Abstract
The enteroendocrine and enteric nervous systems convey signals through an overlapping network of regulatory peptides that act either as circulating hormones or as localized neurotransmitters within the gastrointestinal tract. Because recent studies invoke an important role for vasoactive intestinal peptide (VIP) as a downstream mediator of glucagon-like peptide-2 (GLP-2) action in the gut, we examined the importance of the VIP-GLP-2 interaction through analysis of Vip−/− mice. Unexpectedly, we detected abnormal villous architecture, expansion of the crypt compartment, increased crypt cell proliferation, enhanced Igf1 and Kgf gene expression, and reduced expression of Paneth cell products in the Vip−/− small bowel. These abnormalities were not reproduced by antagonizing VIP action in wild-type mice, and VIP administration did not reverse the intestinal phenotype of Vip−/− mice. Exogenous administration of GLP-2 induced the expression of ErbB ligands and immediate-early genes to similar levels in Vip+/+ vs. Vip−/− mice. Moreover, GLP-2 significantly increased crypt cell proliferation and small bowel growth to comparable levels in Vip+/+ vs. Vip−/− mice. Unexpectedly, exogenous GLP-2 administration had no therapeutic effect in mice with dextran sulfate-induced colitis; the severity of colonic injury and weight loss was modestly reduced in female but not male Vip−/− mice. Taken together, these findings extend our understanding of the complex intestinal phenotype arising from loss of the Vip gene. Furthermore, although VIP action may be important for the antiinflammatory actions of GLP-2, the Vip gene is not required for induction of a gene expression program linked to small bowel growth after enhancement of GLP-2 receptor signaling.
Enteroendocrine cells are distributed throughout the stomach and small and large intestine and constitute an important cellular network coordinating the control of nutrient ingestion, gall bladder emptying and pancreatic exocrine secretion, gut motility, energy absorption, and nutrient disposal. These actions are accomplished by the regulated synthesis and secretion of dozens of regulatory peptides that act in a paracrine, neural, and endocrine manner to control energy intake and assimilation. Accordingly, there is considerable interest in understanding how the enteroendocrine network originates signals that communicate with local and distant targets.
Proglucagon-derived peptides (PGDP) represent a subset of enteroendocrine-derived hormones that have attracted considerable interest due to their actions on control of food intake, gastrointestinal motility, and insulin and glucagon secretion (1). Tissue-specific posttranslational processing of proglucagon in pancreas, intestine, and brain underlies the complexity of PGDP production in mammals (2). In the pancreas, the major bioactive PGDP is glucagon, whereas enteroendocrine cells produce oxyntomodulin, glicentin, and two glucagon-like peptides (GLP), GLP-1 and GLP-2 (3). Within the gastrointestinal tract, GLP-1 engages the enteric nervous system, leading to control of gut motility and activation of a gut-brain axis that regulates blood flow, insulin secretion, and glucose disposal in peripheral tissues (4). The diverse glucoregulatory actions of GLP-1 underlie the development of medications based on potentiation of GLP-1 action for the treatment of type 2 diabetes (5).
GLP-2 cosecreted together with GLP-1 from L cells acts more proximally in the gut to enhance nutrient absorption (6). Exogenous GLP-2 administration also expands the mucosal surface area of the small bowel by stimulating crypt cell proliferation and inhibiting enterocyte apoptosis (7). GLP-2 also engages antiinflammatory pathways in the intestinal mucosa, and administration of GLP-2 receptor (GLP-2R) agonists attenuates intestinal inflammation in multiple preclinical models of gut injury (8–15). The actions of GLP-2 to promote nutrient absorption, reduce gut motility, and decrease intestinal injury have prompted assessment of whether GLP-2R agonists might be useful for the treatment of human subjects with short bowel syndrome or inflammatory bowel disease (16, 17).
Despite a considerable number of studies describing actions of GLP-2 on crypt cells, immune cells, and enterocytes, the precise mechanisms mediating the actions of GLP-2 within the gut mucosa remain incompletely understood (18). A single receptor transducing the actions of GLP-2 has been identified that exhibits considerable amino acid and structural identity with related members of the class B G protein-coupled receptor family (19). Localization of the GLP-2R to specific cell types in the gut has been challenging, in part due to the low level of GLP-2R expression, and the quality of antisera required for detection of the GLP-2R with high sensitivity and specificity. The GLP-2R has been localized to enteric neurons, myofibroblasts, and subsets of enteroendocrine cells in studies of the rodent, porcine, and human gastrointestinal tract (18). Surprisingly, however, GLP-2Rs have not been localized to crypt cells or enterocytes, implying that many of the effects of GLP-2 are indirect, generated by one or more downstream effectors liberated from GLP-2R+ cell types.
Analyses of mechanisms mediating GLP-2 action in the small and large intestine have employed receptor antagonists, immunoneutralizing antisera, and knockout mice. To date, keratinocyte growth factor, IGF-I, and members of the ErbB superfamily have been implicated as growth factors transducing the proliferative effects of GLP-2 in the gut (20–22). In contrast, the mechanisms through which GLP-2 exerts its antiinflammatory actions are less well understood. However, several studies have identified vasoactive intestinal peptide (VIP) as a GLP-2-activated target that contributes to amelioration of inflammation after GLP-2 administration in the 2,4,6-trinitrobenzene sulfonic acid (TNBS) or dextran sulfate (DS) models of rat colitis (11).
VIP is a 28 amino acid peptide that is widely expressed in the central, peripheral, and enteric nervous systems. Within the gut, VIP regulates gut motility, and disturbances of VIP innervation have been implicated in the pathophysiology of irritable bowel syndrome. VIP also exhibits cytoprotective and vasoactive actions and displays immunomodulatory activity in experimental models of inflammation, predominantly in the central and peripheral nervous system. Many of the actions ascribed to VIP overlap those identified for pituitary adenylate cyclase-activating polypeptide (PACAP), a 28-amino-acid neuropeptide that exhibits considerable amino acid identity with VIP. Furthermore, both VIP and PACAP are capable of activating three structurally related receptors, albeit with varying potency, providing considerable complexity in attribution of precise biological actions to a single peptide and receptor (23–25). Because VIP and GLP-2 exhibit an overlapping spectrum of actions that encompass effects on blood flow, inflammation, and cell proliferation and survival, and VIP has been implicated as a downstream target of GLP-2 action (11, 26), we have now examined the requirement for VIP in a spectrum of GLP-2 actions in Vip−/− mice.
Materials and Methods
Animals
C57BL/6 VIP/peptide histidine isoleucine knockout mice (Vip−/−) have been described previously (27). Vip+/+ littermates were used as controls for all studies involving Vip−/− mice. Except where indicated, studies were performed on female mice aged 8–12 weeks bred at the Toronto Centre for Phenogenomics animal facility. Wild-type (WT) mice of the C57BL/6 background were obtained from the in house colony at the Toronto Centre for Phenogenomics. C57BL/6J-ApcMin/+ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and crossed with Vip−/− mice to generate ApcMin/+:Vip+/− mice, which were then bred to generate ApcMin/+/Vip+/+ and ApcMin/+/Vip−/− mice. Polyp burden was assessed in littermate female mice at 14–15 wk of age. All animal experiments were approved by the Animal Care Committee of the Mount Sinai Hospital.
Peptide and drug treatments
Human [Gly2] GLP-2, hence referred to as GLP-2, was from Pepceutical Ltd. (Nottingham, UK), VIP was purchased from Bachem (Torrance, CA) and the VIP receptor antagonist [Lys1-Pro2,5-Arg3,4-Tyr6] VIP (VIP hybrid) (28) from Sigma-Aldrich (Oakville, Ontario, Canada). Peptides were dissolved in PBS (vehicle) and administered to mice by sc injection. Experiments involving analysis of DS-induced colitis were carried out as previously described (15). A clinical disease activity index ranging from 0–4 (29) was determined by scoring stool consistency, presence or absence of fecal blood, and weight loss. To assess intestinal crypt cell proliferation, 5-bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich; 100 mg/kg) dissolved in PBS was injected ip to mice 1 h before death.
Tissue collection, morphometry, immunohistochemistry, and polyp evaluation
Small intestine and colon were collected, flushed with PBS to remove luminal contents, and weighed, and lengths were measured under constant tension. Adjacent 2-cm intestinal segments were obtained from jejunum and colon and fixed in 10% neutral-buffered formalin and paraffin embedded or snap-frozen in liquid nitrogen and stored at −70 C. Digital image acquisition and morphometry were done on 5-μm histological sections stained with hematoxylin and eosin as described (22, 30, 31). Immunohistochemistry was carried out using indirect immunoperoxidase detection with NovaRED substrate (Vector Laboratories, Burlington, Ontario, Canada) followed by hematoxylin counterstaining. A rabbit polyclonal antilysozyme antibody (DakoCytomation, Mississauga, Ontario, Canada) was used to detect Paneth cells. BrdU immunopositivity was detected using a mouse monoclonal anti-BrdU antibody (Invitrogen Canada, Burlington, Ontario, Canada). The incidence of BrdU staining at each cell position within the crypt was scored in a minimum of 100 half-crypts per mouse. To assess polyp burden in ApcMin/+ mice, the small and large intestines were removed and flushed with PBS. The small intestine was divided into three equal segments (proximal, middle, and distal). Then, the intestines were opened longitudinally, laid flat on Whatman paper, and fixed in 10% neutral-buffered formalin for 24 h. Fixed intestines were stained with methylene blue and examined for polyps with the use of a dissection microscope equipped with an eyepiece micrometer.
Western blot analysis
Whole-tissue extracts were prepared by homogenization of intestinal segments in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate in PBS) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich), 5 mm sodium fluoride, and 200 μm sodium orthovanadate. Protein (30–45 μg) was used for Western blot analysis as described (22, 31). The rabbit polyclonal antibody reactive to lysozyme was from DakoCytomation. A mouse monoclonal antibody against heat-shock protein 90 (BD Biosciences, Mississauga, Ontario, Canada) was used as a loading control.
RNA isolation and quantitative real-time RT-PCR
Total RNA from intestinal tissue was extracted by the guanidinium thiocyanate method and cDNA synthesis performed with random hexamers and SuperScript II (Invitrogen Canada). Real-time quantitative PCR was performed on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) with TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assays (Applied Biosystems) for amphiregulin (Mm00437583_m1), cryptdin 5 (Mm00651548_g1), defcr-rs1 (Mm00655850_m1), egf (Mm00438696_m1), egr-1 (Mm00656724_m1), epiregulin (Mm00514794_m1), c-fos (Mm00487425_m1), glp2r (Mm01329473_m1), hb-egf (Mm-00439307_m1), igf-1 (Mm00439560_m1), kgf (Mm00433291_m1), lysozyme P (Mm00657323_m1), pacap (Mm00437433_m1), phlda-1, (Mm00456345_g1), proglucagon (Mm00801712_m1), regIIIα (Mm00441121_m1), tff3 (Mm00495590_m1), and tgfa (Mm00446232_m1). Relative quantification of transcript levels was performed by the 2−ΔCt method using the cycle threshold (Ct) values obtained from the PCR amplification kinetics with the ABI PRISM SDS version 2.1 software. The 18S rRNA was used for normalization because its intestinal expression remained unaltered regardless of mouse genotype or treatment.
Statistics
Results are expressed as mean ± se. Statistical significance was assessed by ANOVA followed by the Bonferroni post hoc test and, where appropriate, by unpaired Student's t test using GraphPad Prism version 4 (GraphPad Software, San Diego, CA).
Results
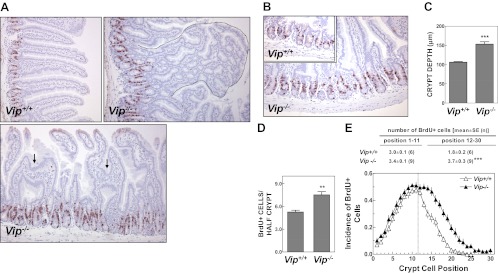
Because previous experiments assessing the importance of VIP as a downstream mediator of GLP-2 action have employed peptide antagonists that may incompletely attenuate VIP action (11, 32), we initiated studies using Vip−/− mice (27). We first examined the baseline phenotype of the Vip−/− gut. Unexpectedly, Vip−/− jejunum exhibited aberrant villous architecture, with a high incidence of spongiform polyps and intervillous bridges, consistent with villous fusion, a rare histological abnormality occasionally observed in the setting of enteritis (Fig. 1A). Crypt cell proliferation within the murine small bowel is classically quantified by assessing the position and number of proliferating cells expressing endogenous markers of cell cycle progression (Ki67, or proliferating cell nuclear antigen) or by determination of the number of cells that have taken up BrdU (33). Both crypt depth (Fig. 1, B and C) and crypt cell proliferation (Fig. 1D) were significantly increased in the jejunum of Vip−/− mice. Analysis of the number and distribution of proliferating cells within the crypt compartment revealed a significant shift in the number and position of BrdU+ cells in Vip−/− jejunum (Fig. 1E).
Fig. 1.
Abnormal villus-to-crypt axis architecture in the jejunum of the Vip−/− mouse. A and B, Photomicrographs illustrating typical villous aberrations (A), including intervillous bridges (arrows) and a spongiform polyp (outlined with a dotted line), and the distinct enlarged crypt compartment (B) in the jejunum of the Vip−/− mouse. Images were obtained from tissue sections stained for BrdU and counterstained with hematoxylin. C–E, Crypt depth (C) and crypt cell proliferation (D and E) in the jejunum of Vip−/− mice and WT littermate controls. Tissue sections were scored for total number of BrdU+ cells per half-crypt (D) and incidence of BrdU staining at each cell position within the crypt (E). Position 1 corresponds to the base of the crypt. For C–E, n = 6–9 mice per group combined from two independent experiments. **, P < 0.0; ***, P < 0.001, Vip−/− vs. Vip+/+.
The disordered control of small bowel growth in Vip−/− mice prompted us to examine the mRNA levels of key peptides and growth factors in the small vs. the large bowel of Vip−/− vs. Vip+/+ mice. Levels of PACAP, Igf1, and KGF mRNA transcripts were significantly up-regulated, whereas levels of proglucagon were reduced in the Vip−/− small bowel (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). In contrast, both proglucagon (Gcg), and Glp2r mRNA transcripts were increased in the Vip−/− colon, whereas no changes in levels of TGFα, EGF, or Tff3 mRNA transcripts were detected in the small or large bowel of Vip−/− mice.
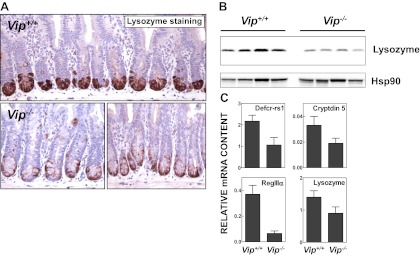
A reduced intensity of lysozyme-positive Paneth cells was observed by immunohistochemical analysis at the crypt base in the Vip−/− small bowel (Fig. 2A). Consistent with these findings, levels of lysozyme were clearly reduced in jejunal extracts from Vip−/− compared with Vip+/+ mice (Fig. 2B). We recently described reduced expression of antimicrobial gene products and reduced bactericidal activity in mucosal extracts of Glp2r−/− mice, findings consistent with a defect of Paneth cell activity (34). Because Paneth cells play an important specialized role in the defense against microbial-induced intestinal injury within the gastrointestinal tract (35), we assessed the expression of mRNA transcripts for key Paneth cell genes in the Vip−/− small bowel. Analysis of Paneth cell gene products revealed reduced levels of not only lysozyme P but also of Defcr-rs1, cryptdin 5, and RegIIIα mRNA transcripts in RNA from Vip−/− jejunum (Fig. 2C). Taken together, these findings extend previous descriptions of the basal intestinal phenotype of Vip−/− mice (36).
Fig. 2.
Reduced expression of Paneth cell-specific products in the jejunum of the Vip−/− mouse. A, Immunohistochemical detection of lysozyme in the jejunal crypt compartment of mice of the indicated Vip genotype. Photomicrographs are representative of four to six mice per group. B, Western blot analysis of lysozyme expression in whole-tissue jejunal extracts from four Vip−/− mice and four WT littermates. Anti-heat-shock protein 90 (Hsp90) antibody was used to monitor loading and transfer conditions. C, Relative mRNA levels of Paneth cell markers in the jejunum of Vip−/− and Vip+/+ mice as assessed by real-time quantitative RT-PCR (n = 8–12 mice per group combined from three independent experiments).
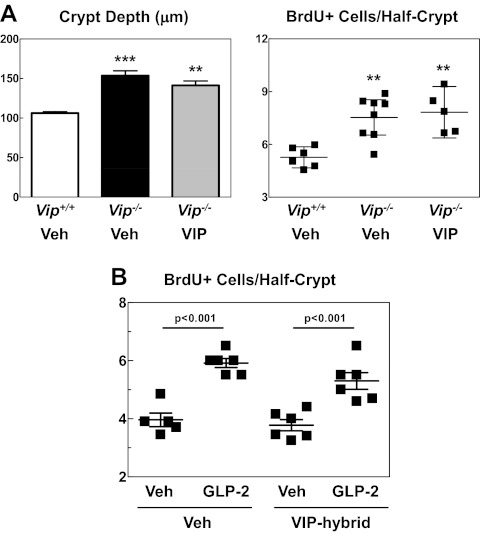
The increased rates of crypt cell proliferation in Vip−/− jejunum may reflect ongoing VIP deficiency and/or developmental changes arising from embryonic absence of Vip gene products during mouse development. To determine the reversibility of this phenotype, we treated mice with exogenous VIP and reassessed crypt cell proliferation. VIP administration had no effect on crypt depth or crypt cell proliferation in Vip−/− mice (Fig. 3A). To ascertain whether diminution of VIP action in WT mice would recapitulate the intestinal findings observed in Vip−/− mice, we treated WT mice with a VIP peptide hybrid antagonist, 100 μg/kg, for 25 d (11). Administration of the VIP hybrid alone for 25 d had no effect on crypt cell proliferation in WT mice (Fig. 3B). Furthermore, the ability of GLP-2 to robustly increase jejunal crypt cell proliferation was unaltered in mice chronically treated with the antagonist VIP hybrid (Fig. 3B). Hence, reduction of VIP action does not affect basal or GLP-2-stimulated control of crypt cell proliferation. To ascertain the potential biological significance of the increased rate of crypt cell proliferation observed in Vip−/− mice in a sensitized model of tumor formation, we generated ApcMin/+:Vip−/− mice and assessed the polyp burden within the intestines. No significant differences in polyp number or size were observed in ApcMin/+:Vip−/− vs. ApcMin/+:Vip+/+ mouse intestines (Supplemental Fig. 2).
Fig. 3.
A, Jejunal crypt depth and crypt cell proliferation after the administration of VIP (2 nmol per mouse) or vehicle alone (Veh) every other day for 11 d in Vip−/− and Vip+/+ littermates as indicated (n = 5–8 mice per group). Each data point in the right panel corresponds to one mouse. **, P < 0.01; ***, P < 0.001, Vip−/−, either VIP- or vehicle-treated, vs. Vip+/+. B, Basal (vehicle-treated) vs. GLP-2-stimulated jejunal crypt cell proliferation in WT C57BL6 mice administered VIP hybrid (100 μg/kg once a day) or vehicle alone (Veh) for 25 d. BrdU labeling was assessed after treatment for 6 h with GLP-2 (0.2 mg/kg, injected every 3 h) or vehicle. Each data point corresponds to one mouse (n = 5–6 mice per group). The statistical significance for the comparison GLP-2 vs. vehicle is indicated.
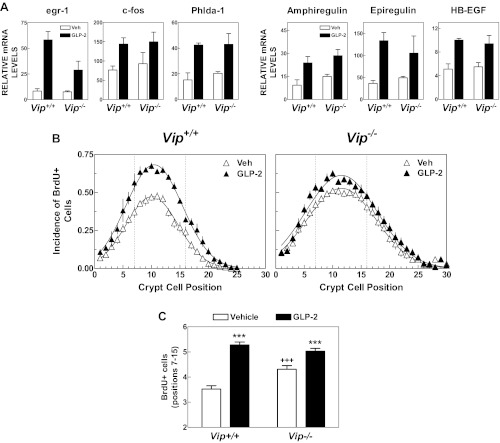
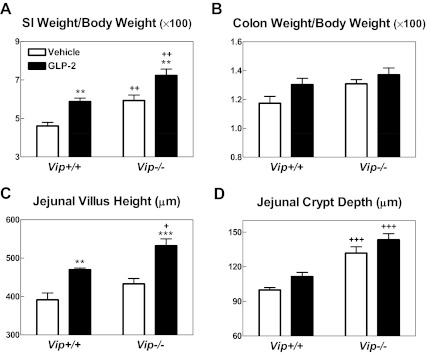
Because GLP-2 and VIP exert antiinflammatory, proliferative, and cytoprotective actions in the gastrointestinal tract, we further assessed the requirement for VIP as a downstream mediator of GLP-2 action in the murine gut. Acute GLP-2 administration robustly increased the abundance of mRNA transcripts for immediate-early genes and ErbB ligands, including egr-1, c-fos, Phlda-1, amphiregulin, epiregulin, and Hb-EGF to a comparable level in Vip+/+ vs. Vip−/− mice (Fig. 4A). Consistent with previous findings, acute GLP-2 administration significantly increased crypt cell proliferation in the jejunum of Vip+/+ mice (Fig. 4B). Although the basal rate of crypt cell proliferation was higher in Vip−/− vs. Vip+/+ mice, GLP-2 significantly increased crypt cell proliferation in Vip−/− mice (Fig. 4, B and C). Furthermore, administration of GLP-2 (0.2 mg/kg) once daily for 9 d produced significant increases in small bowel weight and jejunal villous height in both Vip+/+ and Vip−/− mice (Fig. 5). Jejunal crypt depth and colon weight trended higher but were not significantly different in GLP-2-treated mice (Fig. 5, B and D). Hence, VIP is not required for the GLP-2-stimulated induction of ErbB ligands, immediate-early gene expression, crypt cell proliferation, or small bowel growth.
Fig. 4.
The acute jejunal response to GLP-2 is preserved in the Vip−/− mouse. A, Levels of mRNA transcripts of immediate-early genes (egr-1, c-fos, and Phlda-1) and ErbB ligands (amphiregulin, epiregulin, and HB-EGF) were quantified by real-time quantitative RT-PCR in total RNA from jejunum of Vip−/− mice and WT littermates treated for 45 min with GLP-2 (0.2 mg/kg) or vehicle alone (n = 4 mice per group combined from two independent experiments). B and C, BrdU labeling in the jejunum of Vip−/− mice and Vip+/+ littermates after treatment for 6 h with GLP-2 (0.2 mg/kg, injected every 3 h) or vehicle alone (Veh). Incidence of BrdU+ cells along the longitudinal crypt axis (B) and total number of BrdU+ cells scored in positions 7–15 (C). Position 1 corresponds to the base of the crypt. Data are combined from two independent experiments with a total of six to nine mice per group. To facilitate visual comparisons, the crypt BrdU positional data were fitted to a Gaussian function that is indicated by a solid line. Two-way ANOVA indicated that the crypt BrdU positional distribution curves from vehicle- and GLP-2-treated mice were significantly different (P < 0.001) for both Vip genotypes. ***, P < 0.001, GLP-2 vs. vehicle; +++, P < 0.001, Vip−/− vs. Vip+/+.
Fig. 5.
Relative small intestine (SI) (A) and colonic (B) weight and jejunal histomorphometry (C and D) after the administration of vehicle or GLP-2 (0.2 mg/kg once a day) for 9 d in Vip+/+ and Vip−/− littermates (n = 7 mice per group combined from two independent experiments). **, P < 0.01; ***, P < 0.001, GLP-2 vs. vehicle; +, P < 0.05, ++, P < 0.01; +++, P < 0.001, Vip−/− vs. Vip+/+. No differences in the small intestine length were found between genotypes or treatments.
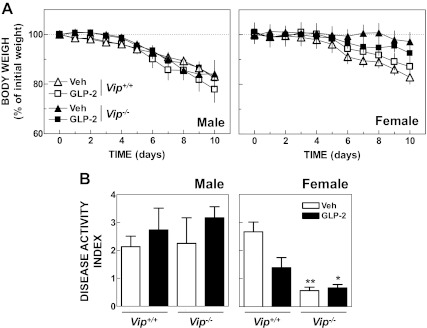
Although GLP-2 action is comparatively greater in the small relative to the large intestine, exogenous GLP-2 administration stimulates colonic growth (20) and attenuates injury to the colonic mucosa in mice with DS-induced colitis (10, 15). Furthermore, previous studies have implicated VIP as an essential downstream antiinflammatory mediator of GLP-2 action in rodents with experimental intestinal injury (11). Accordingly, we assessed the severity of intestinal injury in Vip+/+ vs. Vip−/− mice with DS-induced colitis in the presence or absence of concomitant GLP-2 administration. No difference in weight loss (Fig. 6A) or in the severity of colitis (Fig. 6B) was observed across genotypes in the presence or absence of GLP-2 administration, although untreated female Vip−/− mice exhibited resistance to DS administration. In contrast, GLP-2 treatment stimulated small intestinal growth in mice given DS (Supplemental Fig. 3).
Fig. 6.
A, Relative body weight from Vip+/+ and Vip−/− male (n = 4–5 per group) and female mice (n = 6–7 per group) given dextran sodium sulfate (2.5% for males and 3.0% for females) in the drinking water for 10 d and administered GLP-2 (0.2 mg/kg) or vehicle once (female) or twice (male) daily. Two-way ANOVA indicated that the weight loss curves from vehicle-treated Vip+/+ and Vip−/− female mice were significantly different (P < 0.001). B, Disease activity index in Vip+/+ and Vip−/− male and female mice after 10 d of oral DS and vehicle or GLP-2 treatment. *, P < 0.05; **, P < 0.01, GLP-2- and vehicle-treated, respectively, Vip−/− vs. vehicle-treated Vip+/+.
Discussion
The Vip−/− mouse exhibits a number of interesting phenotypes, including disturbances of circadian rhythm (27), impaired or enhanced inflammatory responses (37, 38), and dysglycemia with abnormal sweet taste preference (39). Very little is known about the basal intestinal phenotype of Vip−/− mice; however, increased small bowel weight and smooth muscle thickening, together with increased villous length and reduced staining of mucus in the small bowel has been described in the Vip−/− mouse (36). Moreover, older Vip−/− mice appeared susceptible to the development of intestinal obstruction. Genetic elimination of the VIP receptor type 1 (VPAC1) gene results in impaired neonatal growth, increased mortality of mice around the time of weaning, and increased mucosal cell proliferation and thickening of the bowel wall, detected in mice analyzed at 8 wk of age (40). In contrast, although VPAC2 knockout mice exhibit enhanced susceptibility to DS-induced colitis (41), a detailed analysis of the basal phenotype of the VPAC2−/− small bowel has not yet been reported. Similarly, although genetic elimination of the PACAP gene results in enhanced sensitivity to both small and large bowel injury (42, 43), a small bowel phenotype arising in uninjured PACAP−/− mice has not yet been described.
We now extend our understanding of the importance of Vip gene products for small bowel growth by demonstrating enhanced crypt cell proliferation and reduced levels of Paneth cell-specific products in the Vip−/− small bowel. Furthermore, the increased crypt cell proliferation was not reversible by VIP replacement and was not reproduced in WT mice treated with VIP hybrid, a VIP antagonist. Hence, this phenotype likely evolves at least in part due to abnormalities arising from deficiency of one or more Vip gene products during development. Interestingly, we demonstrated up-regulation of PACAP gene expression in the jejunum and colon of Vip−/− mice (Supplemental Fig. 1), raising the possibility that the intestinal phenotype resulting from ablation of the Vip gene is partially masked by compensation from related ligands that activate the same family of VIP/PACAP receptors. Furthermore, up-regulation of Igf1 and KGF gene expression in the Vip−/− small bowel raises the possibility that one or both of these growth factors contributes to the Vip−/− phenotype of increased crypt cell proliferation.
We were interested to examine the importance of VIP as a downstream mediator of GLP-2 action after reports linking VIP to the antiinflammatory actions of GLP-2. Immunohistochemistry and in situ hybridization identified a subset of GLP-2R+ enteric neurons in the porcine and human jejunum, the majority of which also exhibited VIP or endothelial nitric oxide synthase immunoreactivity (44). Subsequent studies demonstrated a functional role for VIP signaling as a downstream mediator of GLP-2 action in rats with TNBS-induced ileitis (11). Both VIP and GLP-2 independently reduced weight loss and myeloperoxidase activity in the inflamed bowel, and in coadministration experiments, a VIP antagonist blocked the antiinflammatory actions of GLP-2 in TNBS-induced enteritis, as documented by increased myeloperoxidase activity, enhanced tissue IL-1β, and failure to suppress IL-10, compared with the effects of GLP-2-treatment alone. Moreover, acute GLP-2 administration significantly increased nuclear c-Fos immunoreactivity in VIP+ ileal submucosal neurons. Quantification of neuronal populations demonstrated that GLP-2 treatment increases the number of VIP+ neurons in the absence of inflammation and prevented the loss of VIP+ neurons in the context of active inflammation (26). VIP has also been proposed as a mediator of the gastric myorelaxant activity induced by GLP-2 in the isolated mouse stomach (32).
Previous studies invoking VIP as an essential downstream target for GLP-2 action have employed the VIP receptor antagonist [Lys1-Pro2,5-Arg3,4-Tyr6] VIP (VIP hybrid) (11, 26, 32). However, this molecule is not completely specific for VIP action but binds to multiple VIP/PACAP receptors, predominantly VPAC1 and VPAC2 (45, 46). Hence, this hybrid antagonist molecule blocks not only endogenous VIP but also endogenous PACAP, and one or both peptides may be critical functional mediators of GLP-2 action. We used Vip−/− mice to identify the critical role of Vip gene products as downstream targets for GLP-2 action. Our data clearly show that the effects of GLP-2 to induce gene expression, crypt cell proliferation, and small bowel growth do not require the Vip gene. Nevertheless, it seems likely that the antiinflammatory actions of GLP-2 described previously are mediated in part by VIP and/or PACAP signaling (11, 26).
Unexpectedly, we did not observe a therapeutic effect of GLP-2 in mice with DS-induced colitis, a model we and others have previously used to characterize the antiinflammatory actions of GLP-2. Notably, previous studies of GLP-2 action in the DS colitis model have used mice in the CD1 background (10, 15), whereas the current study employed mice in the C57BL/6 background. Hence we were unable to assess whether the antiinflammatory actions of GLP-2 in the DS colitis model required VIP due to the lack of GLP-2 efficacy in both male and female Vip+/+ and Vip−/− mice with colitis. Furthermore, Sigalet and colleagues (11) did not examine the interaction of GLP-2 and the VIP hybrid antagonist in rats with DS colitis. Hence, the importance of the GLP-2-VIP interaction in this model of colonic inflammation requires further analysis.
In summary, we demonstrate that Vip−/− mice exhibit markedly increased crypt cell proliferation, increased Igf1 and KGF expression, reduced expression of Paneth cell products, and abnormal villous architecture; however, these histological abnormalities are not reversible after VIP replacement. Furthermore, attenuation of VIP signaling with the antagonist VIP hybrid does not reproduce these findings in WT mice, strongly suggesting that the basal phenotype arises secondary to developmental loss of Vip gene products. Moreover, although VIP may be an important target for the antiinflammatory actions of GLP-2, the Vip gene is not required for the GLP-2-dependent induction of a gene expression program linked to stimulation of crypt cell proliferation and small bowel growth. Additional studies of the relationship linking GLP-2 action to VIP in models of experimental intestinal inflammation may extend our understanding of the importance of VIP as a downstream target for GLP-2 in vivo.
Supplementary Material
Acknowledgments
We thank Jackie Koehler for her technical assistance with intestinal polyp assessment in Apc Min/+ mice and Wendy So for her technical assistance with histology and immunohistochemistry.
This work was supported by Grant CIHR MOP-14799 and D.J.D. was supported by the Canada Research Chairs Program and a BBDC Novo Nordisk Chair in Incretin Biology.
B.Y. and D.H. carried out the experiments, analyzed the data, and wrote the manuscript. B.Y. and D.J.D. designed the experiments, and D.J.D. also wrote the manuscript. J.A.W. generated VIP−/− mice and edited the manuscript.
Disclosure Summary: None of the authors have any disclosures directly related to this manuscript. NPS Pharmaceuticals, D.J.D., the University of Toronto, and the University Health Network are parties to an agreement for licensing of GLP-2 patents.
Footnotes
- BrdU
- 5-Bromo-2′-deoxyuridine
- DS
- dextran sulfate
- GLP
- glucagon-like peptide
- GLP-2R
- GLP-2 receptor
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- PGDP
- proglucagon-derived peptide
- TNBS
- 2,4,6-trinitrobenzene sulfonic acid
- VIP
- vasoactive intestinal peptide
- VPAC1
- VIP receptor type 1
- WT
- wild type.
References
- 1. Drucker DJ. 2002. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 122:531–544 [DOI] [PubMed] [Google Scholar]
- 2. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. 1986. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 261:11880–11889 [PubMed] [Google Scholar]
- 3. Orskov C, Holst JJ, Poulsen SS, Kirkegaard P. 1987. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 30:874–881 [DOI] [PubMed] [Google Scholar]
- 4. Burcelin R. 2010. The gut-brain axis: a major glucoregulatory player. Diabetes Metab 36(Suppl 3):S54–S58 [DOI] [PubMed] [Google Scholar]
- 5. Lovshin JA, Drucker DJ. 2009. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5:262–269 [DOI] [PubMed] [Google Scholar]
- 6. Brubaker PL, Izzo A, Hill M, Drucker DJ. 1997. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol 272:E1050–E1058 [DOI] [PubMed] [Google Scholar]
- 7. Drucker DJ, Erlich P, Asa SL, Brubaker PL. 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA 93:7911–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ivory CP, Wallace LE, McCafferty DM, Sigalet DL. 2008. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 295:G1202–G1210 [DOI] [PubMed] [Google Scholar]
- 10. L'Heureux MC, Brubaker PL. 2003. Glucagon-like peptide-2 and common therapeutics in a murine model of ulcerative colitis. J Pharmacol Exp Ther 306:347–354 [DOI] [PubMed] [Google Scholar]
- 11. Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. 2007. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293:G211–G221 [DOI] [PubMed] [Google Scholar]
- 12. Arthur GL, Schwartz MZ, Kuenzler KA, Birbe R. 2004. Glucagonlike peptide-2 analogue: a possible new approach in the management of inflammatory bowel disease. J Pediatr Surg 39:448–452; discussion 448–452 [DOI] [PubMed] [Google Scholar]
- 13. Boushey RP, Yusta B, Drucker DJ. 1999. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol 277:E937–E947 [DOI] [PubMed] [Google Scholar]
- 14. Boushey RP, Yusta B, Drucker DJ. 2001. Glucagon-like peptide (GLP)-2 reduces chemotherapy-associated mortality and enhances cell survival in cells expressing a transfected GLP-2 receptor. Cancer Res 61:687–693 [PubMed] [Google Scholar]
- 15. Drucker DJ, Yusta B, Boushey RP, DeForest L, Brubaker PL. 1999. Human [Gly2]-GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol 276:G79–G91 [DOI] [PubMed] [Google Scholar]
- 16. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. 2011. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60:902–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. 2010. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm Bowel Dis 16:962–973 [DOI] [PubMed] [Google Scholar]
- 18. Rowland KJ, Brubaker PL. 2011. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol 301:G1–G8 [DOI] [PubMed] [Google Scholar]
- 19. Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. 1999. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96:1569–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. 2005. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept 124:105–112 [DOI] [PubMed] [Google Scholar]
- 21. Dubé PE, Forse CL, Bahrami J, Brubaker PL. 2006. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131:589–605 [DOI] [PubMed] [Google Scholar]
- 22. Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. 2009. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137:986–996 [DOI] [PubMed] [Google Scholar]
- 23. Dickson L, Finlayson K. 2009. VPAC and PAC receptors: From ligands to function. Pharmacol Ther 121:294–316 [DOI] [PubMed] [Google Scholar]
- 24. Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. 2009. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357 [DOI] [PubMed] [Google Scholar]
- 25. Moody TW, Ito T, Osefo N, Jensen RT. 2011. VIP and PACAP: recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Curr Opin Endocrinol Diabetes Obes 18:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigalet DL, Wallace L, De Heuval E, Sharkey KA. 2010. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol Motil 22:1318–e350 [DOI] [PubMed] [Google Scholar]
- 27. Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. 2003. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol 285:R939–R949 [DOI] [PubMed] [Google Scholar]
- 28. Gozes I, McCune SK, Jacobson L, Warren D, Moody TW, Fridkin M, Brenneman DE. 1991. An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. J Pharmacol Exp Ther 257:959–966 [PubMed] [Google Scholar]
- 29. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69:238–249 [PubMed] [Google Scholar]
- 30. Bahrami J, Yusta B, Drucker DJ. 2010. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138:2447–2456 [DOI] [PubMed] [Google Scholar]
- 31. Koehler JA, Harper W, Barnard M, Yusta B, Drucker DJ. 2008. Glucagon-like peptide-2 does not modify the growth or survival of murine or human intestinal tumor cells. Cancer Research 68:7897–7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amato A, Baldassano S, Serio R, Mulè F. 2009. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol 296:G678–G684 [DOI] [PubMed] [Google Scholar]
- 33. Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB. 1995. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut 36:864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee SJ, Lee J, Li KK, Holland D, Maughan H, Guttman DS, Yusta B, Drucker DJ. 2012. Disruption of the murine Glp2r impairs Paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology 153:1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bevins CL, Salzman NH. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9:356–368 [DOI] [PubMed] [Google Scholar]
- 36. Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, Waschek JA. 2007. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease. Peptides 28:1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, Feng JM, Campagnoni AT, Waschek JA. 2010. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 107:19555–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Girard BM, Malley SE, Braas KM, Waschek JA, May V, Vizzard MA. 2008. Exaggerated expression of inflammatory mediators in vasoactive intestinal polypeptide knockout (VIP−/−) mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM. 2010. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes 59:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fabricius D, Karacay B, Shutt D, Leverich W, Schafer B, Takle E, Thedens D, Khanna G, Raikwar S, Yang B, Desmond ME, O'Dorisio MS. 2011. Characterization of intestinal and pancreatic dysfunction in VPAC1-null mutant mouse. Pancreas 40:861–871 [DOI] [PubMed] [Google Scholar]
- 41. Yadav M, Huang MC, Goetzl EJ. 2011. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol 267:124–132 [DOI] [PubMed] [Google Scholar]
- 42. Ferencz A, Nedvig K, Fekecs T, Rácz B, Wéber G, Hashimoto H, Baba A, Helyes Z, Reglödi D. 2010. Comparison of intestinal cold preservation injury on pituitary adenylate cyclase-activating polypeptide in knockout and wild-type mice. Transplant Proc 42:2290–2292 [DOI] [PubMed] [Google Scholar]
- 43. Azuma YT, Hagi K, Shintani N, Kuwamura M, Nakajima H, Hashimoto H, Baba A, Takeuchi T. 2008. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol 216:111–119 [DOI] [PubMed] [Google Scholar]
- 44. Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Hadsell DL, Nichols BL, Burrin DG. 2006. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130:150–164 [DOI] [PubMed] [Google Scholar]
- 45. Gozes I, Meltzer E, Rubinrout S, Brenneman DE, Fridkin M. 1989. Vasoactive intestinal peptide potentiates sexual behavior: inhibition by novel antagonist. Endocrinology 125:2945–2949 [DOI] [PubMed] [Google Scholar]
- 46. Gozes I, Brenneman DE. 1989. VIP: molecular biology and neurobiological function. Mol Neurobiol 3:201–236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.