [PubMed] [Google Scholar]
- 13. Baker AE, Brautigam VM, Watters JJ. 2004. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology 145:5021–5032 [DOI] [PubMed] [Google Scholar]
- 14. Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. 2001. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci 21:1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodel RC, Du Y, Bales KR, Gao F, Paul SM. 1999. Sodium salicylate and 17β-estradiol attenuate nuclear transcription factor NF-κB translocation in cultured rat astroglial cultures following exposure to amyloid A β(1–40) and lipopolysaccharides. J Neurochem 73:1453–1460 [DOI] [PubMed] [Google Scholar]
- 16. Ghisletti S, Meda C, Maggi A, Vegeto E. 2005. 17β-Estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol 25:2957–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meda C, Vegeto E, Pollio G, Ciana P, Patrone C, Pellicciari C, Maggi A. 2000. Oestrogen prevention of neural cell death correlates with decreased expression of mRNA for the pro-apoptotic protein nip-2. J Neuroendocrinol 12:1051–1059 [DOI] [PubMed] [Google Scholar]
- 18. Wang LS, Huang YW, Liu S, Yan P, Lin YC. 2008. Conjugated linoleic acid induces apoptosis through estrogen receptor α in human breast tissue. BMC Cancer 8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, Chandrasiri UP, Toivanen R, Wang Y, Taylor RA, Risbridger GP. 2012. Estrogen receptor-β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc Natl Acad Sci USA 107:3123–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helguero LA, Faulds MH, Gustafsson JA, Haldosén LA. 2005. Estrogen receptors α (ERα) and β (ERβ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene 24:6605–6616 [DOI] [PubMed] [Google Scholar]
- 21. Siegfried T. 2007. Neuroscience: it's all in the timing. Nature 445:359–361 [DOI] [PubMed] [Google Scholar]
- 22. Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., III 2009. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 5:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henderson VW, Brinton RD. 2010. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res 182:77–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rocca WA, Grossardt BR, Shuster LT. 2011. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 1379:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- 26. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- 27. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, et al. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 28. Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. 2004. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- 29. Manson JE, Bassuk SS, Harman SM, Brinton EA, Cedars MI, Lobo R, Merriam GR, Miller VM, Naftolin F, Santoro N. 2006. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause 13:139–147 [DOI] [PubMed] [Google Scholar]
- 30. Sherwin BB. 2006. Estrogen and cognitive aging in women. Neuroscience 138:1021–1026 [DOI] [PubMed] [Google Scholar]
- 31. Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. 2006. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 147:2263–2272 [DOI] [PubMed] [Google Scholar]
- 32. Ishunina TA, Fischer DF, Swaab DF. 2007. Estrogen receptor α and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiol Aging 28:1670–1681 [DOI] [PubMed] [Google Scholar]
- 33. Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. 1995. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res 700:245–253 [DOI] [PubMed] [Google Scholar]
- 34. Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates, 2nd edition San Diego, CA: Elsevier Academic Press [Google Scholar]
- 35. Stell A, Belcredito S, Ciana P, Maggi A. 2008. Molecular imaging provides novel insights on estrogen receptor activity in mouse brain. Mol Imaging 7:283–292 [PMC free article] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 37. Stell A, Belcredito S, Ramachandran B, Biserni A, Rando G, Ciana P, Maggi A. 2007. Multimodality imaging: novel pharmacological applications of reporter systems. Q J Nucl Med Mol Imaging 51:127–138 [PubMed] [Google Scholar]
- 38. Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J. 2007. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell 131:405–418 [DOI] [PubMed] [Google Scholar]
- 39. Martini PG, Katzenellenbogen BS. 2001. Regulation of prothymosin α gene expression by estrogen in estrogen receptor-containing breast cancer cells via upstream half-palindromic estrogen response element motifs. Endocrinology 142:3493–3501 [DOI] [PubMed] [Google Scholar]
- 40. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garnier M, Di Lorenzo D, Albertini A, Maggi A. 1997. Identification of estrogen-responsive genes in neuroblastoma SK-ER3 cells. J Neurosci 17:4591–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laborda J. 1991. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res 19:3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. 2005. Estrogen and cytokines production—the possible cause of gender differences in neurological diseases. Curr Pharm Des 11:1017–1030 [DOI] [PubMed] [Google Scholar]
- 44. Kanda N, Watanabe S. 2003. 17β-Estradiol inhibits MCP-1 production in human keratinocytes. J Invest Dermatol 120:1058–1066 [DOI] [PubMed] [Google Scholar]
- 45. Seli E, Pehlivan T, Selam B, Garcia-Velasco JA, Arici A. 2002. Estradiol down-regulates MCP-1 expression in human coronary artery endothelial cells. Fertil Steril 77:542–547 [DOI] [PubMed] [Google Scholar]
- 46. Garcia-Segura LM, Arévalo MA, Azcoitia I. 2010. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res 181:251–272 [DOI] [PubMed] [Google Scholar]
- 47. Thakur MK, Sharma PK. 2007. Transcription of estrogen receptor α and β in mouse cerebral cortex: effect of age, sex, 17β-estradiol and testosterone. Neurochem Int 50:314–321 [DOI] [PubMed] [Google Scholar]
- 48. Kawai H, Li H, Avraham S, Jiang S, Avraham HK. 2003. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor α. Int J Cancer 107:353–358 [DOI] [PubMed] [Google Scholar]
- 49. Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, Herman JG, Davidson NE. 2000. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res 60:6890–6894 [PubMed] [Google Scholar]
- 50. Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. 1994. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res 54:2552–2555 [PubMed] [Google Scholar]
- 51. Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. 1994. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet 7:536–540 [DOI] [PubMed] [Google Scholar]
- 52. Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. 1999. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43:985–991 [DOI] [PubMed] [Google Scholar]
- 53. Westberry JM, Prewitt AK, Wilson ME. 2008. Epigenetic regulation of the estrogen receptor α promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience 152:982–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilson ME, Westberry JM, Prewitt AK. 2008. Dynamic regulation of estrogen receptor-α gene expression in the brain: a role for promoter methylation? Front Neuroendocrinol 29:375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. 2003. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13:226–234 [DOI] [PubMed] [Google Scholar]
- 57. Ma ZQ, Santagati S, Patrone C, Pollio G, Vegeto E, Maggi A. 1994. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Mol Endocrinol 8:910–918 [DOI] [PubMed] [Google Scholar]
- 58. Mendez P, Cardona-Gomez GP, Garcia-Segura LM. 2005. Interactions of insulin-like growth factor-I and estrogen in the brain. Adv Exp Med Biol 567:285–303 [DOI] [PubMed] [Google Scholar]
- 59. Power RF, Mani SK, Codina J, Conneely OM, O'Malley BW. 1991. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254:1636–1639 [DOI] [PubMed] [Google Scholar]
- 60. Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A. 2003. In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- 61. Kang HS, Ahn HS, Kang HJ, Gye MC. 2006. Effect of estrogen on the expression of occludin in ovariectomized mouse brain. Neurosci Lett 402:30–34 [DOI] [PubMed] [Google Scholar]
- 62. Gorodeski GI. 2007. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology 148:218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ye L, Martin TA, Parr C, Harrison GM, Mansel RE, Jiang WG. 2003. Biphasic effects of 17-β-estradiol on expression of occludin and transendothelial resistance and paracellular permeability in human vascular endothelial cells. J Cell Physiol 196:362–369 [DOI] [PubMed] [Google Scholar]
- 64. Binko J, Majewski H. 1998. 17 β-Estradiol reduces vasoconstriction in endothelium-denuded rat aortas through inducible NOS. Am J Physiol 274:H853–H859 [DOI] [PubMed] [Google Scholar]
- 65. Zancan V, Santagati S, Bolego C, Vegeto E, Maggi A, Puglisi L. 1999. 17β-Estradiol decreases nitric oxide synthase II synthesis in vascular smooth muscle cells. Endocrinology 140:2004–2009 [DOI] [PubMed] [Google Scholar]
- 66. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. 2011. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nadeau S, Rivest S. 1999. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience 93:1449–1464 [DOI] [PubMed] [Google Scholar]
- 68. Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. 2007. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55:412–424 [DOI] [PubMed] [Google Scholar]
- 69. Johnson AB, Bake S, Lewis DK, Sohrabji F. 2006. Temporal expression of IL-1β protein and mRNA in the brain after systemic LPS injection is affected by age and estrogen. J Neuroimmunol 174:82–91 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.