Abstract
The human endometrium undergoes extensive monthly regeneration in response to fluctuating levels of circulating estrogen and progesterone in premenopausal (Pre-M) women. In contrast, postmenopausal (Post-M) endometrium is thin and quiescent with low mitotic activity, similar to the Pre-M endometrial basalis layer. Clonogenic epithelial stem/progenitor (ESP) cells, likely responsible for regenerating endometrial epithelium, have been identified in Pre-M and Post-M endometrium, but their location is unknown. We undertook transcriptional profiling of highly purified epithelial cells from full-thickness Pre-M and Post-M endometrium to identify differentially regulated genes that may indicate a putative ESP cell population resides in the basalis of Pre-M and basalis-like Post-M endometrium. Of 1077 differentially expressed genes identified, the Wnt signaling pathway, important in endometrial development and stem cell regulation, was one of the main gene families detected, including 22 Wnt-associated genes. Twelve genes were validated using quantitative RT-PCR, and all were concordant with microarray data. Immunostaining showed glandular epithelial location of Wnt-regulated genes, Axin-related protein 2 and β-catenin. Axin2 localized to the nucleus of basalis Pre-M and Post-M and cytoplasm of functionalis Pre-M endometrium, suggesting that it regulates β-catenin. Comparison of our Post-M gene profile with published gene microarray datasets revealed similarities to Pre-M basalis epithelial profiles. This differential expression of multiple Wnt-associated genes in human Pre-M and Post-M endometrial epithelial cells and the similar gene profile of Post-M and Pre-M basalis epithelium suggests that a population of putative endometrial ESP may reside in the basalis of Pre-M endometrium, which may be responsible for regenerating glandular epithelium each month.
Premenopausal (Pre-M) endometrium is highly regenerative, undergoing more than 400 cycles of regeneration, differentiation, and shedding during a woman's reproductive years (1). Full-thickness endometrium consists of the functionalis and basalis layers (Fig. 1A) and is responsive to fluctuating levels of circulating ovarian steroid hormones, estrogen, and progesterone (1, 2). During menstruation, the functionalis is shed, whereas the basalis remains, and 4–10 mm of new functionalis is regenerated in the following cycle. In contrast, postmenopausal (Post-M) endometrium is thin, quiescent, and atrophic (Fig. 1A), with low mitotic activity, and is thought to be similar to the basalis of Pre-M endometrium (2, 3). Because circulating estrogen is very low in Post-M women, the functionalis is virtually absent. However, when hormone replacement therapy is given, Post-M endometrium can regenerate sufficiently to support pregnancy to term (4, 5).
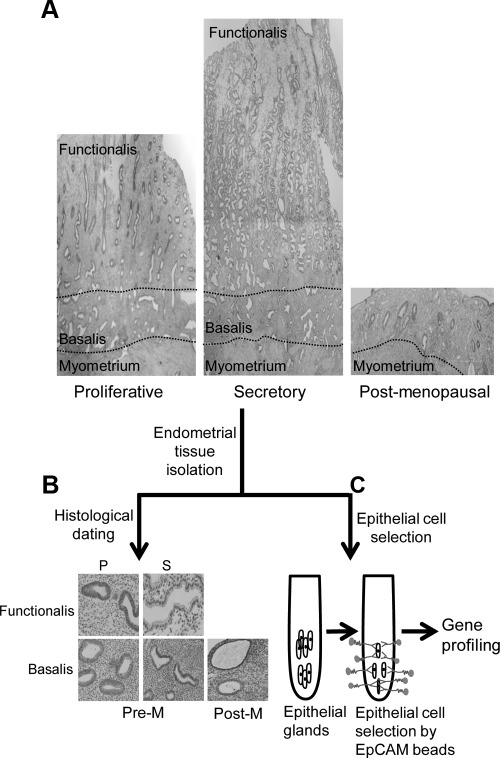
Fig. 1.
Experimental flow chart for investigating epithelial cell gene expression in Pre-M and Post-M human endometrium. A, Full-thickness endometrium showing functionalis and basalis layers in Pre-M endometrium [proliferative (P) and secretory (S) stages] and thin, atrophic, basalis-like Post-M endometrium. B, Histological dating and glandular epithelial morphology of Pre-M (P and S) and Post-M endometrium. C, Epithelial cells of Pre-M and Post-M endometrium were dissociated and purified using magnetic bead sorting with EpCAM-labeled beads for subsequent transcriptome studies.
Given the monthly tissue remodeling of the functionalis, it has been postulated that resident epithelial stem/progenitor (ESP) cells are located in the basalis and are responsible for its remarkable regeneration (2, 6, 7). This concept was strengthened by a kinetic study showing lower cellular proliferation rates in the basalis and in Post-M endometrium compared with the functionalis (8). Evidence for the existence of endometrial ESP cells was first demonstrated by the identification of rare clonogenic epithelial cells in Pre-M and in atrophic, inactive Post-M endometrium (9–11). However, the precise location of clonogenic ESP cells is unknown.
Gene expression profiling of Pre-M endometrium across the menstrual cycle has been studied previously using fresh unfractionated endometrial tissue (12–15). Differential expression of genes between functionalis and basalis epithelial cells has been identified in menstrual endometrium in a laser capture microdissection (LCM) study (16, 17) and between glandular and luminal epithelial compartments in mice (18). Studies using a mouse model have shown that the Wnt signaling pathway is essential for female reproductive tract development (19, 20). Gene profiling studies of endometrial tissue showed that the Wnt signaling pathway was regulated by steroid hormones during its cycles of growth and differentiation (21–24). In human endometrium, Wnt2, Wnt3, Wnt4, Wnt5, Wnt7a, and Wnt7b were expressed in both proliferative and secretory stages of the menstrual cycle (21, 23). Wnt receptor and coreceptors (FZD6 and LRP6) and downstream effectors (DKK1, DVL-1, GSK3β, and β-catenin) were also expressed throughout the menstrual cycle (21). Wnt signaling is a critical downstream mediator of estrogen-induced endometrial proliferation (24). Progesterone counteracts this proliferative effect by inducing differentiation (22, 25, 26). Although these studies highlight the key role of Wnt signaling in endometrial function, there are very few investigations specifically focused on human endometrial luminal and glandular epithelial cells.
This study aimed to investigate the transcriptional profiles of purified epithelial cells isolated from full-thickness Pre-M and basalis-like Post-M endometrium to elucidate differences between the epithelium of the rapidly remodeling functionalis and the quiescent basalis. In this experimental design, functionalis epithelium will dominate the gene expression profile of full-thickness Pre-M endometrium, whereas the Post-M endometrial epithelial gene profile will be similar to the epithelium of the quiescent Pre-M endometrial basalis. Differential gene expression of many Wnt family members was identified. Comparative analysis of our endometrial epithelial gene expression profiles with that of endometrial epithelial cells in remodeling endometrium (16, 17) also provides new evidence that Post-M endometrial epithelium has a similar gene signature to that of basalis epithelium of menstrual endometrium. Our data suggest that the Wnt signaling pathway may have a role in the function of a population of putative ESP cells possibly located in Post-M and the basalis layer of Pre-M endometrium.
Materials and Methods
Patients
Human endometrium attached to the underlying myometrium (n = 40; 13 proliferative, 12 secretory, and 15 Post-M) was obtained from women undergoing hysterectomy for benign gynaecologic conditions, including menorrhagia, prolapse, fibroids, endometriosis, and adenomyosis (Table 1). Ethical approval was obtained from Southern Health Human Research and Ethics Committee B and Monash University Human Research Ethics Committee with informed written consent given by each patient. Histopathological evaluation and menstrual cycle dating was assessed according to well-established criteria (Fig. 1B) (27). Inclusion criteria were normal cycling Pre-M women (30–53 yr old) with benign endometrium, who had not taken steroid hormone treatment within 3 months before surgery, and Post-M (49–70 yr old) women, who had not menstruated for at least 1 yr nor had taken hormones for 3 months before surgery. Exclusion criteria were women undergoing hysterectomy for endometrial pathologies: polyps, hyperplasia, or endometrial cancer.
Table 1.
Information on patient details used in this study
| Sample no. | Age | Menstrual stage | Indication/diagnosis | Assay |
|---|---|---|---|---|
| 1 | 48 | Proliferative | Menorrhagia | M |
| 2 | 52 | Proliferative | Prolapse | M, Q |
| 3 | 49 | Proliferative | Menorrhagia | M, Q |
| 4 | 52 | Proliferative | Fibroids | M, Q |
| 5 | 45 | Secretory | Menorrhagia | M, Q |
| 6 | 48 | Secretory | Fibroids | M, Q |
| 7 | 30 | Secretory | Severe endometriosis | M, Q |
| 8 | 48 | Secretory | Menorrhagia | M, Q |
| 9 | 55 | Post-M | Fibroids | M |
| 10 | 57 | Post-M | Prolapse | M |
| 11 | 49 | Post-M | Unknown | M, Q |
| 12 | 47 | Proliferative | Menorrhagia | Q |
| 13 | 36 | Proliferative | Menorrhagia | Q |
| 14 | 49 | Proliferative | Fibroids | Q |
| 15 | 40 | Proliferative | Fibroids | Q |
| 16 | 41 | Secretory | Prolapse | Q |
| 17 | 41 | Secretory | Prolapse | Q |
| 18 | 47 | Secretory | Prolapse | Q |
| 19 | 46 | Secretory | Menorrhagia | Q |
| 20 | 53 | Post-M | Prolapse | Q |
| 21 | 54 | Post-M | Unknown | Q |
| 22 | 64 | Post-M | Prolapse | Q |
| 23 | 63 | Post-M | Prolapse | Q |
| 24 | 53 | Post-M | Prolapse | Q |
| 25 | 65 | Post-M | Prolapse | Q |
| 26 | 66 | Post-M | Prolapse | Q |
| 27 | 50 | Post-M | Prolapse | Q |
| 28 | 54 | Post-M | Prolapse | Q |
| 29 | 43 | Proliferative | Menorrhagia | I |
| 30 | 53 | Proliferative | Adenomyosis | I |
| 31 | 49 | Proliferative | Adenomyosis | I |
| 32 | 51 | Proliferative | Menorrhagia | I |
| 33 | 46 | Proliferative | Menorrhagia | I |
| 34 | 39 | Secretory | Menorrhagia | I |
| 35 | 38 | Secretory | Adenomyosis | I |
| 36 | 48 | Secretory | Menorrhagia | I |
| 37 | 43 | Secretory | Adenomyosis | I |
| 38 | 53 | Post-M | Prolapse | I |
| 39 | 65 | Post-M | Prolapse | I |
| 40 | 70 | Post-M | Prolapse | I |
M, Microarray; Q, qRT-PCR; I, immunofluorescence.
Endometrial tissue dissociation
The endometrium, including the nonmenstruated basalis layer, was scraped and dissected from the myometrial surface to ensure collection of all endometrial tissue as confirmed microscopically and previously described (9, 11). In Pre-M samples, it was more consistent to remove the entire endometrium comprising functionalis (80–90%) and basalis (10–20%) for comparison with Post-M endometrium than to attempt gross separation of functionalis and basalis of the uterine tissue pieces (∼10 × 10 × 5 mm) made available by the pathologist. Using this reliable, robust method, we were able to consistently obtain samples suitable for analysis. Endometrial tissue was then minced finely and digested in culture medium (sodium bicarbonate buffered DMEM-F12, 10% fetal calf serum, 1% antibiotics, and 1% glutamine; Invitrogen, Carlsbad, CA), 5 mg/ml of collagenase I, and 40 μg/ml deoxyribonuclease (DNase) I (Worthington Biochemical, Freehold, NJ) using a MACSmix rotator (Miltenyi Biotec, Bergisch Gladbach, Germany) at 37 C for 90 min in a 5% CO2 humidified incubator. The digestion was stopped and filtered through a 40-μm sieve (BD Biosciences, Durham, NC). Glandular fragments containing epithelial cells remaining on the filter were backwashed and further digested for 20 min in 0.8 mg/ml collagenase II (Worthington Biochemical) and 40 μg/ml DNase I. Epithelial cell suspensions were then filtered through a 40-μm sieve (BD Biosciences) and centrifuged for 5 min at 209 × g, and the pellets were resuspended in 5-ml medium. Cell counts were performed using a hemocytometer.
Endometrial epithelial cell selection and RNA extraction
Antihuman EpCAM (BerEP4 clone) antibody-coated magnetic Dynabeads (Dynabeads Epithelial Enrich kit; Invitrogen) was used to positively select both luminal and glandular epithelial cells from cell suspensions (9). Briefly, washed epithelial Dynabeads (four beads per cell) were incubated with the cells at 4 C with gentle mixing for 30 min. The cell-bead suspension was placed on the magnet for 5 min to select beaded cells (Fig. 1C), washed twice, and the beaded epithelial cells were resuspended in PBS. Using our established protocol (11), the purity of bead-selected epithelial cells was confirmed by positive staining for the epithelial marker, cytokeratin (>99.8%), and negative for stromal maker, CD90 (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Total RNA was extracted from bead-purified epithelial cells using Ambion RNAqueous Micro kit (Ambion, Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. RNA preparations were DNase treated and purified using DNase inactivation kit (Ambion). RNA purity and integrity was analyzed by A260/A280 nm ratio using a NanoDrop ND1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE) and stored at −80 C until use.
Gene expression profiling
Gene chip hybridization, scanning, and data acquisition were performed at the Australian Genome Research Facility (Melbourne, Australia). Briefly, RNA samples (n = 11) (Table 1) were assessed for quality and integrity using Agilent Bioanalyser 2100 (Agilent, Santa Clara, CA). Samples (500 ng) were labeled using the Ambion TotalPrep RNA amplification kit. A total of 1.5 μg of labeled cRNA was used to prepare a probe cocktail (0.05 μg/μl cRNA and gene expression hybridization (GEX-HYB) buffer according to the manufacturer's protocol) for hybridization to the Sentrix Human-HT12 v3 Beadchip (Illumina, San Diego, CA). The chip was hybridized at 58 C for 16 h on a rocking platform, washed, coupled with Cy3, and scanned using the Illumina BeadArray Reader. BeadStudio scanner software (Illumina) was used to convert signal on the array to an appropriate file for analysis.
Microarray data analyses
The intensity of probe sets and batch normalization were analyzed using Partek Genome Suite 6.5 analysis package (Partek, St Louis, MO). Robust multichip average (RMA) algorithm and one-way ANOVA were applied to adjust background noise, quantile normalization, and data summarization (28, 29). All raw and processed data files have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus dataset with accession no. GSE35221.
Comparison was made between three Post-M samples and eight Pre-M (four proliferative and four secretory). Principal component analysis and hierarchical clustering were performed using Partek software. After RMA normalization, the principal component analysis algorithm in Partek was applied to all 11 samples to identify trends in the data and as a quality control measure. Differential gene expression and hierarchical clustering of the Wnt-associated genes was generated from comparison between Post-M and Pre-M (proliferative and secretory samples combined) endometrial epithelial cells unless stated otherwise.
Biological processes, functional classifications and gene annotations were analyzed using Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Redwood City, CA), database for annotation, visualization and integrated discovery (DAVID) (http://david.abcc.ncifcrf.gov), and GeneCards/Gene ALaCart (http://www.genecards.org). To identify biological processes with significant enrichment, the distribution of genes from our data was compared with a reference annotation gene list for each gene ontology (GO) category generated by IPA or DAVID. Fisher exact P value was used for gene enrichment analysis. The value ranges from 0 to 1, where value equal to zero represents perfect enrichment. P value less than or equal to 0.05 is considered significantly enriched in the annotation categories.
Transcriptional validation
Primers (Supplemental Table 1) were designed for candidate genes using primer bank database and Primer3 (v.0.4.0) software. RNA was reverse transcribed to cDNA using SuperScript III First Strand Synthesis System for RT-PCR (Invitrogen) according to manufacturer's protocols. Quantitative RT-PCR (qRT-PCR) was performed using SYBR Green PCR Master Mix and 7900 HT Fast Real-Time PCR System (Applied Biosystems). Each reaction was run in triplicate and consisted of 100 ng of cDNA, 1–50 μm optimized primers, and 1× Fast Power SYBR Green Mix. Amplification efficiency was determined using serially diluted endometrial epithelial cDNA (200–0.2 ng) using the slope of best fit curve for cycle threshold and concentration. The amplification conditions were 95 C for 10 min, 95 C for 15 sec, and 60 C for 1 min. No-template and no-RT controls were included for each assay to ensure quality and cDNA specificity of the primers. PCR products were verified by agarose gel electrophoresis. Target gene expression was normalized to 18S rRNA and relative gene expression assessed using the 2−ΔΔCT method (30).
Immunofluorescence
Fresh frozen full-thickness Pre-M and Post-M hysterectomy tissues were cryosectioned (5 μm), fixed in 4% paraformaldehyde in 0.1 m PBS (pH 7.4) (Invitrogen) for 20 min at room temperature, permeabilized with 0.2% Triton X-100/PBS for 10 min, washed, and incubated with protein blocking solution (Dako, Carpinteria, CA) for 10 min, followed by overnight incubation at 4 C with primary antibodies: rabbit monoclonal antihuman β-catenin (1:200; Abcam, Cambridge, UK) and rabbit polyclonal antihuman Axin-related protein 2 (Axin2) (1:100; Abcam). Sections were washed and incubated with Alexa Fluor 594 goat antirabbit IgG or Alexa Fluor 488 donkey antirabbit IgG (1:200; Molecular Probes, Eugene, OR) for 1 h at room temperature. Negative controls were stained similarly but without primary antibody. Sections were washed and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) for 15 min and mounted in fluorescence mounting medium (Dako). Images were captured and analyzed using Leica DMR microscope and Leica IM50 version 1.20 Image Manager (Leica Microsystems, Wetzlar, Germany) and ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analyses
Microarray data were analyzed using RMA and ANOVA, and the gene list was filtered using P < 0.05 and fold change more than 2. qRT-PCR validation data were analyzed using Mann-Whitney U test (GraphPad Prism version 5.01; GraphPad, San Diego, CA). Differences of P < 0.05 (95% confidence interval) were considered statistically significant.
Results
Differential gene expression between human Pre-M and Post-M endometrial epithelial cells
To determine whether the epithelial cells in thin, atrophic basalis-like Post-M endometrium expressed a different gene profile compared with the thick, highly proliferative functionalis of Pre-M (proliferative and secretory) endometrium, gene expression profiling of EpCAM magnetic bead-purified endometrial epithelial cells was conducted (Fig. 1). The transcriptional profile of three Post-M and eight full-thickness Pre-M (four proliferative and four secretory) endometrial epithelial samples identified 1077 candidate genes with differential expression. Among these, 275 genes showed higher and 802 lower expression in Post-M compared with Pre-M (proliferative and secretory) endometrium.
To identify biological processes associated with these differentially expressed genes, we used DAVID to map the GO classifications. Fisher exact test was adopted to measure the gene-enrichment annotations. From a list of 275 highly expressed genes identified in Post-M, DAVID annotated 267 genes into six main categories, including immune response, response to wounding, cell death regulation, negative regulation of cell proliferation, and integrin-mediated and Wnt signaling pathways (Table 2). From 802 lowly expressed genes in Post-M, DAVID mapped 749 genes into seven gene families, including protein and intracellular transport, lipid biosynthesis process, protein kinase and tissue remodeling activity, cell cycle, cell migration, and Wnt signaling pathway (Table 2). A complete GO classification and gene list are provided as Supplemental data.
Table 2.
GO classification of enriched biological processes in Post-M vs. Pre-M (proliferative and secretory) endometrial epithelial cells
| Category | Fisher exact P value | Genes involved in category | Percentage (involved genes/total genes) |
|---|---|---|---|
| Post-M > Pre-M (267 genes) | |||
| Immune response | 2.80E-08 | 33 | 12.3 |
| Response to wounding | 2.40E-04 | 21 | 7.9 |
| Cell death regulation | 4.20E-03 | 22 | 8.2 |
| Negative regulation of cell proliferation | 4.40E-03 | 14 | 5.2 |
| Integrin-mediated signaling pathway | 2.40E-02 | 5 | 1.9 |
| Wnt signaling pathway | 4.80E-02 | 8 | 3.0 |
| Post-M < Pre-M (749 genes) | |||
| Protein transport | 1.70E-06 | 63 | 8.4 |
| Intracellular transport | 6.20E-06 | 55 | 7.3 |
| Lipid biosynthetic process | 3.70E-05 | 32 | 4.3 |
| Protein kinase cascade | 3.90E-05 | 35 | 4.7 |
| Cell migration | 5.50E-03 | 11 | 1.5 |
| Cell cycle | 4.80E-02 | 45 | 6.0 |
| Wnt signaling pathway | 6.80E-02 | 11 | 1.5 |
Fisher exact P value for the gene-enrichment category was generated from DAVID reference gene list. Genes involved in each category indicates the number of genes enriched for that category from the input gene list. Percentage indicates the relative number of involved genes from the total number of high or low expressing genes in individual categories. For example, the percentage of immune response is 33/267 = 12.3%.
Wnt-associated signaling pathway
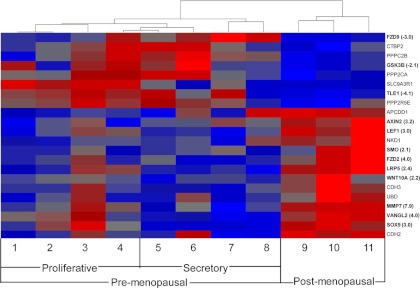
The Wnt signaling pathway was identified as a common GO biological process for endometrial epithelial cells (Table 2), confirming the importance of the Wnt pathway in endometrial development and function (21, 26). Further investigation using IPA software revealed a set of 22 Wnt-associated genes enriched in the differentially expressed gene set between Post-M and Pre-M endometrial epithelial cells (Fig. 2 and Supplemental Fig. 2). The dendrogram shows separate clustering of the Post-M and Pre-M samples for these Wnt-associated genes. For example, a similar differential gene expression pattern was observed between a late proliferative sample (sample 4) and a secretory (sample 5) compared with all three Post-M samples (samples 9–11) (Fig. 2). Of the 22 differentially expressed Wnt pathway genes, 14 showed increased and eight decreased expression levels in Post-M compared with Pre-M (proliferative and secretory). Genes involved directly with the canonical Wnt pathway included a Wnt ligand (WNT10A), several receptors (FZD2 and FZD9), a coreceptor (LRP5), negative regulators of the Wnt pathway (AXIN2 and GSK3β), and downstream targets (LEF1, MMP7, TLE1, and CTBP2). Other differentially expressed genes indirectly involved with the Wnt pathway were transcriptional factors (SMO, SOX9, and VANGL2).
Fig. 2.
Differentially expressed genes involved in the Wnt signaling pathway in Pre-M and Post-M endometrial epithelial cells. Hierarchical cluster analysis of 22 Wnt-associated genes showing P < 0.05 (ANOVA) and a fold change of more than 2 comparing proliferative (samples 1–4), secretory (samples 5–8), and Post-M (samples 9–11) endometrial epithelial cells. Each column shows the relative gene expression of a single patient sample for the Wnt-associated genes, which are labeled on the abscissa. Higher expression (red) and lower expression (blue) is indicated by color and intensity. qRT-PCR validation of selected genes and fold change are identified by bold lettering. Dendrogram and heat map were generated by Partek Genomics Suite software.
Given the distinct distribution and expression of Wnt signaling genes in epithelial cells in the proliferative and secretory phase of the menstrual cycle and the paucity of data on these genes in Post-M endometrial epithelial cells, the Wnt-associated genes were further analyzed. We found three Wnt-associated genes were differentially expressed between Post-M and proliferative stage epithelial cells, three differentially expressed between Post-M and secretory stage, and nine were different between the proliferative and secretory stages of the menstrual cycle (Supplemental Table 2).
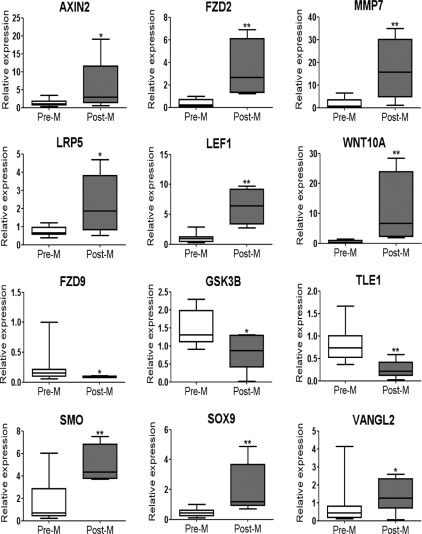
Validation of Wnt-associated genes
Nine genes of the canonical Wnt pathway and three transcription factors were validated by qRT-PCR (Fig. 3). AXIN2, FZD2, MMP7, LEF1, LRP5, SMO, SOX9 VANGL2, and WNT10A were significantly increased in epithelial cells of Post-M compared with Pre-M (proliferative and secretory) endometrium. FZD9, GSK3β, and TLE1 had significantly lower expression. This validation was concordant with the gene profile data using RNA both from the original microarray samples and from an extra set of patient samples (Table 1). This showed the sensitivity of identifying the differentially expressed genes between Post-M and Pre-M endometrium.
Fig. 3.
Validation of selected Wnt canonical pathway and transcription factor genes by qRT-PCR. All 12 genes examined showed statistically significant differences between Pre-M (proliferative and secretory, n = 14, white bar) and Post-M (n = 5, gray bar). Relative expression was normalized to 18S. Data are presented as whisker plots showing medians and minimum and maximum range (95% confidence interval). **, P < 0.001; *, P < 0.05.
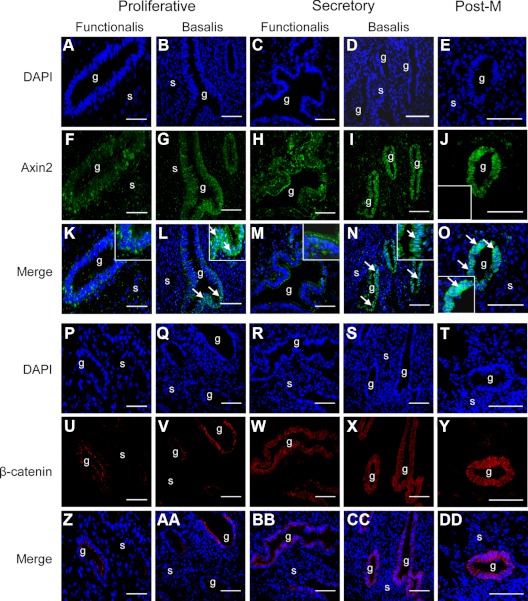
Cellular localization of key regulators of the Wnt pathway, Axin2, and β-catenin
Because Axin2 is a key negative regulator of β-catenin in the canonical Wnt/β-catenin pathway (31, 32), the location of both Axin2 and β-catenin in full-thickness Pre-M and Post-M endometrium was investigated. Immunofluorescence revealed cytoplasmic staining of Axin2 in the functionalis of proliferative (Fig. 4, F and K) and secretory (Fig. 3, H and M) endometrial glandular epithelia. In contrast, nuclear Axin2 staining was observed in the basalis of proliferative (Fig. 4, G and L) and secretory (Fig. 4, I and N) Pre-M and in Post-M endometrial epithelia (Fig. 4, J and O).
Fig. 4.
Immunofluorescence images of Axin2 and β-catenin in Pre-M and Post-M endometrium. Nuclear staining is shown with DAPI (A–E and P–T). Localization of Axin2 in the functionalis (F and H) and basalis (G and I) glandular epithelium of proliferative (F and K) and secretory (I and N) Pre-M and in Post-M (J and O) endometrium. Colocalization of DAPI and Axin2 shows cytoplasmic staining in the functionalis (K and M) and nuclear staining in the basalis epithelium (L and N) of proliferative and secretory, and Post-M endometrium (O). Insets, Axin2 negative control (J), enlargement of Axin2 cytoplasmic localization in the functionalis (K and M) and nuclear staining in basalis of Pre-M (L and N) and in Post-M endometrium (O). Arrows, Axin2 nuclear staining. Localization of cytoplasmic β-catenin in the functionalis (U, W, Z, and BB) and basalis (V, X, AA, and CC) glandular epithelium of proliferative and secretory Pre-M and in Post-M (Y and DD) endometrium. Colocalization of DAPI and β-catenin shows cytoplasmic and plasma membrane staining in the functionalis (Z and BB), basalis (AA and CC) of proliferative and secretory, and Post-M (DD) endometrium. g, Glands; s, stroma. All images were taken at ×40 magnification and are representative of five proliferative, four secretory, and three Post-M endometrial samples.
β-Catenin was located in the cytoplasm and on the plasma membrane but not the nucleus of both functionalis (Fig. 4, U, W, Z, and BB) and basalis (Fig. 4, V, X, AA, and CC) of proliferative and secretory Pre-M, and Post-M endometrial glandular epithelium (Fig. 4, Y and DD). β-Catenin staining was more prominent in Post-M endometrium.
Enriched biological pathways
In this study, we also identified differentially expressed genes that are associated with stem cell signaling networks, stem cell regulation, and cell fate determination, including bone morphogenetic protein (BMP), NOTCH, Hedgehog, and fibroblast growth factor (FGF) signaling pathways (Table 3). To determine whether stem cell networks were menstrual cycle dependent, the data from Pre-M samples separated into proliferative and secretory were compared with each other and individually with Post-M (Supplemental Table 3). This comparison showed 28 differentially expressed genes between Post-M and proliferative endometrial epithelial cells distributed across the BMP (four), NOTCH (six), Hedgehog (four), and FGF (14) signaling pathways (Supplemental Table 3). It showed 19 differentially expressed genes between Post-M and secretory samples in the BMP (seven), NOTCH (two), Hedgehog (three), and FGF (seven) pathways but only three between proliferative and secretory Pre-M samples (one NOTCH and two FGF genes) (Supplemental Table 3). This finding highlights that the greatest number of significant differentially expressed stem cell network genes was between Post-M and Pre-M proliferative, and Post-M and Pre-M secretory epithelial cells.
Table 3.
Biological pathways associated with stem cell networks in the differentially expressed genes of human Post-M vs. Pre-M endometrial epithelial cells
| Gene symbol | Gene description | Post-M vs. Pre-M |
|
|---|---|---|---|
| P value | Fold change | ||
| BMP signaling pathway | |||
| NFKB1 | Nuclear factor of κ light polypeptide gene enhancer in B-cells 1 | 0.028 | −2.1 |
| PRKAG2 | Protein kinase, AMP-activated, γ 2 noncatalytic subunit | 0.005 | −2.5 |
| MAPK13 | MAPK 13 | 0.014 | −2.9 |
| BMP6 | Bone morphogenetic protein 6 | 0.026 | −3.2 |
| MAP2K1 | MAPK kinase 1 | 0.010 | −3.3 |
| NOTCH signaling pathway | |||
| DTX3 | Deltex homolog 3 (Drosophila) | 0.001 | 3.5 |
| NOTCH4 | Notch 4 | 0.017 | 2.3 |
| HEY1 | Hairy/enhancer-of-split related with YRPW motif 1 | 0.013 | −3.9 |
| Hedgehog signaling pathway | |||
| SMO | Smoothened homolog (Drosophila) | 0.015 | 2.1 |
| DYRK1A | Dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase 1A | 0.030 | −2.0 |
| PRKAG2 | Protein kinase, AMP-activated, γ 2 noncatalytic subunit | 0.005 | −2.5 |
| FGF signaling pathway | |||
| FGF9 | Fibroblast growth factor 9 (glia-activating factor) | 0.015 | 4.7 |
| FGF18 | Fibroblast growth factor 18 | 0.001 | 2.6 |
| CRK | v-crk sarcoma virus CT10 oncogene homolog (avian) | 0.018 | −2.3 |
| PIK3C2A | Phosphoinositide-3-kinase, class 2, α polypeptide | 0.040 | −2.4 |
| MAP2K3 | MAPK kinase 3 | 0.002 | −2.7 |
| MAP2K1 | MAPK kinase 1 | 0.010 | −3.3 |
| RPS6KA5 | Ribosomal protein S6 kinase, 90 kDa, polypeptide 5 | 0.006 | −3.5 |
| Stem cell fate determination | |||
| SOX9 | SRY (sex determining region Y)-box 9 | 0.004 | 3.0 |
| DACH1 | Dachshund homolog 1 (Drosophila) | 0.006 | 2.4 |
| KLF5 | Kruppel-like factor 5 (intestinal) | 0.031 | −2.1 |
| Stem cell communication regulators | |||
| ASCL2 | Achaete-scute complex homolog 2 (Drosophila) | 0.036 | 2.2 |
| GJB1 | Gap junction protein, β 1, 32 kDa | 0.023 | −3.2 |
| PPARG | Peroxisome proliferator-activated receptor γ | 0.005 | −6.0 |
| Pluripotency maintenance and regulation | |||
| IL6ST | IL-6 signal transducer (gp130, oncostatin M receptor) | 0.004 | −2.8 |
| EDNRB | Endothelin receptor type B | 0.021 | −3.5 |
| SPHK1 | Sphingosine kinase 1 | 0.000 | −8.1 |
Fold change are for comparison between Post-M vs. Pre-M endometrial epithelial cells. Positive values indicate higher and negative values indicate lower expression. Gene annotations were obtained from Gene ALaCart. P < 0.05.
A set of nine genes involved in endometrial cancer progression was differentially expressed. This included four differentially expressed genes between Post-M and proliferative, four between Post-M and secretory, and three between proliferative and secretory (Supplemental Fig. 3). Among these, several genes were associated with the Wnt signaling pathway, suggesting that Wnt pathway is important in endometrial development, function, and proliferative diseases.
Other enriched gene families in endometrial epithelial cells
Genes of the GO category negative regulation of cell proliferation (indicative of cell quiescence) were enriched in Post-M endometrial epithelial cells, suggestive of minimal epithelial cell proliferation, including the putative ESP population, in Post-M endometrium. These genes included RARRES1 (12.9-fold) and RXRA (2.6-fold), DMBT1 (9.5-fold), CEBPA (2.7-fold), and ADAMST1 (2.6-fold) (Table 4). Immune/wounding response genes, including S100A8 (11.8-fold), S100A9 (11.4-fold), and WDFC2 (2.6-fold), were also higher in Post-M compared with Pre-M endometrial epithelium (Table 4).
Table 4.
List of highly enriched gene families differentially expressed between Post-M and Pre-M (proliferative and secretory) endometrial epithelial cells
| Gene symbol | Gene description | Post-M vs. Pre-M |
|
|---|---|---|---|
| P value (<0.05) | Fold change | ||
| Regulation of cell proliferation | |||
| RARRES1 | Retinoic acid receptor responder (tazarotene induced) 1 | 0.005 | 12.90 |
| DMBT1 | Deleted in malignant brain tumors 1 | 0.031 | 9.48 |
| VANGL2 | Vang-like 2 (van gogh, Drosophila) | 0.002 | 3.96 |
| SOX9 | SRY (sex determining region Y)-box 9 | 0.004 | 2.99 |
| CEBPA | CCAAT/enhancer binding protein (C/EBP), α | 0.001 | 2.77 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 motif, 1 | 0.006 | 2.64 |
| RXRA | Retinoid X receptor, α | 0.030 | 2.61 |
| SMO | Smoothened homolog (Drosophila) | 0.015 | 2.06 |
| Defense response | |||
| S100A9 | S100 calcium binding protein A9 | 0.027 | 11.83 |
| S100A8 | S100 calcium binding protein A8 | 0.003 | 11.43 |
| WFDC2 | WAP four-disulfide core domain 2 | 0.001 | 2.65 |
| CCL4L1 | Chemokine (C-C motif) ligand 4-like 1 | 0.031 | 2.45 |
| Cell cycle regulators | |||
| CDKN1C | Cyclin-dependent kinase inhibitor 1C | 0.018 | 2.00 |
| CDK7 | Cyclin-dependent kinase | 0.025 | −2.20 |
| CCNE1 | Cyclin E1 | 0.024 | −2.30 |
| WEE1 | WEE1 homolog (S. pombe) | 0.014 | −2.33 |
| CDC5 liter | Cell division cycle 5-like | 0.015 | −2.40 |
| CABLES1 | Cdk5 and Ab1 enzyme substrate 1 | 0.000 | −5.16 |
| Lipid biosynthesis process | |||
| CH25H | Cholesterol 25-hydroxylase | 0.000 | 4.67 |
| HMGCS1 | 3-Hydroxy-3-methylglutaryl-CoA synthase 1(soluble) | 0.011 | −2.80 |
| HMGCR | 3-Hydroxy-3-methylglutaryl-CoA reductase | 0.015 | −3.64 |
| PPARG | Peroxisome proliferator-activated receptor γ | 0.005 | −6.00 |
| HSD17B2 | Hydroxysteroid (17-β) dehydrogenase 2 | 0.001 | −26.88 |
| Secretoglobin family | |||
| SCGB3A1 | Secretoglobin, family 3A, member 1 | 0.002 | 6.19 |
| SCGB2A2 | Secretoglobin, family 2A, member 2 | 0.040 | −6.00 |
| SCGB1D2 | Secretoglobin, family 1D, member 2 | 0.005 | −31.11 |
| SCGB1D4 | Secretoglobin, family 1D, member 4 | 0.002 | −90.07 |
| Metallothionein family | |||
| MT1E | Metallothionein 1E | 0.015 | −2.40 |
| MTE | Metallothionein 1I (pseudogene) | 0.028 | −2.73 |
| MT1F | Metallothionein 1F | 0.003 | −3.38 |
| MT1H | Metallothionein 1H | 0.000 | −6.95 |
| MT1G | Metallothionein 1G | 0.001 | −7.57 |
| Extracellular matrix, tissue remodeling | |||
| MMP7 | Matrix metallopeptidase 7 (matrilysin, uterine) | 0.015 | 7.91 |
| MMP11 | Matrix metallopeptidase 11 (stromelysin 3) | 0.030 | 4.65 |
| MMP25 | Matrix metallopeptidase 25 | 0.045 | −2.39 |
| MAP4K5 | MAPK kinase kinase kinase 5 | 0.016 | −2.11 |
| MAP4K2 | MAPK kinase kinase kinase 2 | 0.003 | −2.12 |
| MAP2K3 | MAPK kinase 3 | 0.002 | −2.71 |
| MAP4K3 | MAPK kinase kinase kinase 3 | 0.043 | −2.79 |
| MAPK13 | MAPK 13 | 0.014 | −2.94 |
| MAP2K1 | MAPK kinase 1 | 0.010 | −3.27 |
| Keratin family | |||
| KRT17 | Keratin 17 | 0.015 | 7.80 |
| KRT18 | Keratin 18 | 0.042 | −2.10 |
| KRTAP4-7 | Keratin-associated protein 4-7 | 0.045 | −2.17 |
| KRT19 | Keratin 19 | 0.016 | −2.49 |
| KRT80 | Keratin 80 | 0.025 | −2.82 |
| KRT13 | Keratin 13 | 0.004 | −3.53 |
Fold change for each gene is shown; positive values indicate higher and negative values indicate lower expression between Post-M and Pre-M (proliferative and secretory) endometrial epithelial cells. Data were generated from IPA and DAVID databases. Gene annotations were obtained from Gene ALaCart. P < 0.05 and fold change >2.
As expected, cell cycle regulatory genes CDK7 (−2.2-fold), CCNE1 (−2.3-fold), WEE1 (−2.3-fold), CDC5L (−2.4-fold), and CABLES1 (−5.2-fold) and lipid/steroid hormone molecules, HMGCS1 (−2.8-fold), HMGCR (−3.6-fold), PPARG (−6.0-fold), and HSD17B2 (−26.9-fold), were decreased in Post-M compared with Pre-M endometrial epithelium (Table 4), indicating the lack of epithelial growth in the absence of endogenous circulating estrogen. It also confirmed that Pre-M endometrial epithelium is modulated by hormones during the transition from estrogen stimulated proliferation to progesterone induced differentiation (18, 33).
Transporter molecules, the secretoglobin family, SCGB2A2 (−6.0-fold), SCGB1D2 (−31.1-fold), and SCG1D4 (−90.1-fold), and the metallothionein family, MT1E (−2.4-fold), MTE (−2.7-fold), MT1F (−3.4-fold), MTH (−7.0-fold), and MT1G (−7.6-fold), were down-regulated in Post-M compared with Pre-M (proliferative and secretory) endometrial epithelial cells. Tissue remodeling molecules, including members of the matrix metalloproteinase family, MMP7 (7.9-fold), MMP11 (4.6-fold), and MMP25 (−2.4-fold), and the MAPK kinase family (fold change from −3.3 to −2.1) were also differentially expressed between Post-M and Pre-M endometrial epithelial cells. This confirmed the role of matrix metalloproteinases in the monthly tissue remodeling of the endometrium and was consistent with earlier studies (17, 34). In addition, the keratin family, KRT17 (7.8-fold), KRT18 (−2.1-fold), KRTA4–7, (−2.2-fold), KRT19 (−2.5-fold), KRT80 (−2.8-fold), and KRT13 (−3.5-fold), also showed differential expression between Post-M and Pre-M, suggesting possible novel epithelial-specific markers that could distinguish between epithelium in the functionalis and basalis endometrium.
Differentially regulated endometrial epithelial genes in common with other published literature
We propose that Post-M endometrium is similar to the basalis of Pre-M endometrium and that an ESP cell population resides in the basalis. However, the gene profile of epithelial cells in Post-M and the basalis of Pre-M endometrium has not been previously examined. We therefore compared our gene set with published microarray datasets on endometrial epithelial cells isolated from functionalis vs. basalis using LCM (16, 17). From a list of 71 genes identified in the Gaide Chevronnay et al. study (16) comparing epithelial cells from functionalis and basalis layer, 21 genes were found in common with Post-M and Pre-M epithelial samples of this study (Supplemental Table 4). Seven of the 21 genes showed increased and 14 genes decreased expression in Post-M compared with Pre-M. This represents a 6.5-fold enrichment of Pre-M basalis genes in Post-M epithelium than would be expected for random distribution.
In another comparative analysis from a list of 416 differentially expressed genes between endometrial explants cultured with and without sex steroid hormones (17), a set of 57 genes was found in common with the present study comparing uncultured Post-M and Pre-M (proliferative and secretory) endometrial epithelial cells. Of these, 12 genes showed increased and 45 decreased expression in Post-M compared with Pre-M respectively (Supplemental Table 5). Lists of higher and lower differentially expressed genes between Post-M and Pre-M and their respectively GO classifications are provided in Supplemental Tables 6–9.
Discussion
In this study, we compared gene expression profiles of purified epithelial cells isolated from full-thickness Pre-M (proliferative and secretory) and basalis-like Post-M endometrium to identify gene pathways that distinguish the quiescent Pre-M basalis and Post-M epithelium from the rapid remodeling functionalis epithelium. Hierarchical clustering analyses revealed major gene differences between Post-M and Pre-M endometrial epithelial cells, and in particular 22 Wnt-associated genes were modulated in Post-M compared with Pre-M (proliferative and secretory) endometrial epithelial cells. These genes included a Wnt ligand (WNT10A), Wnt receptors (FZD2 and FZD9) and coreceptor (LRP5), negative regulators of the Wnt pathway (AXIN2 and GSK3β), and downstream transcriptional factor/targets of the Wnt pathway (MMP7, LEF1, TLE1, and CTBP2). The differential expression of several members of the canonical Wnt pathway within the endometrial epithelial compartment was striking, especially in Post-M, suggesting that Wnt signaling is likely down-regulated in Post-M endometrium, owing to the nuclear location of the negative regulator, Axin2. This would explain the quiescent state of Post-M endometrial epithelium until it is reexposed to estrogen (5, 24). Furthermore, components of the Wnt cascade are hormonally regulated, because a large number of genes were differentially expressed between cycling (Pre-M) and noncycling (Post-M) endometrium, confirming and extending previous studies in mouse and human endometrium (19, 23). These findings suggest that the Wnt signaling pathway is important in endometrial epithelial growth and regression, possibly through down-regulating the proliferative activity of ESP cells postulated to reside in the basalis. In addition, comparison of our data with that of Gaide Chevronnay et al.(16, 17) demonstrates and confirms that Post-M endometrial epithelium has a similar gene expression profile to the basalis of Pre-M endometrium.
Our identification of cytoplasmic β-catenin expression in glandular epithelial cells of Post-M and proliferative and secretory stages of Pre-M endometrium is consistent with previous reports (21, 24). Nuclear location of Axin2 but not β-catenin was observed in our study, suggesting that Axin2 may work with glycogen synthase kinase 3β to retain β-catenin in the proteosomal degradation complex (35, 36). More importantly, nuclear staining of Axin2 in Post-M endometrial epithelium indicates negative feedback action on the canonical Wnt pathway to maintain cytoplasmic β-catenin and subsequent down-regulation of downstream transcriptional targets (31, 32, 37). Nuclear Axin2 was selectively found in the basalis of proliferative and secretory Pre-M endometrial epithelial cells, indicating the similarity in Wnt pathway activity between the basalis of Pre-M and Post-M endometrium. Hierarchical clustering showed similar gene expression patterns between proliferative and secretory epithelium in comparison with Post-M endometrial epithelium. Together, these findings suggest that Axin2 regulates Wnt signaling (31, 32) to maintain the relative quiescent state of Post-M glandular epithelium. Wnt signaling is essential for maintaining stem/progenitor cell function, particularly in the intestine, where it is involved in stem cell self-renewal and Paneth cell differentiation (38). Similarly, Wnt signaling is involved in endometrial growth and differentiation during the menstrual cycle (26, 39), thus we suggest that it may have a role in regulating the endometrial ESP cell compartment.
The differential expression and enrichment of BMP, NOTCH, Hedgehog, and FGF signaling pathway genes identified in this study suggests the possible existence of stem cell signaling networks. More stem cell pathway genes were identified when Pre-M samples were separated into proliferative and secretory and individually compared with Post-M (Supplemental Table 3), emphasizing the importance of stem cell signaling pathways in Post-M epithelium. The coexistence of Wnt signaling molecules and BMP, NOTCH, Hedgehog, and FGF signaling cascades suggests cross talk between these pathways, which may function to regulate adult stem cell fate (40–42). It is possible that cross talk between Wnt and these developmental pathways may have a role in regulating and maintaining ESP cells to control epithelial homeostasis, further strengthening the hypothesis that this population may be located within the basalis layer of the endometrium. However, it is important to acknowledge that the ESP cell compartment is very small compared with the remaining epithelial cells, even in Post-M endometrium, and that these stem cell signaling pathways may have roles in “basalis-like” epithelial cells.
It is of interest that negative regulation of cell proliferation (indicates quiescence) genes were highly expressed in basalis-like Post-M endometrial epithelium. Highly prominent genes in this category were the cell proliferation regulator, RXRA, whose expression was expected in atrophic glands (43, 44), a tissue remodeling gene ADAMST1 and an estrogen-modulated gene, DMBT1, essential for epithelial proliferation and regeneration (45, 46). The annotation of these highly expressed genes in Post-M endometrium suggests their possible functional role in regulating endometrial epithelial cell proliferation in the basalis of Pre-M and their potential roles in regulating putative resident ESP cells. This study also found that cell cycle regulatory genes had lower expression in Post-M compared with Pre-M endometrial epithelium, confirming previous reports showing the slow proliferation rate and reduced mitotic activity of Post-M and basalis of Pre-M endometrium (2, 6, 8, 47).
HSD17β2, a key target of estrogen metabolism and regulated by progesterone, was highly expressed in Pre-M, consistent with previous reports (14, 45, 48). The low expression level of HSD17β2 in basalis-like Post-M endometrium reflects the low estrogen hormonal status (2, 3). The extracellular matrix/tissue remodeling genes found in the present study agree with others (17, 49, 50), indicating the active involvement of these molecules in remodeling the endometrium throughout a women's reproductive years (17). The diminished expression of transporter molecules and the secretoglobin family in Post-M compared with Pre-M endometrium (34, 46, 51) indicates the quiescent state of Post-M endometrial epithelium and confirms the high level of cellular nutrient uptake in cycling functionalis endometrium and their important role in endometrial receptivity. Similarly, the metallothionein family found lowly expressed in Post-M compared with Pre-M (proliferative and secretory) endometrial epithelial cells, suggest their important role in detoxification and protection of proliferating cells from heavy metal toxicity in the latter (34, 52). Members of the keratin family identified in this study could be useful to distinguish basalis and functionalis epithelial cells in cycling and noncycling endometrium. Further characterization of these members is required to determine their specific role in endometrial epithelium.
In this study, the differential gene expression data generated from human endometrial epithelial cells was concordant with a LCM gene array study comparing basalis and functionalis epithelium in menstruating endometrium (16, 17). Our comparative analysis showed that differentially expressed genes obtained from Post-M samples were similar to gene profiles of epithelial cells in the basalis layer of menstrual Pre-M endometrium (16). Furthermore, highly expressed genes in Post-M endometrial epithelial cells identified in our study were found in common and similar with gene profiles of endometrial glands in explants cultured in the absence of hormones (17). These two key comparisons between our study and that of Gaide Chevronnay et al. (16, 17) further support our proposal that Post-M is similar to the basalis of Pre-M endometrium and that it operates independently of sex steroid hormones (3). The few differences in gene expression observed between the comparative studies could be due to different experimental design, method of epithelial cell isolation and microarray platform used.
To our knowledge, the gene expression data presented here is the first to analyze and compare purified epithelial cells isolated from Pre-M and Post-M endometrium of hysterectomy tissue using highly specific magnetic bead sorting. The identification of endometrial epithelial-specific genes (MMP7 and WNT7A) and the notable absence of stromal-specific genes (WNT4 and DKK) validates our approach. These epithelial-specific genes are consistent with previous reports (16, 17, 21), indicating that our technique used for isolating homogenous human endometrial epithelial cells was highly selective. Our experimental design comparing full-thickness, hormone-responsive cycling Pre-M endometrium with basalis-like, hormone-depleted Post-M endometrium has elucidated a likely gene profile for basalis endometrial epithelium, which would normally be masked by genes contributed by the more active and abundant functionalis layer. We also reasoned that the ESP population would be enriched in Post-M samples, because there was no functionalis to dilute their contribution to the gene expression as in Pre-M samples. The identification of many differentially expressed stem cell network genes between Post-M and Pre-M, including the 22 Wnt signaling pathway genes, supports this contention. Similarly, given the relatively small contribution of the basalis in Pre-M samples, the gene expression signal for any given gene is diluted by this proportion. It is expected that a slight diminution in fold change is relative to the proportion of basalis compared with the functionalis of Pre-M samples. However, the main variable for obtaining statistical significance for differential expression is consistency between samples.
This study lays the groundwork for several immediate investigations, including the identification of endometrial ESP cell markers and examining the role of ESP cells in endometrial epithelial regeneration. Previous studies have shown that regeneration of the functionalis epithelium originates from remaining gland stumps located in the basalis (53, 54). Rare clonogenic epithelial cells likely have a role (9, 10). However, the exact origin and location of endometrial ESP cells remain unknown. Several morphological studies suggest that regeneration of new endometrial epithelium during menses results from stromal differentiation (55, 56). Alternatively, vascular endothelial growth factor may have a role, because it is important for luminal epithelial migration during repair and endometrial regeneration in primate and mouse models (57). We suggest that basalis gene products contribute to endometrial regeneration via regulating the ESP cell compartment, whereas functionalis gene products may be involved in amplifying the stem cell response, differentiation, and endometrial breakdown (2, 58, 59). The data presented in this study could be used to extend previous reports on endometrial regeneration (55–57) to elucidate the origin and location of ESP cells. The involvement of epithelial-specific genes in hormone-induced regeneration could be broadened by comparing this study with that of previous published gene expression profiles of Post-M endometrium treated with hormones (5, 60).
This study comparing the transcriptome of basalis-like Post-M and full-thickness Pre-M endometrial epithelial cells provides evidence suggesting a similar molecular gene signature between Post-M and Pre-M basalis epithelium. However, the specific gene contribution from the luminal and glandular epithelium was not addressed. Future studies using specific markers distinguishing luminal and glandular epithelium are needed to determine the relative roles of these two epithelial compartments in endometrial epithelial regeneration. Our quantitative data were consistent for all samples examined, because the underlying nonendometrial pathologies of prolapse, fibroids, and menorrhagia showed no apparent effect, and adenomyosis samples were only used for immunohistochemical analysis.
An important finding of this study is that an intact canonical Wnt signaling cascade is present within the endometrial epithelial compartment in both Pre-M and Post-M endometrium. At the transcript level, these Wnt molecules are differentially expressed between Post-M and Pre-M endometrial epithelial cells. At a protein level, we have shown that Axin2, a key negative regulator of the canonical Wnt signaling pathway likely shuttles between cytoplasmic and nuclear compartments, suggesting that Axin2 is actively involved in β-catenin regulation in human endometrium. This finding indicates that the Wnt signaling pathway is important in endometrial epithelial function, especially in Post-M and in the basalis of Pre-M endometrium, where there is minimum proliferative activity. The Wnt signaling pathway may influence endometrial epithelial regeneration through regulating cell fate decisions of the ESP cell population possibly present in the basalis through interactions with niche cells. Given the essential role of Wnt signaling in stem cell biology, current investigations in our laboratory are extending the present finding, by identifying markers of endometrial ESP cells and examining the role of Wnt signaling in these cells.
Supplementary Material
Acknowledgments
We thank Professor Neil Watkins for his advice on the experimental design, Dr. Helen Abud for helpful advice on Wnt signaling molecules, Dr. Hirotaka Masuda for critical reading of the manuscript and together with Dr. Camden Lo (Monash Micro Imaging) for advice on immunofluorescence protocol and analyses, Dr. Aidan Sudbury and Dr. Jason Li for statistical and bioinformatics advice, respectively, and Ms. Frances Walker, Ms. Pamela Mamers, and Ms. Yao Han for collection of endometrial tissues.
This work was supported by Australian National Health and Medical Research Council Grants, R.D Wright Career Development Award 465121 and 1021127 (C.E.G.) and 145780 and 288713 (C.N.S.), the Australian Government Department of Health and Ageing Grant 1002743 (C.N.S.), National Institutes of Health U19 A1067773-07 (C.N.S.), the Australian National Breast Cancer Foundation (C.N.S.), and the Victorian Government's Operational Infrastructure Support Program and an Australian Postgraduate Awards (H.P.T.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Axin2
- Axin-related protein 2
- BMP
- bone morphogenetic protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- DAVID
- database for annotation, visualization and integrated discovery
- DNase
- deoxyribonuclease
- ESP
- epithelial stem/progenitor
- FGF
- fibroblast growth factor
- GO
- gene ontology
- IPA
- Ingenuity Pathway Analysis
- LCM
- laser capture microdissection
- Post-M
- postmenopausal
- Pre-M
- premenopausal
- qRT-PCR
- quantitative RT-PCR
- RMA
- robust multichip average.
References
- 1. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. 2006. Endocrine regulation of menstruation. Endocr Rev 27:17–46 [DOI] [PubMed] [Google Scholar]
- 2. Gargett CE. 2007. Uterine stem cells: what is the evidence? Hum Reprod Update 13:87–101 [DOI] [PubMed] [Google Scholar]
- 3. Kurman RJ, Ellenson LH, Ronnett BM, McCluggage WG. 2011. Benign diseases of the endometrium. In: Blaustein's pathology of the female genital tract. New York: Springer; 305–358 [Google Scholar]
- 4. Paulson RJ, Boostanfar R, Saadat P, Mor E, Tourgeman DE, Slater CC, Francis MM, Jain JK. 2002. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA 288:2320–2323 [DOI] [PubMed] [Google Scholar]
- 5. Klaassens AH, van Wijk FH, Hanifi-Moghaddam P, Sijmons B, Ewing PC, Ten Kate-Booij MJ, Kooi GS, Kloosterboer HJ, Blok LJ, Burger CW. 2006. Histological and immunohistochemical evaluation of postmenopausal endometrium after 3 weeks of treatment with tibolone, estrogen only, or estrogen plus progestagen. Fertil Steril 86:352–361 [DOI] [PubMed] [Google Scholar]
- 6. Padykula HA, Coles LG, Okulicz WC, Rapaport SI, McCracken JA, King NW, Jr, Longcope C, Kaiserman-Abramof IR. 1989. The basalis of the primate endometrium: a bifunctional germinal compartment. Biol Reprod 40:681–690 [DOI] [PubMed] [Google Scholar]
- 7. Padykula HA. 1991. Regeneration in the primate uterus: the role of stem cells. Ann NY Acad Sci 622:47–56 [DOI] [PubMed] [Google Scholar]
- 8. Ferenczy A, Bertrand G, Gelfand MM. 1979. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol 133:859–867 [DOI] [PubMed] [Google Scholar]
- 9. Chan RW, Schwab KE, Gargett CE. 2004. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 70:1738–1750 [DOI] [PubMed] [Google Scholar]
- 10. Schwab KE, Chan RW, Gargett CE. 2005. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril 84 1124–1130 [DOI] [PubMed] [Google Scholar]
- 11. Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. 2009. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 80:1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T, Okai T. 2005. Differences in gene expression in the proliferative human endometrium. Fertil Steril 83:1206–1215 [DOI] [PubMed] [Google Scholar]
- 13. Yanaihara A, Otsuka Y, Iwasaki S, Koide K, Aida T, Okai T. 2004. Comparison in gene expression of secretory human endometrium using laser microdissection. Reprod Biol Endocrinol 2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 15. Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PAW. 2004. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod 10:879–893 [DOI] [PubMed] [Google Scholar]
- 16. Gaide Chevronnay HP, Galant C, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. 2009. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 150:5094–5105 [DOI] [PubMed] [Google Scholar]
- 17. Gaide Chevronnay HP, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. 2010. Ovarian steroids, mitogen-activated protein kinases, and/or aspartic proteinases cooperate to control endometrial remodeling by regulating gene expression in the stroma and glands. Endocrinology 151:4515–4526 [DOI] [PubMed] [Google Scholar]
- 18. Niklaus AL, Pollard JW. 2006. Mining the mouse transcriptome of receptive endometrium reveals distinct molecular signatures for the luminal and glandular epithelium. Endocrinology 147:3375–3390 [DOI] [PubMed] [Google Scholar]
- 19. Miller C, Pavlova A, Sassoon DA. 1998. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 76:91–99 [DOI] [PubMed] [Google Scholar]
- 20. Parr BA, McMahon AP. 1998. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707–710 [DOI] [PubMed] [Google Scholar]
- 21. Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. 2003. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 88:3860–3866 [DOI] [PubMed] [Google Scholar]
- 22. Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. 2006. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91:1453–1461 [DOI] [PubMed] [Google Scholar]
- 23. Bui TD, Zhang L, Rees MC, Bicknell R, Harris AL. 1997. Expression and hormone regulation of Wnt2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer 75:1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou X, Tan Y, Li M, Dey SK, Das SK. 2004. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC, Kim JJ, Grootegoed JA, Burger CW, Fodde R, Blok LJ. 2009. Progesterone inhibition of Wnt/β-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res 15:5784–5793 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, van der Zee M, Fodde R, Blok LJ. 2010. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget 1:674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noyes R, Hertig A, Rock J. 1975. Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- 28. Bolstad BM, Irizarry RA, Astrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 [DOI] [PubMed] [Google Scholar]
- 29. Sprung CN, Li J, Hovan D, McKay MJ, Forrester HB. 2011. Alternative transcript initiation and splicing as a response to DNA damage. PLoS One 6:e25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Cong F, Varmus H. 2004. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of β-catenin. Proc Natl Acad Sci USA 101:2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stacey K, Beasley B, Wilce PA, Martin L. 1991. Effects of female sex hormones on lipid metabolism in the uterine epithelium of the mouse. Int J Biochem 23:371–376 [DOI] [PubMed] [Google Scholar]
- 34. Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. 2002. Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- 35. Cadigan KM, Peifer M. 2009. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1:a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caspi M, Zilberberg A, Eldar-Finkelman H, Rosin-Arbesfeld R. 2008. Nuclear GSK-3β inhibits the canonical Wnt signalling pathway in a β-catenin phosphorylation-independent manner. Oncogene 27:3546–3555 [DOI] [PubMed] [Google Scholar]
- 37. Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. 2002. Activation of AXIN2 expression by β-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem 277:21657–21665 [DOI] [PubMed] [Google Scholar]
- 38. van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. 2002. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250 [DOI] [PubMed] [Google Scholar]
- 39. Nei H, Saito T, Yamasaki H, Mizumoto H, Ito E, Kudo R. 1999. Nuclear localization of β-catenin in normal and carcinogenic endometrium. Mol Carcinog 25:207–218 [PubMed] [Google Scholar]
- 40. Katoh M. 2007. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev 3:30–38 [DOI] [PubMed] [Google Scholar]
- 41. Nakamura T, Tsuchiya K, Watanabe M. 2007. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol 42:705–710 [DOI] [PubMed] [Google Scholar]
- 42. Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. 2011. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472:110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alfaro JM, Fraile B, Lobo MV, Royuela M, Paniagua R, Arenas MI. 2003. Immunohistochemical detection of the retinoid X receptors α, β, and γ in human prostate. J Androl 24:113–119 [PubMed] [Google Scholar]
- 44. Wu Y, Cai Y, Aguilo J, Dai T, Ao Y, Wan Y-JY. 2004. RXRα mRNA expression is associated with cell proliferation and cell cycle regulation in Hep3B cell. Exp Mol Pathol 76:24–28 [DOI] [PubMed] [Google Scholar]
- 45. Tan YF, Li FX, Piao YS, Sun XY, Wang YL. 2003. Global gene profiling analysis of mouse uterus during the oestrous cycle. Reproduction 126:171–182 [DOI] [PubMed] [Google Scholar]
- 46. Ace CI, Okulicz WC. 2004. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod Biol Endocrinol 2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Felix JC, Farahmand S. 1997. Endometrial glandular proliferation and estrogen receptor content during the normal menstrual cycle. Contraception 55:19–22 [DOI] [PubMed] [Google Scholar]
- 48. Mustonen MV, Isomaa VV, Vaskivuo T, Tapanainen J, Poutanen MH, Stenbäck F, Vihko RK, Vihko PT. 1998. Human 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J Clin Endocrinol Metab 83:1319–1324 [DOI] [PubMed] [Google Scholar]
- 49. Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. 2002. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8:871–879 [DOI] [PubMed] [Google Scholar]
- 50. Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen KG. 1994. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest 94:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Müller-Schöttle F, Classen-Linke I, Alfer J, Krusche C, Beier-Hellwig K, Sterzik K, Beier HM. 1999. Expression of uteroglobin in the human endometrium. Mol Hum Reprod 5:1155–1161 [DOI] [PubMed] [Google Scholar]
- 52. Ioachim EE, Kitsiou E, Carassavoglou C, Stefanaki S, Agnantis NJ. 2000. Immunohistochemical localization of metallothionein in endometrial lesions. J Pathol 191:269–273 [DOI] [PubMed] [Google Scholar]
- 53. Ferenczy A. 1976. Studies on the cytodynamics of human endometrial regeneration. I. Scanning electron microscopy. Am J Obstet Gynecol 124:64–74 [DOI] [PubMed] [Google Scholar]
- 54. Ludwig H, Metzger H. 1976. The re-epithelization of endometrium after menstrual desquamation. Arch Gynakol 221:51–60 [DOI] [PubMed] [Google Scholar]
- 55. Garry R, Hart R, Karthigasu KA, Burke C. 2009. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod 24:1393–1401 [DOI] [PubMed] [Google Scholar]
- 56. Garry R, Hart R, Karthigasu KA, Burke C. 2010. Structural changes in endometrial basal glands during menstruation. BJOG-Int J Obstet Gy 117:1175–1185 [DOI] [PubMed] [Google Scholar]
- 57. Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. 2008. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J 22:3571–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chan RW, Gargett CE. 2006. Identification of label-retaining cells in mouse endometrium. Stem Cells 24:1529–1538 [DOI] [PubMed] [Google Scholar]
- 59. Chan RW, Kaitu'u-Lino T, Gargett CE. 2012. Role of label retaining cells in estrogen-induced endometrial regeneration. Reprod Sci 19:102–114 [DOI] [PubMed] [Google Scholar]
- 60. Hanifi-Moghaddam P, Boers-Sijmons B, Klaassens AH, van Wijk FH, den Bakker MA, Ott MC, Shipley GL, Verheul HA, Kloosterboer HJ, Burger CW, Blok LJ. 2007. Molecular analysis of human endometrium: short-term tibolone signaling differs significantly from estrogen and estrogen + progestagen signaling. J Mol Med 85:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.