Abstract
The Ron receptor tyrosine kinase (macrophage stimulating 1 receptor) is overexpressed in approximately 50% of human breast cancers. Transgenic mice overexpressing Ron in the mammary epithelium [mouse mammary tumor virus driven (MMTV)-Ron expressing mice] develop mammary tumors that exhibit up-regulation of β-catenin and β-catenin target genes. β-Catenin has been shown to be a mediator of mammary tumorigenesis in various breast cancer models, including downstream of Ron. However, the in vivo impact of a conditional loss of β-catenin downstream of Ron receptor overexpression on the onset, growth, turnover, and metastasis of mammary tumors has not been addressed. To determine the significance of β-catenin in the context of Ron overexpression, we conditionally deleted β-catenin in mammary epithelial cells of MMTV-Ron mice. Conditional deletion of β-catenin in the mammary epithelium, through the use of whey acidic protein (WAP)-Cre transgenic mice, significantly delayed the onset of mammary hyperplastic nodules, the presence of palpable mammary tumors, and ultimately decreased liver metastasis. β-Catenin loss in this model was also associated with decreased expression of cyclin D1. In total, these studies support an important role for β-catenin downstream of Ron receptor signaling during the development of mammary tumorigenesis.
Breast cancer is the second leading cause of cancer death among women in the United States (American Cancer Society, 2009–2010). Standard treatment options for breast cancer include radiation therapy, chemotherapy, hormone therapy, and/or targeted therapy. Although the use of specific monoclonal antibodies and tyrosine kinase inhibitors in combination with chemotherapy has shown promise in clinical studies, breast tumor recurrence and the lack of continued disease-free survival persist (1, 2). Therefore, the identification of new biomarkers and drug targets remains a high priority.
The Ron receptor tyrosine kinase, also referred to as macrophage stimulating 1 receptor (Mst1R), is up-regulated in about half of human breast cancers (3, 4). In addition to breast cancer, Ron overexpression is also seen in several human epithelial cancers, including lung, stomach, colon, pancreas, and prostate cancers (2, 5–7). Overexpression of Ron has been shown to induce proliferation and migration of cells in vitro and tumor formation in vivo (2, 8, 9). Recently, expression of Ron, hepatocyte growth factor-like protein (HGFL) (the Ron ligand), and matriptase, a serine protease that cleaves pro-HGFL, were associated with poor overall survival among patients without metastatic disease at the time of primary tumor resection (10). Ron activation by HGFL has been shown to trigger activation of a number of signaling cascades, including phosphatidylinositol 3-kinase/Akt, MAPK, c-Jun NH2-terminal kinase, and β-catenin, which affect cell proliferation, differentiation, and migration (6, 7, 11–13).
We have previously shown that overexpression of Ron in the mouse mammary epithelium induces tumor formation in 100% of female mice and is associated with elevated levels of β-catenin and the up-regulation of β-catenin target genes, including cyclin D1 and c-myc (9). Increased β-catenin, localized to the nuclear and cytoplasmic compartments, is observed in about 40% of primary breast tumors and is associated with poor prognosis and poor patient survival (14). Dysregulated β-catenin signaling leads to perturbation of mammary stem and progenitor cell dynamics as well as mammary tumor formation in mice (15–18). The Ron receptor is also an important regulator of pubertal mouse mammary gland development, because mice with a targeted deletion of the Ron tyrosine kinase domain exhibit alterations in mammary ductal extension and branching morphogenesis (19).
Based on these reports, we hypothesized that β-catenin is important for Ron-induced mammary tumor initiation, progression, and metastatic disease in vivo. Here, we show that decreasing the expression of β-catenin within the mammary epithelium of transgenic mice overexpressing Ron induces a delay in palpable mammary tumor development compared with control mice. These differences were associated with a delay in the onset of mammary hyperplasia and the down-regulation of the β-catenin target gene cyclin D1. Our findings demonstrate and further support an important link between the Ron receptor and its downstream signaling mediator, β-catenin, in regulating mammary gland tumorigenesis in vivo.
Materials and Methods
Generation of mice
Mice with mammary-specific Ron overexpression, referred to as mouse mammary tumor virus driven (MMTV)-Ron mice, and mice with a floxed β-catenin allele (β-catF/F) were previously described (9, 20). These two mouse lines were crossed to produce mice overexpressing Ron in the mammary gland and having β-catenin floxed (MMTV-Ron β-catF/F). β-Catenin deletion in the mammary glands of MMTV-Ron β-catF/F mice was achieved by crossing MMTV-Ron β-catF/F mice to mice that expressed the whey acidic protein (WAP)-Cre transgene (21). Our study groups consisted of MMTV-Ron β-catF/F (control) and MMTV-Ron β-catF/F WAP-Cre mice. Genotyping of the transgenic mice was performed by PCR analysis with the following temperature conditions: annealing, 59 C and elongation, 72 C; and primer sets: β-catenin primers forward, 5′-ACT GCC TTT GTT CTC TTC CCT TCT G-3′ and reverse, 5′-CAG CCA AGG AGA GCA GGT GAG G-3′ and WAP-Cre forward, 5′- CAT CAC TCG TTG CAT CGA CC-3′ and reverse 5′-TAG AGC TGT GCC AGC CTC TTC-3′. All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Tumor latency
To accelerate mammary tumorigenesis in our MMTV-Ron model and to offer the most frequent expression of WAP-Cre and ultimately β-catenin deletion, our study animals were maintained as continuous breeding pairs, starting at 8 wk of age until palpable tumor development. Female MMTV-Ron transgenic mice are unable to nurse their offspring to weaning. Therefore, all pups were removed within 1–2 d after parturition from the transgenic dams and breeding continued. Two weeks before a scheduled harvest date, all study animals were isolated and monitored to ensure that dams were not pregnant. If any dams appeared pregnant, they were then scheduled for a later time point. After parturition, pups were removed (if applicable), and dams underwent a minimal 10-d involution period before mammary gland and tissue harvest. Animals were palpated weekly for over 400 d, and palpable tumor incidence was plotted against time. Tumor development in MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were subjected to Kaplan-Meier analysis using GraphPad Prism (GraphPad Software, San Diego, CA) as previously described (9, 11). All mice were injected with bromodeoxuridine (BrdU) 2 h before being euthanized by CO2 asphyxiation, and mammary glands, mammary tumors, liver, and lungs were removed, frozen, or processed for histological examination.
Tissue histology and immunohistochemistry
Thoracic mammary glands were snap frozen for RNA and protein isolation. Any palpable tumor within the thoracic gland was excised, cut into 5-mm slices, and processed for RNA and protein separately from the normal/hyperplastic mammary glands. Inguinal mammary glands were used for whole-mount analysis and histological assessment. Formalin-fixed mammary glands were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin Y for routine histological assessment. To detect β-catenin, formalin-fixed paraffin-embedded sections were incubated in citrate buffer for antigen retrieval as recommended by the manufacturer. After rinsing in PBS, slides were incubated overnight with a polyclonal rabbit antibody directed against β-catenin (RB-9035; Neomarkers, Fremont, CA). β-Catenin was visualized using a biotinylated-conjugated antirabbit secondary antibody and the ABC technique (Vector Laboratories, Burlingame, CA) followed by diaminobenzidine (Sigma, St. Louis, MO). Slides were counterstained with Harris modified hematoxylin (Harris modified; Fisher Scientific, Pittsburgh, PA). A minimum of three animals was assessed per genotype.
Mitotic and cell death indices were assessed in normal ductal epithelium, hyperplastic nodules, and tumors by quantitative morphometric analysis of BrdU incorporation and in situ terminal transferase-mediated 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL). For the mitotic index, mice were injected with BrdU 2 h before harvest, and BrdU was localized in sections with a mouse monoclonal biotinylated anti-BrdU antibody (Zymed Laboratories, Inc., San Francisco, CA), and the ABC technique followed by diaminobenzidine. DNA fragmentation was assessed by TUNEL assay using a commercially available kit (Millipore, Billerica, MA) after optimization of pretreatment conditions and reagent dilutions to ensure that labeling was restricted to cells with apoptotic morphology. For both assays, tissues were counterstained with hematoxylin.
BrdU incorporation and TUNEL were quantified in normal ductal epithelium, hyperplastic nodules, and tumors within tissue sections viewed and photographed at ×400 magnification. A minimum of three animals was assessed per genotype, with three to six fields per section and a minimum of 300 epithelial cells assessed per slide. The photographs were analyzed, and the percentage of epithelial cells that labeled positive for BrdU or TUNEL was calculated.
For whole-mount analysis, mammary glands were fixed in Carnoy's fixative and stained overnight in carmine alum. Samples were dehydrated, cleared in xylene, mounted, and examined on a stereoscope equipped with an Axiovert digital camera.
Quantitative real-time (qRT)-PCR
Total RNA was isolated from tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). qRT-PCR was performed using an ABI7900HT (Applied Biosystems by Life Technologies, Carlsbad, CA) as previously described (19, 22). Briefly, independent mammary gland or tumor RNA preparations from six mice of each genotype were made at the time points indicated. Total RNA was reverse transcribed using the High Capacity kit (Applied Biosystems), and a cDNA stock was generated from each RNA sample. Each cDNA was independently analyzed in duplicate (60 ng of cDNA/well) using specific primer sets that were designed with Primer Express 3.0 software (Applied Biosystems). The primers used the default annealing/elongation temperature (60 C), and a SYBR green (Roche Diagnostics, Indianapolis, IN) dissociation curve was performed for each primer set to ensure that a single gene product was being amplified with each primer set. The resulting primer sets were: β-catenin forward, 5′-TCCCTGAGACGCTAGATGAGG-3′ and reverse, 5′-CGTTTAGCAGTTTTGTCAGCTC-3′ and cyclin D1 forward, 5′- GCGTACCCTGACACCAATCTC-3′ and reverse, 5′- CTCCTCTTCGCACTTCTGCTC-3′. Gene expression values were normalized to β-glucuronidase mRNA: forward, 5′-TTGAGAACTGGTATAAGACGCATCAG-3′ and reverse, 5′-TCTGGTACTCCTCACTGAACATGC-3′ as an internal control and are reported as normalized gene expression. For data presentation, duplicate values from each run were averaged, and the six values were then averaged to generate one value for each genotype/time point. The level of expression was determined using a standard curve (log range −104) dilution series method comparison. The final data are expressed as the mean ± se of six animals per time point.
Western blot analysis
Protein was isolated from tissues as described previously (9). Proteins were separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore). After transfer, the membranes were probed with a rabbit monoclonal anti-β-catenin antibody (1:1000; Cell Signaling, Danvers, MA). The membranes were stripped and reprobed with a mouse monoclonal anticyclin D1 antibody (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Actin or tubulin expression was used as the loading control. A minimum of three animals was assessed per genotype per time point with multiple assessments analyzed per mammary gland.
Statistical analysis
Statistical analysis for the detection of hyperplastic nodules was determined using the χ2 test. Statistical significance for BrdU incorporation, TUNEL analysis, qRT-PCR, and tumor metastasis was determined by the Student's t test using GraphPad Prism software. Data on hyperplastic nodules and tumor development were subjected to Kaplan-Meier analysis using GraphPad Prism software. Means were considered significantly different if a P value of less than 0.05 was obtained.
Results
β-Catenin expression in MMTV-Ron β-catF/F WAP-Cre mice during involution
To determine the impact of altering β-catenin expression within the mammary epithelium during Ron-induced mammary tumorigenesis, we used the MMTV-Ron transgenic mice that were shown previously to develop aggressive and highly metastatic breast cancer as a result of Ron receptor overexpression in the mammary epithelium (9). We crossed the MMTV-Ron mice with mice containing a β-catF/F (20) to produce MMTV-Ron β-catF/F mice. MMTV-Ron β-catF/F mice were subsequently crossed with the well-characterized WAP-Cre mice to conditionally delete β-catenin (21). Study animals consisted of female MMTV-Ron β-catF/F mice and MMTV-Ron β-catF/F WAP-Cre mice.
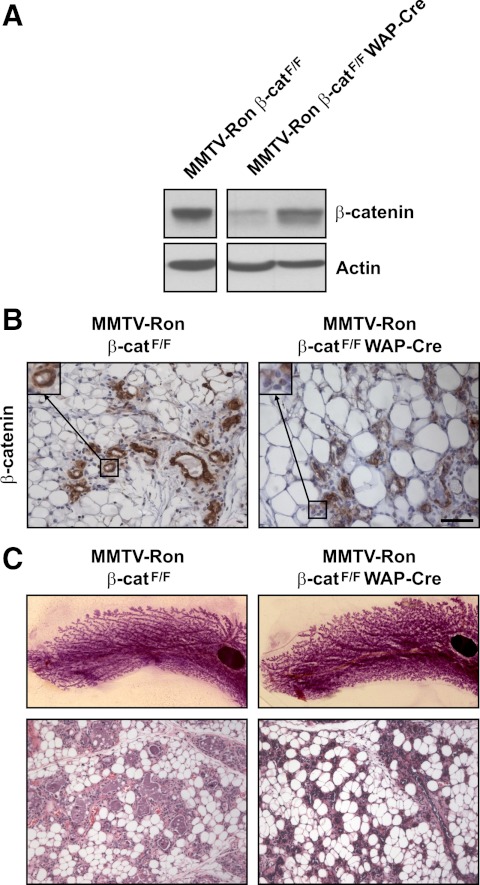
To examine the extent of the conditional loss of β-catenin in MMTV-Ron β-catF/F WAP-Cre mice, mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre were isolated from age-matched, 12- to 14-wk-old, female mice during their first involution cycle at involution d 6. As depicted in Fig. 1A, mammary glands from MMTV-Ron β-catF/F WAP-Cre mice exhibited reduced β-catenin expression as analyzed by Western blot analyses compared with control mice. The reduction in β-catenin expression was evident but variable among the MMTV-Ron β-catF/F WAP-Cre mice. The variability in the reduction of β-catenin expression is depicted in Fig. 1A, where β-catenin expression from mammary glands from two separate MMTV-Ron β-catF/F WAP-Cre mice is shown compared with that observed from a control MMTV-Ron β-catF/F gland. Immunohistochemical labeling of mammary glands from MMTV-Ron β-catF/F WAP-Cre mice also demonstrated a reduction in β-catenin expression compared with the control mammary glands (Fig. 1B) with a mosaic pattern of β-catenin expression being observed throughout the gland. No overt abnormalities were identified in the involuting mammary glands of MMTV-Ron β-catF/F WAP-Cre mice compared with controls based on whole-mount and histological analyses (Fig. 1C). To accelerate mammary tumorigenesis in our MMTV-Ron model and to offer the most frequent expression of WAP-Cre and ultimately β-catenin deletion, our study animals were maintained as continuous breeding pairs to optimize transgene promoter expression, and newly born pups were removed within 48 h after parturition.
Fig. 1.
Efficient deletion of β-catenin in MMTV-Ron β-catF/F WAP-Cre mice at involution d 6. Mammary glands from age-matched MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were isolated at involution d 6 and examined for the extent of β-catenin deletion by Western blot analysis (A) and immunohistochemistry (B). Insets show a 2-fold magnification of a representative mammary duct immunolabeled for β-catenin. Whole-mount and histological (hematoxylin and eosin staining) analysis of corresponding mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice displayed no appreciable differences in mammary gland morphology (C). Scale bar, 50 μm.
Deletion of β-catenin delays the onset of mammary hyperplasia in MMTV-Ron mice
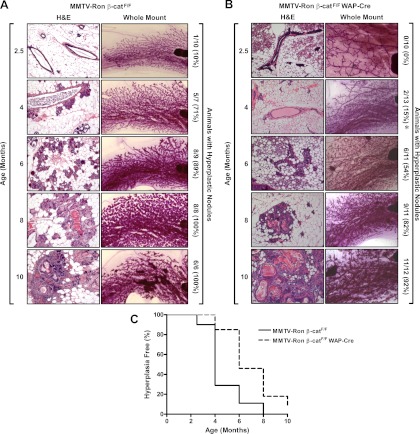
To define the impact that deletion of β-catenin has on the incidence, the initiation of hyperplastic nodules, and the presence of palpable mammary tumors in MMTV-Ron mice, we isolated mammary glands at 2.5, 4, 6, 8, and 10 months of age from MMTV-Ron β-catF/F (control) and MMTV-Ron β-catF/F WAP-Cre mice. Female mice were housed with male mice up until 2 wk before their scheduled harvest date. If any dams appeared pregnant, then they were scheduled for a later time point, allowing for at least a 10-d involution period before mammary gland harvest. Inguinal mammary glands were examined by whole-mount and histological analysis for the presence of hyperplastic nodules. Hyperplastic nodules were determined by the presence of a congregated cell mass around the ducts of the mammary gland. Hyperplastic areas were identified in control MMTV-Ron β-catF/F mammary glands as early as 2.5 months of age with the majority of animals exhibiting mammary hyperplasia by 4 months of age (Fig. 2A). In contrast, no hyperplasia was evident in MMTV-Ron β-catF/F WAP-Cre glands at 2.5 months and only sparsely detectable by 4 months of age (Fig. 2B). A significant difference in the percentage of mice with hyperplastic nodules was found at 4 months comparing the MMTV-Ron β-catF/F WAP-Cre vs. the MMTV-Ron β-catF/F control mice. The percentage of MMTV-Ron β-catF/F WAP-Cre animals with detectable hyperplastic nodules remained less throughout the time course but did not reach statistical significance. By 8 months of age, the extent of hyperplastic nodules within the mammary glands of MMTV-Ron β-catF/F WAP-Cre mice still had not approached that of the control mice, which exhibited mammary hyperplastic nodules in 100% of the mice at this time point. In total, mammary glands from MMTV-Ron β-catF/F WAP-Cre (Fig. 2B) mice had a delay in the onset of mammary gland hyperplastic nodules compared with control mice at every time point examined with statistical differences observed between groups at 4 months of age. The delay in the onset of mammary gland hyperplasia in the MMTV-Ron β-catF/F WAP-Cre mouse model is depicted using a Kaplan-Meier graph with mammary hyperplastic nodules as the end point (Fig. 2C). Thus, the onset of Ron-induced mammary tumorigenesis is delayed in the presence of WAP-Cre expression and a reduction in β-catenin expression.
Fig. 2.
Time course to assess the onset of mammary hyperplasia in MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mammary glands. Mammary hyperplasia was evaluated from inguinal mammary glands taken at 2.5, 4, 6, 8, and 10 months of age in MMTV-Ron β-catF/F (A) and MMTV-Ron β-catF/F WAP-Cre (B) mice by whole-mount and histological analysis. The number of mice that displayed mammary hyperplastic nodules by either whole-mount or histological analysis is indicated along with the total number of mice evaluated per group. The numbers in parentheses indicate the percent of mice that exhibited mammary hyperplastic nodules at the time point indicated. Although a reduction in the percentage of mice with hyperplastic nodules was consistently observed throughout the time course in the MMTV-Ron β-catF/F WAP-Cre mice, only at 4 months was there a significant difference in the percentage of mice with hyperplastic nodules compared with MMTV-Ron β-catF/F control mice. *, P < 0.05 compared with the corresponding time point from MMTV-Ron β-catF/F mice. C, β-Catenin deletion in WAP-Cre transgenic mice significantly delayed the development of hyperplastic nodules. The percent of mammary glands without evidence of the development of hyperplastic nodules is plotted over time. A statistically significant difference in hyperplasia development was observed between groups (P < 0.05). H&E, Hematoxylin and eosin staining.
WAP-Cre-induced deletion of β-catenin delays palpable mammary tumor development in MMTV-Ron mice
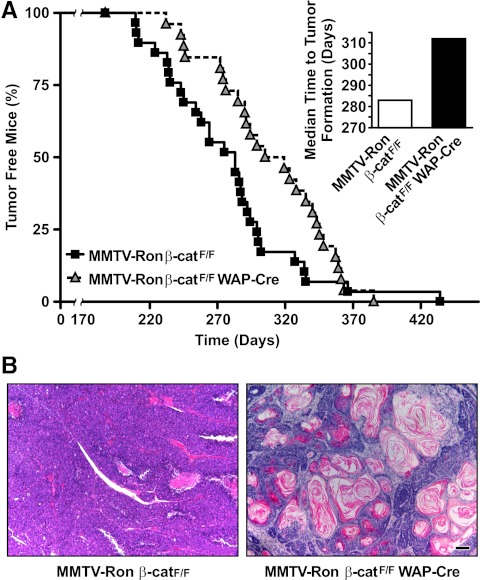
Palpable mammary tumor kinetics as a result of β-catenin loss by WAP-induced expression of Cre is depicted in Fig. 3A. Although mammary tumors form in 100% of the mice from both groups, palpable tumor development was significantly delayed in the MMTV-Ron β-catF/F WAP-Cre mice vs. the control mice. The MMTV-Ron β-catF/F WAP-Cre mice had a median time to palpable mammary tumor formation of 312 d vs. the MMTV-Ron β-catF/F mice having a median of 283 d.
Fig. 3.
β-Catenin loss in MMTV-Ron β-catF/F WAP-Cre mice delays the onset of palpable mammary tumor formation in MMTV-Ron mice. A, β-Catenin deletion in WAP-Cre transgenic mice significantly delayed mammary tumor formation. The percent of tumor-free mice is plotted over time and results in a statistically significant delay in palpable tumor formation between groups (P ≤ 0.05). Tumor incidence in MMTV-Ron β-catF/F control and MMTV-Ron β-catF/F WAP-Cre mice was similar with 100% of the animals developing mammary tumors. The median time to palpable mammary tumor formation in MMTV-Ron β-catF/F WAP-Cre mice (n = 26) was 312 d compared with 283 d in the control MMTV-Ron β-catF/F mice (n = 29). B, Representative images of mammary tumors from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice are shown and were taken from mice when tumors represented approximately 10% body weight in each genotype. Note the presence of keratin pearl deposits in tumors from MMTV-Ron β-catF/F WAP-Cre mice vs. well-differentiated mammary tumor histology in tumors from MMTV-Ron β-catF/F mice. Scale bar, 200 μm.
WAP-Cre-induced β-catenin deletion results in an altered mammary tumor phenotype
WAP-Cre-induced β-catenin deletion produced mammary tumors that displayed a different histology compared with mammary tumors from MMTV-Ron β-catF/F mice. MMTV-Ron β-catF/F WAP-Cre mammary tumors had a high propensity to contain keratin pearls that consisted of concentric layers of abnormal squamous cells with high deposition of cytokeratins (Fig. 3B). This phenotype was present in 12 out of 18 tumors analyzed (67%). Mammary tumors from MMTV-Ron β-catF/F mice displayed phenotypes consistent with adenocarcinomas with varying degrees of large cells and regions of desmoplasia as previously described (9). Interestingly, at around 280 d, or at about 9 months of age, approximately 50% of the MMTV-Ron β-catF/F mice developed palpable tumors, whereas only about 25% of the MMTV-Ron β-catF/F WAP-Cre mice displayed palpable tumors at this same time frame. By approximately 300 d, the majority (over 80%) of the control mice contained palpable mammary tumors compared with less than 50% of the MMTV-Ron β-catF/F WAP-Cre mice. These results suggest that the conditional loss of β-catenin in this experimental system is associated with an early reduction in hyperplastic nodules (Fig. 2C) by about 4 months of age and a corresponding reduction in the formation of palpable mammary tumors by about 9 months of age. However, 100% of all the mice subsequently develop mammary tumors by about 14 months of age, suggesting that β-catenin loss may be important early in the development of the mammary tumors in this model.
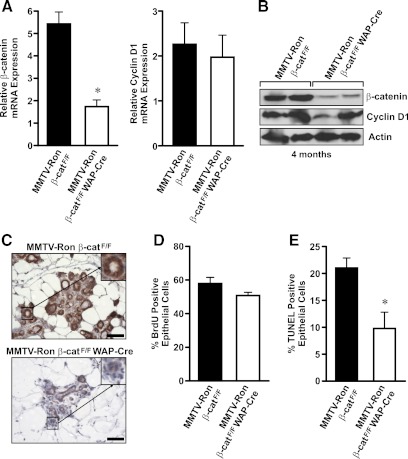
Characterization of β-catenin expression in mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice at 4 months of age
By 4 months of age, 71% of control mammary glands showed the presence of hyperplastic nodules. In contrast, only 15% of the mammary glands from MMTV-Ron β-catF/F WAP-Cre mice displayed hyperplastic nodules at this time point. To correlate this difference to changes in β-catenin expression, thoracic mammary glands from 4-month-old MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were used to isolate RNA and protein to compare β-catenin and cyclin D1 levels. A significant decrease in β-catenin mRNA and protein expression was observed in MMTV-Ron β-catF/F WAP-Cre glands compared with MMTV-Ron β-catF/F glands at this time point (Fig. 4, A and B). At this early time point, there were no changes in cyclin D1 protein or RNA levels. Immunohistochemical detection of β-catenin expression and localization was also performed on MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre glands (Fig. 4C), which also showed a decrease in β-catenin expression in glands from the MMTV-Ron β-catF/F WAP-Cre compared with glands from the control mice. At this early time point, there were no differences observed in the percentage of cells staining positive for BrdU incorporation. Interestingly, however, there was a decrease in the number of TUNEL staining mammary epithelial cells between genotypes with MMTV-Ron β-catF/F WAP-Cre glands exhibiting less cell death (Fig. 4, D and E). These results show efficient β-catenin deletion in the mammary epithelium at 4 months of age, a time point when hyperplastic nodules are less prevalent in the MMTV-Ron β-catF/F WAP-Cre glands.
Fig. 4.
β-Catenin expression is decreased in mammary glands isolated from MMTV-Ron β-catF/F WAP-Cre mice at 4 months of age. A, qRT-PCR of β-catenin and cyclin D1 expression was performed on mammary glands isolated at 4 months of age from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice. mRNA expression values were normalized to β-glucuronidase as an internal control, and the relative expression of β-catenin is depicted. *, P < 0.05. B, Western blot analysis for β-catenin and cyclin D1 expression was performed on mammary glands isolated at 4 months of age from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice. Actin was used as a loading control. C, Immunohistochemical labeling for β-catenin was performed on mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice isolated at 4 months of age. Representative images are shown. Insets show a 2-fold magnification of a representative mammary duct immunolabeled for β-catenin. D and E, Mammary glands from 4-month-old MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were examined for the extent of epithelial cell proliferation by BrdU immunohistochemistry (D) or for epithelial cell death by TUNEL staining (E). The percent of mammary epithelial cells positive for BrdU and TUNEL labeling was quantitated. A reduction in TUNEL staining is observed in the mammary epithelium from the MMTV-Ron β-catF/F WAP-Cre mice compared with controls at this time point.
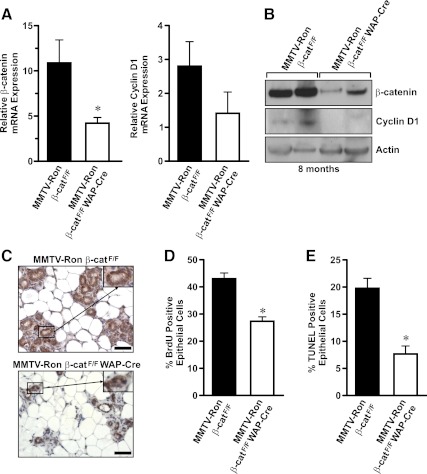
Reduced β-catenin expression in mammary glands from MMTV-Ron β-catF/F WAP-Cre mice at 8 months
Hyperplastic nodules were present in a majority of the mammary glands assessed from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice by 8 months of age. To determine the extent of β-catenin deletion at this time point, thoracic mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were used to isolate RNA and protein to examine β-catenin and cyclin D1 expression by qRT-PCR (Fig. 5A) and Western blot analysis (Fig. 5B). β-Catenin expression was also examined by immunohistochemistry (Fig. 5C). A significant reduction in β-catenin mRNA and protein expression was observed in MMTV-Ron β-catF/F WAP-Cre glands compared with controls, which also corresponded with a slight reduction in cyclin D1 mRNA and protein expression, although this latter trend was not significant. In addition, MMTV-Ron β-catF/F WAP-Cre mammary glands had significantly reduced numbers of epithelial cells undergoing proliferation, as indicated by a lower percentage of cells having BrdU incorporation. The glands from MMTV-Ron β-catF/F WAP-Cre mice also had a reduction in the number of cells undergoing apoptosis, quantified by TUNEL staining in comparison with control glands at this time point (Fig. 5, D and E). These results suggest that the early and continued conditional loss of β-catenin at 8 months of age is associated with reduced mammary cell proliferation, albeit with a consistent decrease in cell death, which is also associated with decreased palpable tumor formation as depicted in Fig. 3A.
Fig. 5.
β-Catenin expression is significantly reduced in mammary glands from 8-month-old MMTV-Ron β-catF/F WAP-Cre mice compared with controls. A, qRT-PCR was performed on mRNA isolated from mammary glands of MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice isolated at 8 months of age. B, Total protein extracts from mammary glands of MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre were analyzed for β-catenin and cyclin D1 expression. C, Tissue sections of mammary glands from 8-month-old mice were used for immunohistochemical analysis of β-catenin. Insets show a 2-fold magnification of a representative mammary duct immunolabeled for β-catenin. The extent of epithelial cell proliferation by BrdU immunohistochemistry (D) or for epithelial cell apoptosis by TUNEL staining (E) was examined in 8-month-old mammary glands from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice. *, P < 0.05. Scale bar, 50 μm.
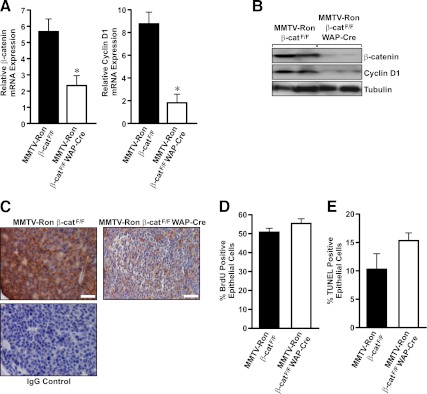
β-Catenin expression in end-stage mammary tumors from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice
By 10 months of age, hyperplastic nodules were similarly detectable in glands from MMTV-Ron β-catF/F or MMTV-Ron β-catF/F WAP-Cre mice. However, only 60% of the MMTV-Ron β-catF/F WAP-Cre mice had at least one palpable tumor at this time point compared with 80% of the control mice having palpable tumors. At the time of harvest, palpable tumors were separated from the adjacent mammary gland and processed as designated in Materials and Methods. To define the extent of β-catenin deletion within the mammary tumors at this time point, mRNA and protein were isolated from cells resting in the outer edge of the growing tumor to avoid necrotic or desmoplastic regions within the tumors. As shown in Fig. 6, A and B, end-stage mammary tumors (defined as when the tumors represented ∼10% body weight) from MMTV-Ron β-catF/F WAP-Cre mice exhibited a significant loss of β-catenin mRNA and protein expression compared with tumors from control mice. Similarly, mammary tumors from MMTV-Ron β-catF/F WAP-Cre mice displayed a corresponding decrease in cyclin D1 expression compared with tumors from control mice (Fig. 6, A and B). Immunolabeling for β-catenin within end-stage tumors also demonstrated a significant reduction in β-catenin expression in the MMTV-Ron β-catF/F WAP-Cre tumors compared with control tumors, although β-catenin expression was observed to be mosaic in regions of the MMTV-Ron β-catF/F WAP-Cre tumors (Fig. 6C). At this late stage of tumor development, there were no significant differences between groups observed in the mammary tumor mitotic or apoptotic indices as determined by quantifying BrdU and TUNEL immunolabeling (Fig. 6, D and E).
Fig. 6.
End-stage tumors from MMTV-Ron β-catF/F WAP-Cre mice maintain β-catenin loss. A, qRT-PCR analysis of β-catenin and cyclin D1 expression was performed on mRNA isolated from end-stage mammary tumors of control and MMTV-Ron β-catF/F WAP-Cre mice. *, P < 0.05. B, Western blot analysis was performed on mammary tumor lysates from control and MMTV-Ron β-catF/F WAP-Cre mice. The tumor lysates were analyzed for expression of β-catenin and cyclin D1. Tubulin was used as the loading control. C, Representative immunohistological images of end-stage mammary tumors from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice showing β-catenin expression are depicted. An IgG control of the MMTV-Ron β-catF/F image is provided to show secondary cross reactivity with an isotype control antibody. D, Quantification of BrdU incorporation to assess proliferation rates of mammary tumors in MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice is shown. E, Quantification of TUNEL positive cells to assess the apoptotic rate in end-stage mammary tumors isolated from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice is shown. Scale bar, 50 μm.
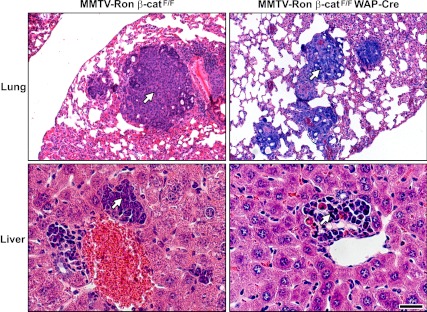
Decreased metastatic burden to the liver in MMTV-Ron mammary tumors with β-catenin deleted
Serial sections of the lung and liver from control MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were examined histologically for the presence of mammary tumor metastasis. A high frequency of metastasis to the lungs and liver was observed in both groups (Table 1). Interestingly, the incidence of liver metastasis in MMTV-Ron β-catF/F WAP-Cre mice was significantly reduced compared with that observed in the MMTV-Ron β-catF/F control mice. Figure 7 shows representative images of lung and liver metastases from control MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice.
Table 1.
End-stage number of mice with metastasis to the lung and liver represented by genotype
| MMTV-Ron β-catF/F | MMTV-Ron β-catF/F WAP-Cre | |
|---|---|---|
| Lung | 28/31 (90%) | 22/23 (95.6%) |
| Liver | 21/24 (87.5%) | 14/22 (63.6%)a |
The number of mice with metastasis to the particular tissue/total number of mice evaluated for the select tissue is indicated. The percentage of mice with metastasis to the select tissue is provided in parenthesis.
P < 0.05 compared with liver metastasis observed in the MMTV-Ron β-catF/F mice.
Fig. 7.
Metastatic dissemination in MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice. Representative images of metastatic lesions within the lungs and livers from MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice are shown. Arrows indicate metastatic foci. Scale bar, 200 μm.
Discussion
We have previously shown that Ron overexpression in the mammary epithelium results in mammary tumors in 100% of MMTV-Ron transgenic mice, and these tumors are associated with elevated β-catenin expression (9). In this study, we examined the effect of a conditional deletion of β-catenin in the context of Ron overexpression within the mammary gland to assess the impact β-catenin deletion has on the development and progression of Ron-induced mammary tumorigenesis. Here, we show that the development of mammary hyperplasia is delayed in MMTV-Ron mice when β-catenin is deleted through the use of WAP-Cre transgenic mice. The onset of palpable mammary tumor formation in MMTV-Ron β-catF/F WAP-Cre mice was also significantly delayed compared with the control mice with a median tumor latency of 312 d in the MMTV-Ron β-catF/F WAP-Cre mice compared with 283 d in the control mice.
The delay in mammary tumorigenesis is consistent with our data showing a decrease in the proliferation rate of the MMTV-Ron β-catF/F WAP-Cre hyperplastic nodules, corresponding with the loss or reduction in β-catenin and cyclin D1 expression in the mammary tumors of the MMTV-Ron β-catF/F WAP-Cre mice compared with controls. Additionally, we showed a decrease in cell death based on TUNEL staining in the mammary glands of MMTV-Ron β-catF/F WAP-Cre mice compared with MMTV-Ron β-catF/F mice in hyperplastic nodules. We speculate that the reduced number of TUNEL positive epithelial cells in the MMTV-Ron β-catF/F WAP-Cre mice may coincide with the proportion of cells that express β-catenin, although further investigation into this difference is needed. Together, our studies support the supposition that conditional loss of β-catenin has an important effect on our model system, particularly during early stages of Ron-induced tumorigenesis.
Along these lines, at 4 months of age, there were no noted differences between genotypes in the percentage of tumor-free mice over time with neither genotype exhibiting palpable tumors at this stage. However, there was a significant increase in the percentage of glands from MMTV-Ron β-catF/F mice that contained hyperplastic nodules compared with the MMTV-Ron β-catF/F WAP-Cre mice. In contrast, by 8 months of age, conditional loss of β-catenin results in a significant difference in the number of mice with palpable mammary tumors as well as a reduction in mammary gland proliferation when compared with MMTV-Ron β-catF/F mice. After about 10 months of age through end-stage tumor analysis, β-catenin and cyclin D1 expression levels were reduced in the MMTV-Ron β-catF/F WAP-Cre compared with controls, but the tumor proliferation and apoptotic rates became more similar in the end-stage samples. Thus, these results suggest that β-catenin may play an important role during early tumor development, after which there may be limited further consequences of β-catenin loss.
Despite the importance of the conditional loss of β-catenin in the development of hyperplastic nodules and palpable mammary tumors, the late stage mammary tumors did not differ in proliferation rates between genotypes even with a reduction in β-catenin and a decrease in cyclin D1 expression in the MMTV-Ron β-catF/F WAP-Cre mice. The apparent paradox in the low levels of cyclin D1 without a change in proliferation in our late stage tumors is similar to previous reports, which have shown no significant differences in proliferative activity between high and low cyclin D1 expressing tumors in human papillary superficial bladder cancers (23). Further, both cyclin D1 and Ki67 (a proliferation marker) were shown to be independent predictors of reduced disease-free survival with patients having low cyclin D1, low p27kip1, and high Ki67 expression defining a “high-risk” group of patient with an increased risk of disease recurrence. Others have also suggested that a determining factor for cell proliferation may be the balance between regulators of cell proliferation including p27kip1 and cyclin D/cdk4 levels (24).
In addition to differences in the onset of hyperplasia and palpable tumor formation, we also report a delay in mammary tumor cells metastasizing to the liver in the MMTV-Ron β-catF/F WAP-Cre mice compared with the rate of metastasis in our control mice. Contrary to the liver metastasis, metastasis to the lung was not different between the two groups. However, because lung metastasis was only assessed at the end-stage time point and both genotypes had greater than 90% of the mice with metastatic burden to the lung at this time, it remains unknown whether lung metastasis may have differed between the MMTV-Ron β-catF/F WAP-Cre and control mice at an earlier time point. Lung metastasis may have occurred as an earlier event in the MMTV-Ron-induced mammary tumor model compared with liver metastases, or these data could also suggest that β-catenin expression may be important in the establishment or growth of liver metastases. In total, these studies suggest that continued suppression of β-catenin within the mammary epithelium results in a delay in mammary tumor onset and decreased overall metastatic tumor burden.
Deregulation of β-catenin signaling within luminal progenitor cells has been found to produce tumors of mixed lineage (17, 25). Histologically, MMTV-Ron β-catF/F WAP-Cre mammary tumors were also highly associated with foci of central keratinization found within concentric layers of abnormal squamous cells. Breast cancer from patients and mice with alterations in BRCA1 frequently exhibit basal-like phenotypes, which contain metaplastic elements consisting of neoplastic spindle cells or squamous cells with the presence of keratin pearls (26–28). These similarities suggest that mammary tumors from the MMTV-Ron β-catF/F WAP-Cre mice may have attributes of a murine model of basal-like breast cancer, although further investigation into these tumors is warranted to determine whether this model may prove useful for examining new treatment modalities that target BRCA1-driven and/or basal-like carcinomas.
Although the tumors in both the MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre mice were very aggressive, the disparate tumor phenotypes in the MMTV-Ron β-catF/F and MMTV-Ron β-catF/F WAP-Cre models may be due to a combination of factors. Although efficient β-catenin loss was observed in MMTV-Ron β-catF/F WAP-Cre mammary tumors compared with those from MMTV-Ron β-catF/F mice, it is important to note that the pattern of β-catenin deletion was mosaic. This point is important, because β-catenin has been shown to play pivotal roles in the mammary gland regulating stem cell self renewal and multipotency, as well as promoting proliferation and cell survival during various stages of mammary gland development (17, 29, 30). Further studies are needed to determine whether the phenotypes observed reflect β-catenin loss in different mammary epithelial cell types, the precise timing of this loss, and/or the extent of deletion of this protein during MMTV-Ron-induced tumorigenesis.
Overall, our data support previous studies demonstrating that Ron overexpression leads to the development of highly aggressive, metastatic mammary tumors (4, 9). More specifically, previous work showed that Ron overexpression in the mammary epithelium of mice lead to mammary tumors that exhibited increases in β-catenin expression, activation, and tyrosine phosphorylation. Recently published studies have further identified an important role of the Ron receptor tyrosine kinase, and the Ron ligand HGFL, in the activation of β-catenin in breast cancer cells through tyrosine phosphorylation of tyrosine residues 654 and 670 in β-catenin (4). In this report, the WAP-Cre transgenic model provided a process by which we could investigate the consequences of a conditional loss of β-catenin within the in vivo mammary gland after glandular development had completed. In this experimental system, the extent of β-catenin loss was mosaic but provided important evidence that β-catenin loss in vivo during mammary tumor formation leads to a delay in the development of hyperplastic nodules and palpable mammary tumors. In our previous studies, using mammary tumor cell lines with a complete elimination of β-catenin, we demonstrated that complete loss of this protein in Ron-overexpressing mammary tumor cell lines was able to inhibit tumor cell growth both in vitro and using an orthotopic xenograph model (4). These combined studies support an important role for β-catenin downstream of Ron receptor signaling during mammary tumorigenesis.
In an examination of human breast cancer specimens, a statistically significant correlation between high Ron expression and high β-catenin expression was observed (4). Breast cancer patients with high Ron and high β-catenin expression exhibited significantly reduced survival within a 30-month follow-up period and more lymph node metastasis compared with patients with low Ron and β-catenin expression. In this report, we show that tumor formation in MMTV-Ron mice is regulated by β-catenin in vivo, wherein reduced β-catenin expression leads to increases in tumor latency, decreases in tumor growth, and a reduction in the metastatic burden to the liver. The extent and timing of β-catenin expression may play an important role in tumor growth, may regulate aspects of tumor histology, and may possibly alter ensuing responses to breast cancer treatment. Further understanding of the complex spatiotemporal expression of β-catenin will be required to provide insights into the development and eventual treatment of patients that exhibit activation of this pathway in breast cancer.
Acknowledgments
The authors thank Dr. Nikolaos Nikolaidis, William Stuart, Stephen Ching, and Soumya Kashinkunti for their assistance with animal maintenance, proof reading, and tissue collection; Glenn Doerman for graphical assistance; the assistance of Dr. Margaret Collins for the histopathological assessment of the mammary tumors that developed in these transgenic models.
This work was supported by Public Health Service Grants CA100002 (to S.E.W.) and HD051953 (to G.M.Z.) from the National Institutes of Health, the Cincinnati Veteran's Administration Medical Center Grant 1I01BX000803 (to S.E.W.), and by a University of Cincinnati Cancer Center grant (S.E.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxuridine
- β-catF/F
- floxed β-catenin allele
- HGFL
- hepatocyte growth factor-like protein
- MMTV
- mouse mammary tumor virus
- qRT
- quantitative real time
- TUNEL
- terminal transferase-mediated 2′-deoxyuridine, 5′-triphosphate nick end labeling
- WAP
- whey acidic protein.
References
- 1. Easson AM, McCready DR. 2004. Management of local recurrence of breast cancer. Expert Rev Anticancer Ther 4:219–226 [DOI] [PubMed] [Google Scholar]
- 2. O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M, Gottfredsen KJ, Thobe MN, Cheng Y, Li Y, Hicklin DJ, Zhu Z, Waltz SE, Hayman MJ, Ludwig DL, Pereira DS. 2006. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res 66:9162–9170 [DOI] [PubMed] [Google Scholar]
- 3. Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, Di Renzo MF, Costantino A, Sismondi P, Comoglio PM. 1998. Overexpression of the RON gene in human breast carcinoma. Oncogene 16:2927–2933 [DOI] [PubMed] [Google Scholar]
- 4. Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE. 2011. β-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene 30:3694–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thobe MN, Gurusamy D, Pathrose P, Waltz SE. 2010. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene 29:214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE, Lowy AM. 2007. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res 67:6075–6082 [DOI] [PubMed] [Google Scholar]
- 7. Wagh PK, Peace BE, Waltz SE. 2008. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res 100:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peace BE, Hughes MJ, Degen SJ, Waltz SE. 2001. Point mutations and overexpression of Ron induce transformation, tumor formation, and metastasis. Oncogene 20:6142–6151 [DOI] [PubMed] [Google Scholar]
- 9. Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA, Peace BE, Beauman SR, Collins MH, Waltz SE. 2006. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with β-catenin activation. Cancer Res 66:11967–11974 [DOI] [PubMed] [Google Scholar]
- 10. Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, Bishop JM. 2007. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci USA 104:7570–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peace BE, Toney-Earley K, Collins MH, Waltz SE. 2005. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res 65:1285–1293 [DOI] [PubMed] [Google Scholar]
- 12. Ma Q, Guin S, Padhye SS, Zhou YQ, Zhang RW, Wang MH. 2011. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol Cancer 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. 2001. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the β-catenin pathway. Mol Cell Biol 21:5857–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. 2000. β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97:4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Incassati A, Chandramouli A, Eelkema R, Cowin P. 2010. Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res 12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyoshi K, Hennighausen L. 2003. β-Catenin: a transforming actor on many stages. Breast Cancer Res 5:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, Tan LK, Rosen JM, Varmus HE. 2003. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA 100:15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heuberger J, Birchmeier W. 2010. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2:a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer SE, Zinser GM, Stuart WD, Pathrose P, Waltz SE. 2009. The Ron receptor tyrosine kinase negatively regulates mammary gland branching morphogenesis. Dev Biol 333:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. 2001. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- 21. Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25:4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. 2011. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology 53:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sgambato A, Migaldi M, Faraglia B, De Aloysio G, Ferrari P, Ardito R, De Gaetani C, Capelli G, Cittadini A, Trentini GP. 2002. Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer 97:671–678 [DOI] [PubMed] [Google Scholar]
- 24. Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O'Hare MJ, Lu X. 1997. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 94:6380–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, Cowin P. 2009. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One 4:e4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brodie SG, Deng CX. 2001. BRCA1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet 17:S18–22 [DOI] [PubMed] [Google Scholar]
- 27. McCarthy A, Savage K, Gabriel A, Naceur C, Reis-Filho JS, Ashworth A. 2007. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol 211:389–398 [DOI] [PubMed] [Google Scholar]
- 28. Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. 1999. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet 22:37–43 [DOI] [PubMed] [Google Scholar]
- 29. Zeng YA, Nusse R. 2010. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatsell S, Rowlands T, Hiremath M, Cowin P. 2003. β-Catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–158 [DOI] [PubMed] [Google Scholar]