Abstract
Estrogens affect a diversity of peripheral and central physiological endpoints. Traditionally, estrogens were thought to be peripherally derived transcription regulators (i.e. slow acting). More recently, we have learned that estrogens are also synthesized in neuronal cell bodies and synaptic terminals and have potent membrane effects, which modulate brain function. However, the mechanisms that control local steroid concentrations in a temporal and spatial resolution compatible with their acute actions are poorly understood. Here, using differential centrifugation followed by enzymatic assay, we provide evidence that estrogen synthesis within synaptosomes can be modulated more dramatically by phosphorylating conditions, relative to microsomes. This is the first demonstration of a rapid mechanism that may alter steroid concentrations within the synapse and may represent a potential mechanism for the acute control of neurophysiology and behavior.
Along with their well-known actions as transcription regulators, estrogens also produce membrane-initiated, rapid, and transient effects on signaling pathways affecting physiological and behavioral endpoints, including social behaviors, energy balance, and cognitive processes (1, 2). Fluctuations in circulating steroids occur relatively slowly (3) and do not provide the temporal or anatomical resolution required for the acute activation of specific brain circuits (4–6). Estrogens' ability to rapidly and transiently regulate physiology implies that their synthesis may be rapidly fine tuned. Unfortunately, the mechanisms that control local steroid concentrations in a time and spatial resolution compatible with their acute actions are poorly understood.
Estrogens are synthesized from androgens by aromatase, a P450 enzyme traditionally associated with microsomes of discrete neuronal populations in the brain of vertebrates (7, 8). Aromatase is also found in presynaptic terminals, suggesting that 17β-estradiol (E2) synthesis may be achieved with spatial specificity (9–11). Further, brain aromatase activity (AA) is rapidly and reversibly modulated by calcium-dependent phosphorylations resulting from neuronal depolarizations or glutamate release (12–14). Thus, transient and activity-dependent regulation of aromatase may alter its ability to synthesize estrogens. A recent in vivo microdialysis study has indeed suggested that fluctuations in the synthesis of estrogens induced by neuronal activity may be a reflection of presynaptic aromatization (13). However, microdialysis is unable to unequivocally discriminate between microsomal and synaptosomal E2 synthesis. Because estrogens are lipophilic and thus cannot be stored, rapid modulation of their presynaptic synthesis is one mechanism that could account for local and rapid changes in bioavailability. However, whether aromatase can be acutely regulated in specific ultrastructural compartments is unclear, and the mechanism for this potential regulation is unknown.
To clarify this issue, zebra finch telencephalon, tissue that contains the highest concentration of neuronal aromatase in any vertebrate tested, was subjected to differential centrifugation to separate synaptosomal and microsomal fractions. The modulation of AA across subcellular compartments and sexes was then evaluated using the tritiated water assay via exposure to phosphorylating conditions with or without protein kinase inhibition.
Materials and Methods
Animals
Fifteen male and 12 female zebra finches (n = 3 per sample, five male and four female samples) were used. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee guidelines at Lehigh University.
Fractionation
Telencephalons were rapidly removed, weighed, placed into ice-cold KTH buffer (150 mM KCL, 10 mM Tris-Base, Hepes, pH7.2) with sucrose (0.32 M) [0.1 mg of fresh tissue per milliliter] and homogenized, surrounded by ice, with 4 × 5-sec bursts of an electric homogenizer. Briefly, as previously described (11), homogenates were centrifuged for 15 min at 1034 × g. The resulting pellet was discarded and the supernatant (S1) was further centrifuged for 30 min at 10,081 × g. The supernatant (S2) was removed and kept aside, whereas the pellet (P2), containing synaptosomes and mitochondria, was washed twice with 300 μl of KTH-sucrose buffer followed by another 10-min centrifugation at 10,081 × g. The P2 wash solution was added to S2. The resulting mixture was then centrifuged for 1 h at 100,000 × g. The microsomal pellet (P3) and P2 were weighed wet, resuspended in KTH-sucrose (10 and 50 mg of pellet per milliliter, respectively), and stored at −80 C.
Electronic microscopy
After the 10,081 × g spin, some P2 were washed and fixed in 4% glutaraldehyde for 2 h at 4 C. Pellets were kept in 0.1 m phosphate buffer overnight at 4 C, then washed in 0.9% saline (10 min), and exposed to 2% OsO4 in 0.9% saline containing 1.5% KFeCN for 2 h. After serial dehydration, pellets were exposed to propylene oxide (30 min), 1:1 propylene oxide and Epon (2 h), 1:2 propylene oxide and Epon (overnight), and then polymerized in 100% Epon at 65 C for 48 h. Ultrathin sections (50–70 nm) were collected on copper grids, air dried, and examined on a Jeol 1200EX.
Enzymatic assays
AA was quantified by measuring the release of 3H-water produced from each molecule of [1β-3H]androstenedione aromatized (15). All samples and reagents were kept on ice at all times unless stated otherwise. Figure 1 illustrates the sequential incubation steps and concentrations of drugs added. Aliquots (100 μl) were mixed with one volume of KTH buffer (50 μl) containing either the calcium chelator EGTA (8 mm) (15), the specific protein kinase C inhibitor bisindolylmaleimide (BIS) (40 μm) (15, 16) or neither, and another volume of KTH buffer (50 μl) with or without ATP, Mg2+, and Ca2+ (PO4, 4–8 mm; equimolar concentrations of ATP, Mg2+, and Ca2+). This resulted in a repeated design with four treatments reaching final concentrations (indicated in parentheses) in a preincubation volume of 200 μl: control, phosphorylating conditions alone (PO4, 1–2 mm), phosphorylating conditions with EGTA (2 mm), and phosphorylating conditions with BIS (10 μm). Samples were then preincubated for 10 min in a water bath at 37 C to allow for the phosphorylation process. Previous experiments conducted in quail brain homogenates or cultured cells expressing human aromatase demonstrated that incubation in identical conditions (high but physiological concentrations of ATP, Mg2+, and Ca2+) promotes protein phosphorylation (17) and, indeed, results in aromatase phosphorylation (see Refs. 18–20; for further details, see Discussion).
Fig. 1.
Schematic presentation of the experimental protocol. All reagents were used ice-cold and delivered in tubes placed on ice (symbolized by vertical lines). A*, [1β-3H]-androstenedione. NADPH, Reduced nicotinamide adenine dinucleotide phosphate; PO4, equimolar concentrations of ATP, Mg2+ and CA2+; TCA, trichloroacetic acid.
The reaction was stopped by placing the samples on ice and adding EGTA to reach a final concentration of 2 mm in all samples (the concentration added was adjusted to account for the presence of EGTA as a pretreatment in some samples). [1β-3H]androstenedione (specific activity = 26.3 Ci/mmol; PerkinElmer, Waltham, MA), KTH, and reduced nicotinamide adenine dinucleotide phosphate were added to the homogenate to reach final concentrations of 25 nm, 1.2 mm, and 40 μm, respectively. The aromatization reaction was initiated by incubation at 37 C. After 10 min, the reaction was stopped by adding cold 10% trichloroacetic acid containing 2% charcoal. 3H-water was purified by centrifugation followed by Dowex cation exchange column separation and quantified with a scintillation counter.
All samples were assayed in duplicate with an additional tube assayed in the presence of an excess of the aromatase inhibitor Fadrozole (gift from Novartis, Basel, Switzerland), whose activity measured was subtracted from the pooled duplicate value to determine the final specific activity in each sample. Samples were randomly run within three assays for each experiment, each containing a whole homogenate serving as an internal control. AA was expressed in nmol/h · mg fresh weight of pellet after correction of the counts for quenching, recovery, blank values, and percentage of tritium in β-position in the substrate.
Data analysis
Due to a difference in variance between fractions that cannot be verified statistically (tests for sphericity are invalid when there are less than three repeated modalities), results from each fraction were analyzed by separate two-way ANOVA with sex and treatment as independent and repeated factors, respectively. Significant treatment effects were queried with Tukey post hoc tests. All analyses were performed with Statistica 9.1 (Statsoft, Inc., Tulsa, OK).
Results
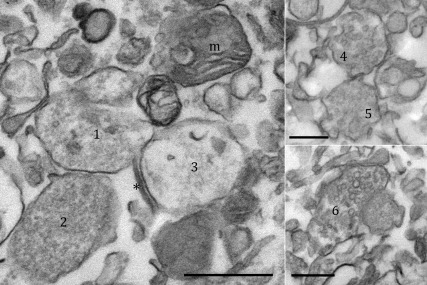
Verification of the authenticity of subfractions by electronic microscopy revealed numerous synaptosomes containing varying amounts of clear neurotransmitter vesicles and mitochondria (Fig. 2). Similar subfractions prepared from quail preoptic-hypothalamic (HPOA) homogenates have been validated previously based on the expression of subcellular-specific enzymatic activities (21). Together, these observations indicate that the AA measured in the P2 pellets is a reflection of synaptosomal aromatization.
Fig. 2.
Electrophotomicrographs of P2 pellets. Electron photomicrographs demonstrating the presence of synaptosomal profiles in the P2 pellets used in the current study. Synaptosomes (1–6) filled with varying numbers of clear vesicles are visible, sometimes, adjacent to a mitochondrion with visible cristae (m). Also visible is a synapto-dendrosome with an associated postsynaptic density (*) but no visible postsynaptic element. Scale bars, 500 nm.
A first experiment tested the effect of a preincubation with 1 mm PO4 with or without EGTA (2 mm; calcium chelator), and BIS (10 μm; protein kinase inhibitor), on AA measured in the two fractions. Separate two-way ANOVA identified a significant effect of treatment in both fractions (P2: F3,21 = 33.802, P < 0.001; P3: F3,21 = 5.462, P = 0.006) but no sex difference (P2: F1,7 = 0.439, P = 0.529; P3: F1,7 = 1.151, P = 0.319) or interaction (P2: F3,21 = 0.758, P = 0.530; P3: F3,21 = 1.267, P = 0.311). Post hoc analyses revealed that preincubation with 1 mm PO4 caused a weak but significant reduction of AA in both fractions (∼21–25%) (Fig. 3, A and B). Ca2+ withdrawal partially (but not significantly) blocked this effect in microsomes but significantly raised AA about 40% above control levels in synaptosomes suggesting the existence of an endogenous AA inhibition by Ca2+-dependent processes differentially affecting microsomal and synaptosomal aromatase. Finally, protein kinase blockade completely suppressed PO4-induced enzymatic inhibitions in both fractions. Similar to findings obtained in quail HPOA homogenates (18, 19), telencephalic aromatase (regardless of its subcellular localization) appears to be rapidly altered by Ca2+-dependent phosphorylations. However, the present inhibition was much lower than previously reported with the same concentration of ATP, Mg2+, and Ca2+ in quail HPOA (15).
Fig. 3.
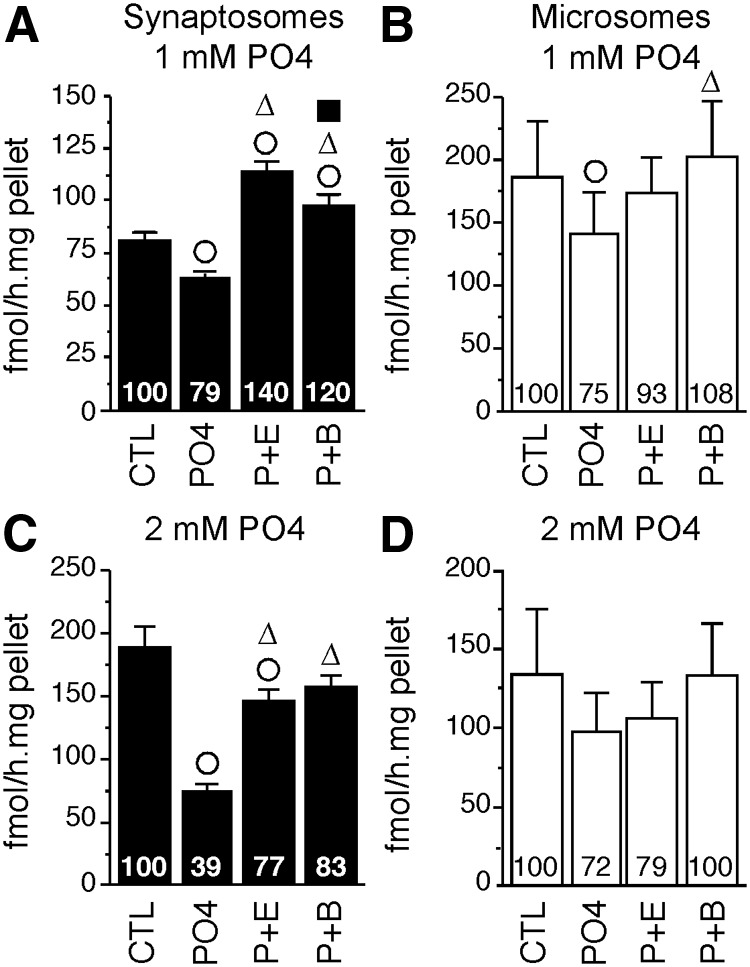
Phosphorylating conditions (PO4) differentially inhibit synaptosomal and microsomal AA (mean ± sem). Effect of preincubation with 1 mm (A and B) and 2 mm (C and D) PO4 with or without 2 mm EGTA (P+E) or 10 μm BIS (P+B) on synaptosomal (black, P2 fraction) and microsomal (white, P3 fraction) AA. Numbers in the bars represent the average percentages of the control mean. ○, Δ, and ■, P < 0.05 for within-fraction comparison with control treatment (CTL), PO4, and P+E, respectively.
Therefore, a higher concentration of PO4 (2 mm) was tested on the same samples. As previously, no sex difference (P2: F1,7 = 0.350, P = 0.529; P3: F1,7 = 0.430, P = 0.533) or interaction between sex and treatment was found (P2: F3,21 = 0.955, P = 0.432; P3: F3,21 = 0.426, P = 0.736). Higher PO4 concentrations yielded a highly significant treatment effect in the synaptosomal fraction (F3,21 = 29.115, P < 0.001). Post hoc analysis indicated that this effect is mainly explained by a robust AA inhibition (−60%) after preincubation with 2 mm PO4 (Fig. 3C). In addition, calcium depletion and kinase inhibition significantly maintained AA to control levels, thus preventing PO4-induced inhibition. By contrast, the weak drop of AA in the microsomal fraction did not reach significance (F3,21 = 2.91, P = 0.058). Together, these results suggest that synaptosomal and microsomal aromatase respond differently to phosphorylating conditions.
Discussion
These findings provide additional support for the idea that the rapid regulation of neural synthesis of estrogens is a conserved vertebrate trait (20), with presynaptic aromatization as a primary locus of influence. This inference is in excellent agreement with earlier hypotheses about the preferential regulation of presynaptic aromatization (13) and offers a temporal and spatial resolution compatible with the rapid effects of estrogens on synaptic physiology (22). Importantly, this property is sexually monomorphic and supports consideration of estrogens as neuromodulators (12) in keeping with the “synaptocrine hypothesis” (23).
In synaptosomes, AA is profoundly (−60%) reduced by high but physiological concentrations of ATP, Mg2+, and Ca2+, an effect prevented by calcium chelation and protein kinase inhibition. Identical regimens (including an array of kinase inhibitors, varying concentrations and durations of preincubation with ATP, Mg2+, or Ca2+) have been used to investigate the kinases involved as well as the sites, extent, and consequences of phosphorylation of the aromatase protein in birds (15, 18, 19, 21). Taken together, these data strongly suggest that the treatments used in the present set of studies may indeed induce changes in aromatization via calcium-dependent phosphorylations (12). This influence may be direct, because aromatase contains consensus phosphorylation sites (19). Direct evidence using antiphospho-residues and radiolabeled phosphate show that phosphorylations target the enzyme itself, rather than other regulatory and/or associated proteins that would secondarily alter AA (20).
In conditions in which the neuronal integrity and connectivity are preserved, decreases in AA of similar magnitude and temporal pattern are elicited by K+-induced depolarization and exposure to glutamate (12–14). These changes are reversible and independent of the enzyme stability, and kinase inhibition completely prevents these activity-dependent enzymatic changes (12, 20). Thus, depolarization and aromatase phosphorylation appear strongly associated, a pattern corroborating the hypothesis that the synaptosomal changes in AA seen here depend on calcium-dependent phosphorylations and are likely involved in the fine tuning of local synaptic E2 synthesis. It is critical, however, to acknowledge that the current preparation does not permit us to unequivocally identify the source of calcium flux, nor does it permit identification of region-specific contributions to variations in synaptosomal aromatization within the songbird telencephalon.
Previous studies suggested that the calcium involved in the AA regulation was likely released from internal stores. Indeed, in HPOA explants, blockers of calcium channels or complete removal of Ca2+ from the extracellular milieu failed to abolish the K+-induced enzymatic inhibition, whereas a similar inhibition was obtained with thapsigargin, a sesquiterpene lactone that increases the intracellular pool of free Ca2+ by blocking its uptake by the endoplasmic reticulum (12). In contrast, it was recently suggested that the acute control of brain estrogen synthesis depends on calcium entry through presynaptic voltage-dependent calcium channels, an inference supported by the observation that the in vivo retrodialysis of ω-conotoxin (a blocker of the presynaptic N-type channels) into the zebra finch caudomedial nidopallium blocks the K+-induced drop in estrogen synthesis (13). Although the present study does reveal an interesting overshoot in EGTA-treated synaptosomes, but not microsomes, with lower concentrations of ATP, Mg2+, and Ca2+ (see Fig. 3), this observation only suggests the existence of a higher calcium concentration in synaptosomes compared with microsomes and, unfortunately, cannot provide any further information concerning the source of calcium in intact neurons.
Although presynaptic aromatase is described in many species (9, 10), its regulation is less well studied. The transcriptional activity of testosterone is thought to regulate the concentration of the enzyme and, thus, its activity, in microsomes and synaptosomes in a region-specific manner (21, 24). However, this is the first study to report a compartment-specific, phosphorylation-dependent regulation of aromatase. The present data strongly suggest that phosphorylations preferentially alter AA in synaptosomes more rapidly than the genomic control of aromatase expression by testosterone (12, 20). Although the molecular underpinnings of this compartment-specific regulation remain unknown, it may reflect the compartmentalization of specific kinases and/or the dynamics of Ca2+ availability. These hypotheses await testing.
The traditional view about the mechanisms of estrogenic action may require refinement, because estrogens are produced by a variety of tissues, including the brain, and act through multiple pathways to affect many physiological and behavioral processes. Mounting evidence indicates that brain E2 synthesis can be regulated in a time frame compatible with the rapid effects described at the cellular and organismal level (1, 2). However, the subcellular dynamics of their synthesis are almost never discussed. Consequently, it is sometimes assumed that estrogens invariantly diffuse away from their site of synthesis, flooding a large extent of their surroundings in a relatively nonspecific manner. The present finding suggests that the acute control of estrogen synthesis (and their release, because their lipophilic nature prevents their storage) is confined to a limited spatial domain offering a spatially restricted delivery mechanism of estrogens, perhaps to fine tune synaptic processes (22). Indeed, manipulations of local estrogen action or synthesis alter neuronal excitability and behavioral output (4–6, 25–28). Moreover, fluctuations in E2 synthesis occur in response to sudden changes in the social or environmental context (14, 29, 30). Thus, it is likely that activity-dependent fluctuations of local, probably synaptic, E2 bioavailability critically impact behavior. Finally, the present demonstration of acute control of AA preferentially in synaptosomes brings further support to the neuromodulatory role played by locally produced estrogens in the songbird telencephalon possibly for regulation of singing and auditory processing (6, 28) and likely extending to other systems and vertebrate species (2).
Acknowledgments
We thank Dr. Jacques Balthazart, Dr. Thierry D. Charlier, and Catherine de Bournonville for their help with data analysis and comments on earlier versions of this manuscript.
This work was supported by National Institutes of Health Grant NS 042767. C.A.C. is a Fonds pour Recherche Scientifique – Fonds National pour la Recherche Scientifique Research Associate.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- Aromatase activity
- BIS
- bisindolylmaleimide
- E2
- 17β-estradiol
- HPOA
- preoptic-hypothalamic.
References
- 1. Roepke TA, Ronnekleiv OK, Kelly MJ. 2011. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci 16:1560–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornil CA, Charlier TD. 2010. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol 22:664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornil CA, Ball GF, Balthazart J. 2006. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res 1126:2–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kow LM, Pfaff DW. 2004. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA 101:12354–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tremere LA, Jeong JK, Pinaud R. 2009. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci 29:5949–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roselli CE, Resko JA. 1997. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol 61:365–374 [PubMed] [Google Scholar]
- 8. Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. 2000. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423:619–630 [DOI] [PubMed] [Google Scholar]
- 9. Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. 1996. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinology 63:149–155 [DOI] [PubMed] [Google Scholar]
- 10. Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. 2005. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci 272:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rohmann KN, Schlinger BA, Saldanha CJ. 2007. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol 67:1–9 [DOI] [PubMed] [Google Scholar]
- 12. Balthazart J, Ball GF. 2006. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 241–249 [DOI] [PubMed] [Google Scholar]
- 13. Remage-Healey L, Dong S, Maidment NT, Schlinger BA. 2011. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci 31:10034–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Remage-Healey L, Maidment NT, Schlinger BA. 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konkle AT, Balthazart J. 2011. Sex differences in the rapid control of aromatase activity in the quail preoptic area. J Neuroendocrinol 23:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. 1991. The bisindolylmalmeide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266:15771–15781 [PubMed] [Google Scholar]
- 17. Albert KA, Helmer-Matyjek E, Nairn AC, Müller TH, Haycock JW, Greene LA, Goldstein M, Greengard P. 1984. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci USA 81:7713–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balthazart J, Baillien M, Ball GF. 2001. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol 13:63–73 [DOI] [PubMed] [Google Scholar]
- 19. Balthazart J, Baillien M, Charlier TD, Ball GF. 2003. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci 17:1591–1606 [DOI] [PubMed] [Google Scholar]
- 20. Charlier TD, Harada N, Balthazart J, Cornil CA. 2011. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology 152:4199–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlinger BA, Callard GV. 1989. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology 49:434–441 [DOI] [PubMed] [Google Scholar]
- 22. Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. 2011. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci 34:16056–16063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saldanha CJ, Remage-Healey L, Schlinger BA. 2011. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32:532–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roselli CE. 1995. Subcellular localization and kinetic properties of aromatase activity in rat brain. J Steroid Biochem Mol Biol 52:469–477 [DOI] [PubMed] [Google Scholar]
- 25. Taziaux M, Keller M, Bakker J, Balthazart J. 2007. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci 27:6563–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. 2006. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav 49:45–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. 2006. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res 66:110–123 [DOI] [PubMed] [Google Scholar]
- 28. Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. 2010. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA 107:3852–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. 2005. Sexual behavior affects preoptic aromatase activity and brain monoamines' levels. Endocrinology 146:3809–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dickens MJ, Cornil CA, Balthazart J. 2011. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology 152:4242–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]