Abstract
1,25-dihydroxyvitamin D3 [1,25(OH)2D3 or calcitriol], the hormonally active vitamin D metabolite, exhibits anticancer actions in models of breast cancer and prostate cancer. Because CYP27B1 (1α-hydroxylase), the enzyme catalyzing 1,25(OH)2D3 formation in the kidney, is also expressed in extrarenal tissues, we hypothesize that dietary vitamin D3 will be converted to 25(OH)D3 in the body and then to 1,25(OH)2D3 locally in the cancer microenvironment in which it will exert autocrine/paracrine anticancer actions. Immunocompromised mice bearing MCF-7 breast cancer xenografts showed significant tumor shrinkage (>50%) after ingestion of a vitamin D3-supplemented diet (5000 IU/kg) compared with a control diet (1000 IU/kg). Dietary vitamin D3 inhibition of tumor growth was equivalent to administered calcitriol (0.025, 0.05, or 0.1 μg/mouse, three times a week). Both treatments equivalently inhibited PC-3 prostate cancer xenograft growth but to a lesser extent than the MCF-7 tumors. Calcitriol at 0.05 μg and 0.1 μg caused modest but statistically significant increases in serum calcium levels indicating that the dietary vitamin D3 comparison was to a maximally safe calcitriol dose. Dietary vitamin D3 did not increase serum calcium, demonstrating its safety at the concentration tested. The vitamin D3 diet raised circulating 1,25 dihydroxyvitamin D levels and did not alter CYP27B1 mRNA in the kidney but increased it in the tumors, suggesting that extrarenal sources including the tumors contributed to the elevated circulating 1,25 dihydroxyvitamin D3. Both calcitriol and dietary vitamin D3 were equipotent in suppressing estrogen synthesis and signaling and other proinflammatory and growth signaling pathways. These preclinical data demonstrate the potential utility of dietary vitamin D3 supplementation in cancer prevention and therapy.
Accumulating evidence suggests that vitamin D3 (cholecalciferol) and its active metabolite calcitriol [1,25(OH)2D3] may reduce the risk of developing cancer and may be useful in the treatment of cancer (1, 2). Epidemiological studies imply that vitamin D (when written without a subscript indicates either D2 or D3) deficiency increases the risk of developing several cancers including breast cancer (BCa), prostate cancer (PCa), and colorectal cancer (3–5). Sunlight exposure, which promotes cutaneous synthesis of vitamin D3 and dietary intake of vitamin D, are inversely associated with cancer risk and mortality (5–9). Data also indicate an inverse association between serum 25-hydroxyvitamin D [25(OH)D] levels and cancer risk (4, 10–12). However, all epidemiological studies do not show an association between cancer risk and vitamin D deficiency (13, 14), and some data even found the risk to be increased with higher circulating levels of serum 25(OH)D, suggesting a U-shaped dose-response curve (15). However, preclinical studies in cell culture and animal models provide strong data clearly supportive of a vitamin D benefit in cancer prevention or treatment (1, 2).
Vitamin D3 is not merely a vitamin but the essential precursor to 1,25(OH)2D3 (calcitriol) (16), a potent steroid hormone that has antiproliferative, antiinflammatory, prodifferentiating, and proapoptotic activities in multiple cancers (1, 2, 17–20). In our study, we will refer to the drug administered to mice as calcitriol but will use 1,25(OH)2D to refer to serum levels because the assay we used did not distinguish between the D2 or D3 forms of the vitamin D metabolites. Vitamin D3 derived from the diet or synthesized in the skin gets hydroxylated in the liver to form 25(OH)D3 by several enzymes including CYP27A1 and CYP2R1 (16, 21). Conversion of 25(OH)D3, the circulating prohormone, to 1,25(OH)2D3 is subsequently accomplished in the kidney in a tightly controlled enzymatic step catalyzed by 1α-hydroxylase (CYP27B1) (16). However, normal and malignant colon, prostate and breast cells also express CYP27B1 that is not tightly regulated and thus circulating 25(OH)D levels determine the extent of 1,25(OH)2D that can be synthesized locally within these tissues (22). These observations provide a rationale for vitamin D3 to exhibit all of the anticancer actions of administered calcitriol and thereby also provide a basis for the protective effect of sunlight and dietary vitamin D against cancer development. Our findings strongly suggest that adequate vitamin D nutrition is important in cancer chemoprevention and treatment (1, 2, 23, 24).
In this study we investigated the beneficial effects of a diet supplemented with vitamin D3 in comparison with injections of calcitriol using xenograft models of human BCa and PCa in nude mice. Our data showed that at the concentrations tested (5000 IU/kg), dietary vitamin D3 was as potent as the active hormone calcitriol in inhibiting tumor growth without significantly altering serum calcium levels, demonstrating its efficacy and safety at this concentration. Dietary vitamin D3 suppressed estrogen synthesis and signaling in BCa xenografts and exerted additional inhibitory effects on other proinflammatory and growth signaling pathways in both BCa and PCa xenografts, which were equivalent to the effects seen with calcitriol. These preclinical data suggest that vitamin D3 supplements may have utility in cancer therapy.
Materials and Methods
Materials
Calcitriol was a kind gift from Milan Uskokovic (BioXell Co., Nutley NJ). The rodent diets were from Research Diets Inc. (New Brunswick, NJ). Tissue culture media, supplements, and fetal bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY) and Mediatech Inc. (Herndon, VA).
Methods
Cell culture
MCF-7 human BCa cells were routinely cultured in DMEM/F12 medium containing 10% FBS, and PC-3 human PCa cells were cultured routinely in RPMI 1640 medium containing 5% FBS. Cultures were maintained at 37 C and 5% CO2 in a humidified incubator and were screened to ensure the absence of murine viruses before inoculation into nude mice. Cell cultures were grown to approximately 75% confluence, washed, and digested with trypsin to obtain cell pellets under sterile conditions. Cell pellets were resuspended (∼10 × 107 cells/ml) in sterile culture media mixed with an equal volume of regular Matrigel (Collaborative Biomedical, Bedford, MA) before injections into mice.
Animal studies
All animal procedures were performed in compliance with the guidelines approved by Stanford University Administrative Panel on Laboratory Animal Care. Four- to 6-wk-old male and female [intact and ovariectomized (OVX)] athymic, nude (Foxnnu/nu) mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in the Research Animal Facility (Stanford University School of Medicine) in a designated pathogen-free area.
Establishment of PCa xenografts
PC-3 human PCa cells (∼2.5 × 106 cells) were injected sc into the right flank of male nude mice. Body weights and tumor volumes were measured weekly. Tumor volumes were calculated from two tumor diameter measurements using a vernier caliper and using the formula: tumor volume = (length × width2)/2 (25). When the tumors reached about 100 mm3 in size, the mice were assigned to the different treatment groups and the treatments were continued over a period of 4 wk.
Establishment of BCa Xenografts
BCa xenografts were established in both intact (premenopausal model) and OVX (postmenopausal model) female nude mice by injecting MCF-7 cells (∼10 million) suspended in a small volume (∼100 μl) of the culture medium-Matrigel mixture. Typically the growth of MCF-7 xenografts in mouse models is supported by estrogen supplementation. However, because one of our main goals was to investigate the effect of calcitriol and dietary vitamin D3 on estrogen synthesis, we did not provide estrogenic supplementation to these mice. In the OVX mice (postmenopausal model), we implanted pellets of the estrogenic precursor androstenedione (15 mg per 90 d time-release pellets from Innovative Research of America, Sarasota, FL) in the intrascapular region to ensure substrate for local estrogen synthesis by aromatase in the breast microenvironment. Orthotopic xenografts were established in OVX mice using a midline abdominal skin incision to visualize the fourth mammary fat pads (inguinal glands) and cell suspensions were injected into the fat pad on both the right and left sides. For pellet implantation and tumor cell injection into the mammary fat pads, mice were manipulated in surgical aseptic conditions under isoflurane anesthesia and received carprofen (5 mg/kg) for analgesia. For the premenopausal model, similar cell suspensions were injected sc into the right flank of intact mice. When the tumors reached approximately 70 mm3 in size in either model, the mice were assigned to the treatment groups and the treatments were continued over the following 4 wk. Body weights and tumor sizes were measured weekly after BCa cell inoculation and continued throughout the experiment.
Treatments
Care was taken to ensure there was no statistically significant difference in tumor volumes between the experimental groups at the beginning of treatment, which was initiated by feeding the mice experimental diets and beginning calcitriol administration. We used the active hormone calcitriol for treatments. We will refer to the drug used for treatment as calcitriol to indicate that we used the vitamin D3 form of the active hormone. The supplement added to the diet was the D3 form of the vitamin. The experimental groups were as follows: 1) mice on control diet (AIN76; Research Diets Inc.; 1000 IU/kg of vitamin D3 diet) receiving injections of vehicle; 2) mice on control diet receiving injections of calcitriol (0.025 and 0. 05 μg/mouse, respectively, for the OVX and intact female mice bearing BCa xenografts and 0.1 μg/mouse for male mice bearing PCa xenografts); and 3) mice on vitamin D3-supplemented diet (5000 IU/kg of vitamin D3 of diet). Stock solutions of calcitriol were made in 100% ethanol and stored at −20 C. Appropriate dilutions were made in sterile saline and were administered by ip injections three times a week (on Mondays, Wednesdays, and Fridays). The mice in the control group received ip injections of 0.1% ethanol in sterile saline (vehicle). The dosage of calcitriol and the intermittent administration regimen were based on our prior work (26) and published observations (27–29). Treatments were continued for 4 wk. At the end of the study, 14 h after the final calcitriol injections, mice were euthanized according to Administrative Panel on Laboratory Animal Care guidelines using CO2. Blood samples were collected by cardiac puncture while under CO2 anesthesia causing exsanguination, and serum samples were prepared and frozen. Tumors and kidneys were harvested and snap frozen in liquid nitrogen for subsequent analysis. Aliquots of the tumor tissue were fixed in formalin and embedded in paraffin for immunohistochemistry (IHC).
Real-time PCR
Total RNA was isolated from tumor and other tissue samples by homogenization using Trizol (Invitrogen, Carlsbad, CA) as described (23). Five micrograms of RNA were subjected to reverse transcription using the SuperScript III first-strand synthesis kit (Invitrogen) and an oligodeoxythymidine primer (30). Relative changes in mRNA levels were assessed by the comparative cycle time (ΔΔCT) method (31). The mRNA expressions of the genes of interest were normalized to either TATA box-binding protein (TBP) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels as described previously (32).
Measurement of estrone and estradiol levels
Protein extracts from tumor tissue and the surrounding mammary fat from the intact and OVX female mice were prepared by homogenizing the tissues in a Tris-EDTA buffer containing high salt as described previously (33). Aliquots of the tissue extracts were used to measure estrone and estradiol levels using enzyme immunoassay kits (Cayman Chemical Co., Ann Arbor, MI) following the manufacturer's protocols. Protein concentration in the extracts was determined by the method of Bradford (34).
Measurement of serum calcium and vitamin D metabolites
Serum calcium levels were measured using the QuantiChrom calcium assay kit (BioAssay Systems, Hayward, CA) following the manufacturer's protocol. Serum vitamin D metabolite levels were measured at Heartland Assays (Ames, IA) by methods previously described for 25(OH)D (35) and 1,25(OH)2D (36). The assay did not distinguish between vitamin D2 or D3 metabolites.
Immunohistochemical analysis
Tumor tissue was fixed in 10% neutral-buffered formalin and processed for IHC studies as described previously (37). Five-micrometer sections were placed on positively charged glass slides and baked at 60 C for approximately 1 h in a standard histology oven (Fisher Scientific, Houston, TX). Antibodies to estrogen receptor (ER)-α (clone 1D5, 1:80; Dako, Carpinteria, CA), aromatase (1:100 mouse antihuman cytochrome P450 aromatase antibody, AbDSerotec, Raleigh, NC), and Ki67 (clone MIB-1, 1:200; Dako) were applied per standard immunohistochemistry (37) practice using a Dako autostainer (Dako). IgG was used as a negative control. ERα staining was quantified according to the commonly used Allred score in BCa patients, which incorporates both intensity of staining and percentage of cells stained (38). In the case of aromatase and Ki76, the percentage of cells positively stained was subjectively estimated from an examination of many fields at high magnification (×200–400).
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 5 software (GraphPad Software, San Diego, CA). Data were evaluated by ANOVA with Scheffé's F test as the post hoc analysis.
Results
Effects of dietary D3 and calcitriol on tumor growth
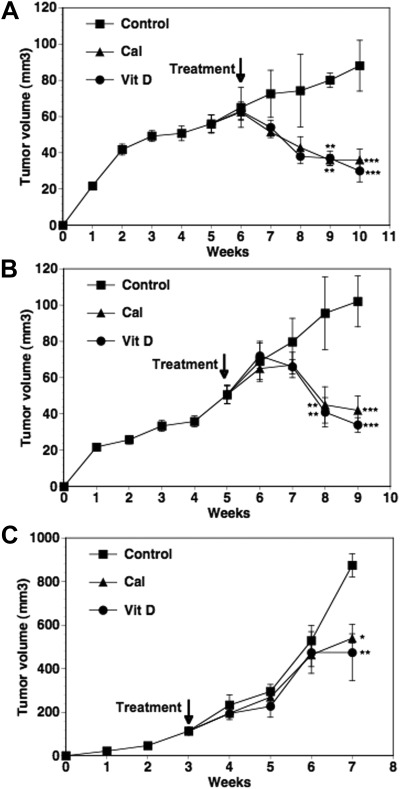
In the intact female mice (premenopausal model), BCa xenografts in the flank grew to approximately 70 mm3 in size by 6 wk after tumor cell inoculation. As shown in Fig 1A, in the control group that received the diet with the basal levels of dietary vitamin D3 (1000 IU/kg) and vehicle injections over the next 4 wk, the tumors continued to grow reaching a mean volume of approximately 90 mm3 by wk 10. In mice receiving either the vitamin D3-supplemented diet (5000 IU/kg) or calcitriol injections (0.05 μg/mouse), decreases in tumor volumes were evident as early as 1 wk after the initiation of treatments. Tumor volumes were significantly lower by the third week of treatment (P < 0.01 compared with control) in both treated groups. By the end of 4 wk, mean tumor volumes were approximately 60% lower in calcitriol-treated mice and approximately 65% decreased in the dietary D3-treated mice compared with the control mice (P < 0.001). Similar comparable tumor shrinkage was seen in the OVX mice (postmenopausal BCa) model (Fig. 1B). The reduction in tumor volumes in these mice treated for 4 wk with calcitriol (0.025 μg/mouse) or dietary vitamin D3 was approximately 59 and 67% lower, respectively, than the vehicle-treated control group (P < 0.001).
Fig. 1.
Effect of calcitriol and dietary vitamin D3 on the growth of xenograft tumors. MCF-7 xenografts were established in the flanks of intact female nude mice (premenopausal model). After 6 wk of tumor growth, the mice were given a vitamin D3-supplemented diet (Vit D) or basal diet + ip injections of calcitriol (0.05 μg/mouse) three times a week (Cal) for the following 4 wk. Mice in the control group (Control) were on the basal diet and received ip injections of the vehicle during this time period. Tumor volumes were measured weekly and calculated as described in Materials and Methods (A). In separate experiments, MCF-7 xenografts were established in the right and left fourth mammary fat pads of OVX nude mice (postmenopausal model) implanted with 60-d time-release androstenedione pellets and allowed to grow for 6 wk. The treatment groups were the same as described in A except that calcitriol was used at 0.025 μg/mouse. Tumor volumes in the experimental groups over the next 4 wk are shown (B). PC-3 xenografts were established in the flanks of intact male nude mice. After 3 wk of tumor growth, treatments were initiated as described in A except that calcitriol was used at 0.1 μg/mouse. Tumor volumes in the experimental groups over the next 4 wk are shown (C). Values represent mean ± se. The number of mice in the various experimental groups were 12–15 in the premenopausal and seven to nine in the postmenopausal models of BCa and six to eight in the PCa xenograft model. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 compared with the corresponding control values.
In comparison with the slower growth rate of MCF-7 xenografts in the absence of estrogen supplements, the rate of growth of the PC-3 xenografts in the male mice was appreciably greater. This is consistent with the more aggressive growth characteristics of PC-3 cells. The PC-3 tumors grew to approximately 150 mm3 in size by 3 wk after the sc inoculation, at which time treatment commenced (Fig. 1C). The tumors reached a mean volume of approximately 900 mm3 in control mice by 7 wk (4 wk after treatment initiation). Statistically significant changes in tumor volume due to calcitriol (0.1 μg/mouse) and dietary vitamin D3 were not apparent until after 4 wk of treatment. At wk 7, the tumor volumes were significantly lower in the calcitriol (∼40% inhibition, P < 0.05) and dietary vitamin D3 (∼50% inhibition, P < 0.01) treatment groups compared with control. At this time point, the experiment was ended because of the large sizes of the tumors in the control mice (>10% body weight).
Effects of dietary D3 and calcitriol on body weights and serum levels of calcium and vitamin D metabolites
There were no changes in the mean body weights between the experimental groups (Table 1), suggesting that the doses of calcitriol and dietary vitamin D3 used in the study were well tolerated by the mice. Blood samples were drawn 14 h after the final calcitriol injections for serum measurements. There were no changes in serum calcium levels in mice receiving 0.025 μg of calcitriol (Table 1). However, small but statistically significant increases were seen in mice receiving 0.05 and 0.1 μg of calcitriol compared with the corresponding controls, even at this late time point after administration of the dose. On the other hand, dietary vitamin D3 supplementation (5000 IU/kg diet) in mice bearing BCa or PCa xenografts did not significantly alter their serum calcium levels (Table 1).
Table 1.
Body weight and serum 25-hydroxyvitamin D3 and serum calcium measurements
| Groups | Body weight (g) | Serum 25-hydroxyvitamin D (ng/ml) | Serum 1,25-dihydroxyvitamin D (pg/ml) | Serum calcium (mg/dl) |
|---|---|---|---|---|
| Breast cancer | ||||
| Premenopausal model | ||||
| Control | 22 ± 0.4 | 21 ± 2 | 77 + 9 | 9.7 ± 0.2 |
| Calcitriol (0.05 μg) | 23.8 ± 0.8 | 15 ± 3 | 32 + 3a | 10.9 ± 0.2a |
| Vitamin D | 23 ± 0.4 | 38 ± 2c,f | 117 + 11a,d | 9.5 ± 0.2 |
| Postmenopausal model | ||||
| Control | 23.7 ± 0.5 | 18 ± 0.6 | 56 ± 3 | 9.8 ± 0.5 |
| Calcitriol (0.025 μg) | 23.4 ± 0.4 | 17 ± 2 | 34 ± 5 | 9.9 ± 0.6 |
| Vitamin D | 23.1 ± 0.6 | 29 ± 1c,f | 82 ± 1b,e | 9.4 ± 0.2 |
| Prostate cancer | ||||
| Control | 27 ± 0.8 | 12.8 ± 2 | 57 + 2 | 8.9 ± 0.5 |
| Calcitriol (0.1 μg) | 25 ± 0.9 | 13.6 ± 2 | 100 + 10a | 10.0 ± 0.2a |
| Vitamin D | 28 ± 0.5 | 30.9 ± 4c,f | 174 + 16b,e | 9.0 ± 0.9 |
| Female mice without tumors | ||||
| Control | 22 ± 1 | 24 ± 2 | 61 + 7 | 9.5 ± 0.1 |
| Calcitriol (0.05 μg) | 21 ± 2 | 11 ± 0.6c | 13 + 1c | ND |
| Vitamin D | 23 ± 1 | 40 ± 1c,f | 72 + 6f | 9.9 ± 0.2 |
ND, Not determined.
P < 0.05 when compared with control.
P < 0.01 when compared with control.
P < 0.001 when compared with control.
P < 0.05 when compared with calcitriol.
P < 0.01 when compared with calcitriol.
P < 0.001 when compared with calcitriol.
As expected, vitamin D3 supplemented-diet caused significant elevations in the serum levels of 25(OH)D, in comparison with the control and calcitriol groups on the standard diet (Table 1). It should be reemphasized that the blood samples were drawn 14 h after the final calcitriol injections, and the data from mice receiving calcitriol therefore represent the nadir values of serum 1,25(OH)2D that peak between 1 and 3 h after injections (39). Interestingly, mice ingesting the vitamin D3-supplemented diet displayed substantial and statistically significant elevations in serum 1,25(OH)2D levels in all the three experimental models. The increases were more modest (∼1.5-fold) in the female BCa models, whereas the increase in the male PCa model was more pronounced (∼3-fold) compared with control. We also determined the effect of the treatments on serum vitamin D metabolite and calcium levels in normal intact female nude mice without any tumors. In mice without tumors receiving calcitriol injections, a significant decrease in nadir serum 1,25(OH)2D levels were seen. Administration of dietary vitamin D3 to mice without tumors did not alter serum 1,25(OH)2D levels in contrast to the elevations seen in the tumor-bearing mice.
Changes in gene expression due to dietary vitamin D3 and calcitriol in BCa xenografts
We next examined the effect of dietary vitamin D3 supplementation and calcitriol administration on gene expression in BCa tumors as a measure of their abilities to regulate vitamin D metabolism [CYP24, CYP27B1, and vitamin D receptor (VDR)] and inhibit estrogen synthesis (aromatase) and signaling (ERα) as well as to exert antiinflammatory [cyclooxygenase (COX)-2, 15-hydroxyprostaglandin dehydrogenase (15-PGDH), prostaglandin E receptor (EP), prostaglandin F receptor (FP)] and antiproliferative [(p21 and IGF binding protein (IGFBP)-3] activities and regulate apoptosis [Bcl-2 associated X protein (Bax) and Bcl2]. For the sake of simplicity and space, we present the gene expression data in the premenopausal BCa model and the PCa model. In general, the treatments resulted in similar changes in the expression of these genes in the postmenopausal BCa model as well (data not shown).
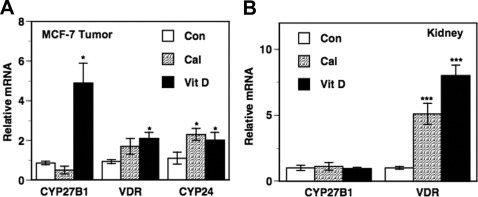
As shown in Fig 2A, calcitriol and dietary vitamin D3 administration to intact mice (premenopausal model) caused modest increases (∼2-fold) in the expression of the canonical calcitriol-responsive gene CYP24 in the tumors at the time point examined (∼14 h after final calcitriol injections). There were no significant changes in tumor CYP27B1 mRNA levels in mice receiving calcitriol (Fig. 2A). Interestingly, dietary vitamin D3 caused an appreciable increase in tumor CYP27B1 mRNA inducing an approximately 5-fold increase compared with control (P < 0.05). Both treatments resulted in modest increases in tumor VDR mRNA levels with the increase due to dietary vitamin D3 achieving statistical significance (Fig. 2A). For comparison and to investigate whether the origin of the elevated levels of serum calcitriol due to the dietary vitamin D3 treatments was from renal or extrarenal sources (Table 1), we determined the changes in renal CYP27B1 mRNA and VDR expression in these mice. There were no changes in renal CYP27B1 mRNA expression levels after either treatment, suggesting the elevated serum 1,25(OH)2D in these mice was more likely from an extrarenal source including the BCa tumor itself than a renal source. Renal VDR mRNA levels (Fig. 2B) showed significant up-regulation after the calcitriol and dietary vitamin D3 treatments, as expected (40, 41).
Fig. 2.
Changes in CYP27B1 and VDR expression in intact nude mice bearing BCa xenograft tumors. Vehicle [control (Con)], calcitriol (Cal; 0.05 μg/mouse), and dietary vitamin D3 (Vit D) were administered to tumor-bearing intact female mice as described in Fig. 1. Total RNA was isolated from harvested tissues and the mRNA levels of CYP27B1, VDR, and CYP24 were determined by quantitative RT-PCR in the tumors (A) and CYP27B1 and VDR in the kidneys (B) as described in Materials and Methods. Relative mRNA expression for each gene in tumors from the control mice was set at 1. Values represent mean ± se from four to six determinations. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 compared with control.
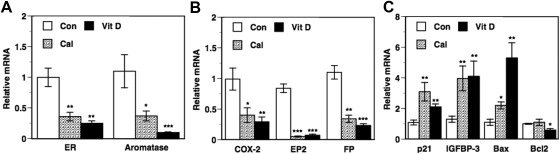
Dietary vitamin D3 and calcitriol administration to intact mice resulted in significant decreases in tumor mRNA levels compared with control for the following genes: ERα, aromatase (Fig. 3A), COX-2, and prostaglandin receptors EP2 and FP (Fig. 3B). The expression of the prostaglandin (PG)-metabolizing enzyme 15-PGDH was too low to be detected in the MCF-7 tumors (data not shown). The reductions in the expression of these genes due to dietary vitamin D3 and calcitriol were comparable except for aromatase mRNA, in which case dietary vitamin D3 elicited a more pronounced reduction. In contrast to inhibition of these genes that would adversely affect the course of BCa, dietary vitamin D3 and calcitriol also caused similar increases in mRNA levels of the antiproliferative genes p21 and IGFBP-3 (Fig. 3C). Calcitriol and dietary vitamin D3 also increased the expression of the proapoptotic gene, Bax. The increase in Bax mRNA due to dietary vitamin D3 was more pronounced than that due to calcitriol (Fig. 3C). We also saw a significant decrease in the expression of the antiapoptotic gene, Bcl2, in mice treated with dietary vitamin D but not calcitriol (Fig. 3C).
Fig. 3.
Changes in gene expression in BCa xenograft in the premenopausal model. Vehicle [control (Con)], calcitriol (Cal; 0.05 μg/mouse), and dietary vitamin D3 (Vit D) were administered to tumor-bearing intact female mice as described in Fig. 1. Total RNA was isolated from harvested tumors, and the mRNA levels of ERα, aromatase (A) COX-2, the PG receptors EP2 and FP (B), p21, IGFBP-3, Bax, and Bcl2 (C) were determined by quantitative RT-PCR as described in Materials and Methods. Relative mRNA expression for each gene in the tumors from the control mice was set at 1. Values represent mean ± se from six to 11 determinations. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 compared with control.
Figure 4 depicts changes in ERα, aromatase, and Ki67 protein expression in BCa tumors in the intact mice as determined by IHC analysis. The treatments did not alter the basic morphology of the tumors. Nuclear staining for ERα protein was seen in tumors from control and treated mice (Fig. 4, A–C). The mean Allred score derived from the tumors in the calcitriol-treated group was lower than the control mice, whereas the dietary vitamin D group had the lowest score, indicating decreased ERα expression in the treated groups. Figure 4, D–F, depicts staining for aromatase protein expression in the three experimental groups. In control tumors 90% of the cells stained strongly for the expression of aromatase and the staining was predominantly cytoplasmic (Fig. 4D). Expression of aromatase decreased in both calcitriol and vitamin D3-treated tumors with the treatments resulting in both decreases in the intensity of staining and the percentage of positively stained cells compared with control (Fig. 4, E and F). Ki67 immunostaining was low, with only approximately 5% positive cells in representative tumor sections from control and treated mice, and we could not detect any differences in Ki67 staining between control tissue and tumors in mice treated with calcitriol or dietary vitamin D3 (Fig. 4, G–I).
Fig. 4.
Effect of calcitriol and dietary vitamin D3 on ERα, aromatase, and Ki67 protein expression in tumors in the premenopausal model. Vehicle [control (Con)], 0.05 μg calcitriol (Cal), and dietary vitamin D3 (Vit D) were administered to intact female mice bearing BCa xenografts as described in Fig. 1. Tumors were harvested and fixed for IHC analysis of aromatase, ERα, and Ki67 expression as described in Materials and Methods. All tumors were immunostained with either anti-ERα antibody (A–C), an antiaromatase antibody (D–F), or an anti-Ki67 antibody (G–I) and are shown at ×400 magnification. No immunostaining was detected with IgG (negative control; not shown). ERα staining is indicated by the Allred score, given in parentheses below each panel. In the case of aromatase and Ki67, the numbers followed by the percent sign indicate percentage of cells showing positive staining.
Effects of dietary vitamin D3 and calcitriol on estrogen levels
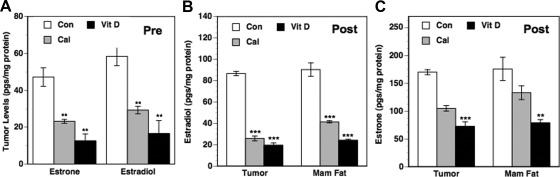
We determined the effect of the treatments on estrogen synthesis by measuring the levels of estrone and estradiol in the tumors. In the premenopausal model, we saw statistically significant decreases in tumor estrone and estradiol levels due to the treatments compared with control (Fig. 5A). The reductions in estrogen levels due to dietary vitamin D3 were comparable or greater in magnitude compared with calcitriol. In the OVX mice (postmenopausal model) receiving supplements of the estrogenic precursor andostenedione, we determined the effect of the treatments on local estrogen synthesis by measuring the levels of estradiol and estrone both in the tumors and the surrounding mammary adipose tissue. When compared with control mice, calcitriol treatment resulted in substantial decreases in estradiol levels in tumors (Fig. 5B; ∼70% decrease; P < 0.001) and the surrounding mammary adipose tissue (Fig. 5B; ∼55% decrease; P < 0.001). The decreases due to dietary vitamin D3 were also substantial (∼77% and ∼73% decreases in the tumors and mammary fat, respectively). Compared with estradiol levels, the decreases in tumor and mammary fat estrone levels due to the treatments were less pronounced but still appreciable and significant (Fig. 5C).
Fig. 5.
Effects of calcitriol and dietary vitamin D3 on estrone and estradiol levels in BCa pre and postmenopausal xenografts and surrounding mammary fat pads. Vehicle [control (Con)], calcitriol (Cal), or dietary vitamin D3 (Vit D) administered to intact female mice representing the premenopausal model (Pre) and to OVX mice receiving androstenedione supplements representing the postmenopausal model (Post) bearing BCa xenografts as described in Fig. 1. Estrone and estradiol levels were determined in extracts of tumors in the premenopausal model (A) and in tumor and mammary fat extracts in the postmenopausal model (B and C) as described in Materials and Methods. Values represent mean ± se from five to 10 determinations. **, P < 0.01 compared with control.
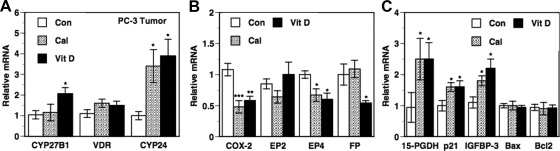
Changes in gene expression due to dietary vitamin D3 and calcitriol in PCa xenografts
Gene expression changes in PCa xenografts due to dietary vitamin D3 and calcitriol treatments were similar to the changes described in BCa xenografts. As expected, calcitriol and dietary vitamin D3 administration caused significant increases (∼3.5- to 4-fold) in the expression of CYP24 mRNA in the PC-3 tumors (Fig. 6A). CYP27B1 mRNA levels were unaltered by calcitriol but significantly increased by dietary vitamin D3 in the PC-3 tumors as well (Fig. 6A). The treatments did not affect tumor VDR mRNA levels (Fig. 6A). Both dietary vitamin D3 and calcitriol decreased COX-2 mRNA and the PG receptor EP4 mRNA to a similar extent (Fig. 6B). A significant reduction in FP mRNA was seen only with dietary vitamin D3, whereas no changes were seen in EP2 mRNA levels with either treatment (Fig. 6B). Both dietary vitamin D3 and calcitriol caused similar increases in 15-PGDH, p21, and IGFBP-3 mRNA levels (Fig. 6C). No changes were seen in the Bax and Bcl2 mRNA levels because of the treatments (Fig. 6C).
Fig. 6.
Changes in gene expression in PCa xenograft tumors due to calcitriol and dietary vitamin D3. Vehicle [control (Con)], calcitriol (Cal; 0.1 μg/mouse), and dietary vitamin D3 (Vit D) were administered to male mice bearing PC-3 tumor xenografts as described in Fig. 1. Total RNA was isolated from harvested tumors and the mRNA levels of CYP27B1, VDR, and CYP24 (A), COX-2, the PG receptors EP2, EP4, and FP (B), 15-PGDH, p21, IGFBP-3, Bax, and Bcl2 (C) were determined by quantitative RT-PCR as described in Materials and Methods. Relative mRNA expression for each gene in tumors from control mice was set at 1. Values represent mean ± se from six to 11 determinations. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 compared with control.
Discussion
Vitamin D3 from the diet is easily converted in the liver to the circulating prohormone 25(OH)D3 (16). Many extrarenal sites including the breast and prostate are fully capable of metabolizing 25(OH)D3 to the active hormone 1,25(OH)2D3 due to the activity of CYP27B1 present in these sites (22). Thus, raising the blood levels of 25(OH)D3 through dietary vitamin D3 supplementation provides increased levels of the substrate for CYP27B1 for the local production of elevated levels of the active hormone 1,25(OH)2D3 in target tissues like breast and prostate, which then exerts autocrine/paracrine anticancer effects at these sites. Although high concentrations of 25(OH)D3 can directly exhibit agonist activity through VDR, its affinity for VDR is approximately 500-fold less than 1,25(OH)2D3 (42, 43), and the levels of free (unbound to vitamin D binding protein) 25(OH)D3 achieved after dietary vitamin D3 ingestion were probably not adequate to contribute much agonist activity without conversion to 1,25(OH)2D3. In our study, a 5-fold increase in vitamin D3 levels in the mouse chow, from 1000 IU/kg in the control diet to 5000 IU/kg in the supplemented diet, resulted in approximately 50% and approximately 145% increase in serum 25(OH)D levels in female and male mice bearing the BCa and PCa xenografts, respectively. At this level of supplementation, dietary vitamin D3 elicited significant shrinkage (∼50–60%) of the xenograft tumors. The dramatic effect of dietary vitamin D3 was equivalent to the tumor shrinkage seen in animals receiving ip calcitriol. Several prior studies have demonstrated the tumor inhibitory effects of the active hormone calcitriol and its structural analogs in nude mice bearing xenografts of human BCa (26, 44–47) and PCa cells (48–51). To the best of our knowledge, our study is the first demonstration of the tumor-inhibitory effects of dietary vitamin D3 in xenograft models of BCa and PCa.
The beneficial effects of dietary vitamin D3 supplements have been reported in experimental models of colon cancer, demonstrating reductions in both colon tumor incidence and multiplicity (52, 53). A previous study has concluded that the 100- to 400-IU/kg diet of vitamin D3 is sufficient to ensure optimal serum concentrations of 25(OH)D in mice and rats (54). Thus, the level of 1000 IU/kg vitamin D3 in the control diet in our study might already represent a low level of vitamin D3 supplementation. Reducing dietary vitamin D3 to levels less than 1000 IU/kg in the control diet might allow an even better assessment of the true extent of tumor inhibition due to dietary vitamin D3 and calcitriol.
Analysis of tumor gene expression revealed equivalent anticancer activities of dietary vitamin D3 and administered calcitriol. The increase in the expression of the CDK inhibitor p21 and IGFBP-3 in MCF-7 xenografts reflect the antiproliferative activity of dietary vitamin D3 and calcitriol. Immunostaining of the nuclear protein Ki67 in the MCF-7 tumors, however, was rather low (below the threshold of ∼10% stained cells) because MCF-7 cells typically form low-grade ER+ tumors, which are associated with low Ki67 expression (55). Any further inhibition of Ki67 due to the treatments was not apparent. The observed low Ki67 expression is also in line with the reported inverse association between Ki67 immunostaining and ERα expression (55, 56). The modest decrease in the expression of the antiapoptotic gene Bcl2 and the appreciable increase in that of the proapoptotic gene Bax suggest an increase in the fraction of tumor cells undergoing apoptosis due to the treatments. In the case of PC-3 tumors, modest increases in p21 and IGFBP-3 expression and no changes in the expression of the apoptosis markers, Bcl2 and Bax, correlated with the relatively lower suppressive effects of the treatments on the growth of this more aggressive cell line. As we have shown before (26, 30, 32), the treatments exhibited antiinflammatory activity in both xenograft models as demonstrated by the suppression of PG synthesis (decreased COX-2) and signaling (increased 15-PGDH and decreased PG receptor expression). A recent study in MMTV-neu transgenic mice fed vitamin D3-supplemented diet demonstrated that vitamin D3 signaling-regulated genes involved in pathways that drive differentiation, alter metabolism, remodel the extracellular matrix, and trigger innate immunity in the mammary tissue (57).
In the case of female mice with BCa xenografts, dietary vitamin D3 was as effective as calcitriol in suppressing tumor aromatase expression in both intact and OVX mice and also in the tumor surrounding mammary fat in the OVX mice, leading to significant suppression of local estrogen synthesis in the breast. These data suggest potential therapeutic utility of dietary vitamin D3 and calcitriol in postmenopausal BCa, in which locally synthesized estrogens play a key role in driving BCa growth (58). In addition to inhibiting estrogen synthesis, dietary vitamin D3 supplementation also decreased tumor ERα levels. Thus, the inhibitory effects of dietary vitamin D3 on estrogen synthesis and signaling were similar to those exerted by calcitriol in cell culture and animal models of BCa (26, 30).
An examination of the expression of genes involved in vitamin D metabolism in the breast and prostate tumors revealed the expected increases in CYP24 mRNA, although the changes were modest at the time point analyzed. There was also a very modest up-regulation of VDR. These data confirm the biological activity of administered calcitriol and dietary vitamin D3 in the tumors. Calcitriol is a known negative regulator of CYP27B1transcription in the kidney (59). However, the regulation of CYP27B1 by calcitriol appears to be tissue specific, and published studies report either no alteration (60, 61) or an increase (62) in CYP27B1 expression in MCF-7 cells treated with calcitriol. In our study, no significant changes were seen in tumor CYP27B1 mRNA levels in mice treated with calcitriol. Interestingly, we found appreciable up-regulation of CYP27B1 mRNA in both MCF-7 and PC-3 tumors after dietary vitamin D3 treatment. An earlier study reported an increase in CYP27B1 mRNA in mouse mammary gland organ cultures exposed to 25(OH)D (63). The mechanism underlying the differential regulation of CYP27B1 expression by the active hormone calcitriol compared with dietary vitamin D3 remains unclear at present, and further research is required to elucidate the mechanism of this effect.
Despite the elevations in serum 25(OH)D levels after dietary vitamin D3 ingestion, we did not expect an increase in circulating 1,25(OH)2D levels because of the tight regulation of renal CYP27B1 by PTH and serum calcium that restricts renal 25(OH)D conversion to 1,25(OH)2D (16). Because extrarenal CYP27B1 is not regulated by PTH (64), we hypothesized that the extent of 25(OH)D conversion to 1,25(OH)2D in the tumors would not be restricted and would be proportional to the dietary levels of vitamin D3. The locally synthesized 1,25(OH)2D3 would be sufficient to exert significant autocrine/paracrine anticancer effects but not high enough to spill into the circulation to raise circulating 1,25(OH)2D levels. However, contrary to our expectation, we observed significant elevations in serum 1,25(OH)2D levels in tumor-bearing mice ingesting dietary vitamin D3 supplements. This observation raises the possibility that the endocrine activity of 1,25(OH)2D due to renal and extrarenal synthesis might also be a contributing factor in the tumor inhibitory effect of dietary vitamin D3, prompting us to examine changes in renal CYP27B1 expression. Dietary vitamin D3 administration to intact female nude mice with MCF-7 tumors did not cause an increase in renal CYP27B1, suggesting that in these mice the origin of the elevated circulating serum 1,25(OH)2D levels was not renal. In a control experiment, non-tumor-bearing intact female mice fed vitamin D3-supplemented diet did not show elevations in circulating 1,25(OH)2D, indicating that the elevated serum 1,25(OH)2D levels in the tumor-bearing mice receiving the vitamin D3-supplemented diet was likely from extrarenal sources and could, at least in part, be tumor derived. Also, serum 1,25(OH)2D levels were lower in mice receiving calcitriol injections compared with control female mice with BCa xenografts. As reiterated before, serum 1,25(OH)2D levels in our study represented nadir values measured 14–16 h after the previous calcitriol injection (39). The induction of CYP24 by levels of 1,25(OH)2D3 that peaked immediately after calcitriol injections would trigger the degradation of 1,25(OH)2D, accounting for the decreases in serum 1,25(OH)2D levels in calcitriol-treated mice compared with controls.
In our study, administration of calcitriol at doses higher than 0.025 μg caused statistically significant increases in serum calcium levels, raising hypercalcemia as a potential side effect of calcitriol therapy in patients (65). However, the ingestion of dietary vitamin D3 at 5000 IU/kg did not increase serum calcium levels, despite the elevations detected in serum calcitriol levels. Although the elevated 1,25(OH)2D levels found in mice receiving the vitamin D3-supplemented diet appeared to be higher than the nadir serum 1,25(OH)2D levels in mice receiving calcitriol, it is likely that they were lower than the elevated serum 1,25(OH)2D levels that peaked shortly after calcitriol injections (39). These elevated peak 1,25(OH)2D levels likely caused the observed increase in serum calcium concentration in the calcitriol-treated mice, which also might have been greater if measured at earlier time points. In other studies, ingestion of diets supplemented with vitamin D3 at levels up to 20,000 IU/kg for up to 7 wk has been shown not to affect the serum calcium levels in the non-tumor-bearing mice (54).
In summary, dietary vitamin D3 exhibited significant tumor inhibitory effects in xenograft models of BCa and PCa, which were equivalent to those mediated by the active hormone calcitriol administered three times a week at graded doses. At the doses tested, dietary vitamin D3 unlike calcitriol did not cause a significant change in serum calcium levels demonstrating its relative safety. We hypothesize that the autocrine/paracrine activity ensuing from local synthesis of 1,25(OH)2D in the tumors after elevations in serum 25(OH)D due to dietary vitamin D3 plays a pivotal role in the anticancer activity of dietary vitamin D3. However, the elevations in circulating 1,25(OH)2D in tumor-bearing mice ingesting a vitamin D3-supplemented diet suggests that the endocrine activity of 1,25(OH)2D, from tumor, extrarenal as well as renal sources, probably also plays a role in mediating the anticancer effects of dietary vitamin D3. Our data support the hypothesis that dietary vitamin D3 is useful in the chemoprevention and treatment of BCa and PCa because it is a very safe, economical, and easily available nutritional agent that is as active as calcitriol in inhibiting tumor growth. Furthermore, adequate vitamin D nutrition and avoidance of vitamin D deficiency would be important in reducing cancer risk. Clinical studies in BCa and PCa patients evaluating the benefits of dietary vitamin D3 supplementation are clearly warranted.
Acknowledgments
We thank Dr. Milan Uskokovic (BioXell Co.) for the kind gift of calcitriol.
This work was supported by Grant CA130991 from the National Cancer Institute (to D.F.), Grant 070101 from the Komen Foundation (to D.F.), and an American Recovery and Reinvestment Act (ARRA) summer student supplement to the parent grant CA130991 from NCI (to J.Y.W.).
Disclosure Summary: Authors S.S., A.V.K., J.Y.W., K.J., M.A.A., and D.F. have nothing to declare. R.H. is the owner/director of Heartland Assays.
Footnotes
- Bax
- Bcl-2 associated X protein
- BCa
- breast cancer
- COX
- cyclooxygenase
- CYP27B1
- 1α-hydroxylase
- EP
- prostaglandin E receptor
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- FP
- prostaglandin F receptor
- IGFBP
- IGF binding protein
- IHC
- immunohistochemistry
- 1,25(OH)2D
- 1,25 dihydroxyvitamin D2 and D3
- 1,25(OH)2D3
- calcitriol
- 25(OH)D
- 25-hydroxyvitamin D
- OVX
- ovariectomized
- PCa
- prostate cancer
- PG
- prostaglandin
- 15-PGDH
- 15-hydroxyprostaglandin dehydrogenase
- VDR
- vitamin D receptor.
References
- 1. Christakos S, DeLuca HF. 2011. Minireview: vitamin D: is there a role in extraskeletal health? Endocrinology 152:2930–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishnan AV, Trump DL, Johnson CS, Feldman D. 2010. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am 39:401–418, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. 2006. The role of vitamin D in cancer prevention. Am J Public Health 96:252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. 2007. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol 103:708–711 [DOI] [PubMed] [Google Scholar]
- 5. Giovannucci E. 2005. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 16:83–95 [DOI] [PubMed] [Google Scholar]
- 6. Coyle YM. 2004. The effect of environment on breast cancer risk. Breast Cancer Res Treat 84:273–288 [DOI] [PubMed] [Google Scholar]
- 7. Cui Y, Rohan TE. 2006. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev 15:1427–1437 [DOI] [PubMed] [Google Scholar]
- 8. Freedman DM, Dosemeci M, McGlynn K. 2002. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med 59:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. 2007. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 16:422–429 [DOI] [PubMed] [Google Scholar]
- 10. Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. 2005. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 14:1991–1997 [DOI] [PubMed] [Google Scholar]
- 11. Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. 2011. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128:1414–1424 [DOI] [PubMed] [Google Scholar]
- 12. Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. 2007. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med 4:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. 2008. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 17:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE. 2009. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res 11:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB. 2008. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst 100:796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feldman D, Malloy PJ, Krishnan AV, Balint E. 2007. Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ, eds. Osteoporosis. 3rd ed San Diego: Academic Press; 317–382 [Google Scholar]
- 17. Deeb KK, Trump DL, Johnson CS. 2007. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700 [DOI] [PubMed] [Google Scholar]
- 18. Gombart AF, Luong QT, Koeffler HP. 2006. Vitamin D compounds: activity against microbes and cancer. Anticancer Res 26:2531–2542 [PubMed] [Google Scholar]
- 19. Stewart LV, Weigel NL. 2004. Vitamin D and prostate cancer. Exp Biol Med (Maywood) 229:277–284 [DOI] [PubMed] [Google Scholar]
- 20. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. 2010. Vitamin D: a pleiotropic hormone. Kidney Int 78:140–145 [DOI] [PubMed] [Google Scholar]
- 21. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. 2004. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101:7711–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewison M, Adams JS. 2011. Extrarenal 1a-hydroxylase. In: Feldman D, Pike JW, Adams JS, eds. Vitamin D. San Diego: Elsevier Academic Press; 777–804 [Google Scholar]
- 23. Chen TC, Holick MF. 2003. Vitamin D and prostate cancer prevention and treatment. Trends Endocrinol Metab 14:423–430 [DOI] [PubMed] [Google Scholar]
- 24. Krishnan AV, Feldman D. 2011. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 51:311–336 [DOI] [PubMed] [Google Scholar]
- 25. Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. 1997. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med 3:402–408 [DOI] [PubMed] [Google Scholar]
- 26. Swami S, Krishnan AV, Wang JY, Jensen K, Peng L, Albertelli MA, Feldman D. 2011. Inhibitory effects of calcitriol on the growth of MCF-7 breast cancer xenografts in nude mice: selective modulation of aromatase expression in vivo. Horm Cancer 2:190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS. 1999. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res 59:2644–2649 [PubMed] [Google Scholar]
- 28. Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. 2001. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 7:1043–1051 [PubMed] [Google Scholar]
- 29. Koshizuka K, Koike M, Asou H, Cho SK, Stephen T, Rude RK, Binderup L, Uskokovic M, Koeffler HP. 1999. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res Treat 53:113–120 [DOI] [PubMed] [Google Scholar]
- 30. Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D. 2010. Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology 151:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 32. Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D. 2005. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 65:7917–7925 [DOI] [PubMed] [Google Scholar]
- 33. Zhao XY, Ly LH, Peehl DM, Feldman D. 1999. Induction of androgen receptor by 1α,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology 140:1205–1212 [DOI] [PubMed] [Google Scholar]
- 34. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- 35. Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. 1993. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- 36. Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. 1996. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42:586–592 [PubMed] [Google Scholar]
- 37. Allred DC, Carlson RW, Berry DA, Burstein HJ, Edge SB, Goldstein LJ, Gown A, Hammond ME, Iglehart JD, Moench S, Pierce LJ, Ravdin P, Schnitt SJ, Wolff AC. 2009. NCCN Task Force Report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Canc Netw 7(Suppl 6):S1–S21; quiz S22–S23 [DOI] [PubMed] [Google Scholar]
- 38. Allred DC, Harvey JM, Berardo M, Clark GM. 1998. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168 [PubMed] [Google Scholar]
- 39. Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. 2004. Pharmacokinetics of 1α,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology 66:62–66 [DOI] [PubMed] [Google Scholar]
- 40. Costa EM, Feldman D. 1986. Homologous up-regulation of the 1,25 (OH)2 vitamin D3 receptor in rats. Biochem Biophys Res Commun 137:742–747 [DOI] [PubMed] [Google Scholar]
- 41. Sandgren ME, DeLuca HF. 1990. Serum calcium and vitamin D regulate 1,25-dihydroxyvitamin D3 receptor concentration in rat kidney in vivo. Proc Natl Acad Sci USA 87:4312–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skowronski RJ, Peehl DM, Feldman D. 1995. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin D3. Endocrinology 136:20–26 [DOI] [PubMed] [Google Scholar]
- 43. Wecksler WR, Okamura WH, Norman AW. 1978. Studies on the mode of action of vitamin D—XIV. Quantitative assessment of the structural requirements for the interaction of 1α, 25-dihydroxyvitamin D3 with its chick intestinal mucosa receptor system. J Steroid Biochem 9:929–937 [DOI] [PubMed] [Google Scholar]
- 44. Colston KW, Mackay AG, James SY, Binderup L, Chander S, Coombes RC. 1992. EB1089: a new vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochem Pharmacol 44:2273–2280 [DOI] [PubMed] [Google Scholar]
- 45. Flanagan L, Packman K, Juba B, O'Neill S, Tenniswood M, Welsh J. 2003. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol 84:181–192 [DOI] [PubMed] [Google Scholar]
- 46. VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. 1998. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology 139:2102–2110 [DOI] [PubMed] [Google Scholar]
- 47. Zinser GM, Tribble E, Valrance M, Urben CM, Knutson JC, Mazess RB, Strugnell SA, Welsh J. 2005. 1,24(S)-dihydroxyvitamin D2, an endogenous vitamin D2 metabolite, inhibits growth of breast cancer cells and tumors. Anticancer Res 25:235–241 [PubMed] [Google Scholar]
- 48. Bhatia V, Saini MK, Shen X, Bi LX, Qiu S, Weigel NL, Falzon M. 2009. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther 8:1787–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blutt SE, Polek TC, Stewart LV, Kattan MW, Weigel NL. 2000. A calcitriol analogue, EB1089, inhibits the growth of LNCaP tumors in nude mice. Cancer Res 60:779–782 [PubMed] [Google Scholar]
- 50. Schwartz GG, Hill CC, Oeler TA, Becich MJ, Bahnson RR. 1995. 1,25-Dihydroxy-16-ene-23-yne-vitamin D3 and prostate cancer cell proliferation in vivo. Urology 46:365–369 [DOI] [PubMed] [Google Scholar]
- 51. Vegesna V, O'Kelly J, Said J, Uskokovic M, Binderup L, Koeffle HP. 2003. Ability of potent vitamin D3 analogs to inhibit growth of prostate cancer cells in vivo. Anticancer Res 23:283–289 [PubMed] [Google Scholar]
- 52. Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. 2010. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol 121:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. 2009. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis 30:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, Adedokun SA. 2008. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr 138:1114–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tan PH, Bay BH, Yip G, Selvarajan S, Tan P, Wu J, Lee CH, Li KB. 2005. Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod Pathol 18:374–381 [DOI] [PubMed] [Google Scholar]
- 56. Coddington R, Cuthbert A, Campbell ID, Herbert A, Theaker JM, Royle GT, Taylor I. 1993. Determination of Ki67 growth fraction and oestrogen receptors in screen-detected breast cancer using cytological preparations. Cytopathology 4:257–266 [DOI] [PubMed] [Google Scholar]
- 57. Matthews D, LaPorta E, Zinser GM, Narvaez CJ, Welsh J. 2010. Genomic vitamin D signaling in breast cancer: insights from animal models and human cells. J Steroid Biochem Mol Biol 121:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. 2002. Aromatase—a brief overview. Annu Rev Physiol 64:93–127 [DOI] [PubMed] [Google Scholar]
- 59. Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S. 2004. Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. EMBO J 23:1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Kemmis CM, Salvador SM, Smith KM, Welsh J. 2006. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr 136:887–892 [DOI] [PubMed] [Google Scholar]
- 61. Turunen MM, Dunlop TW, Carlberg C, Väisänen S. 2007. Selective use of multiple vitamin D response elements underlies the 1α,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res 35:2734–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, Dunstan CR. 2010. Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone 47:795–803 [DOI] [PubMed] [Google Scholar]
- 63. Peng X, Hawthorne M, Vaishnav A, St. Arnaud R, Mehta RG. 2009. 25-Hydroxyvitamin D3 is a natural chemopreventive agent against carcinogen induced precancerous lesions in mouse mammary gland organ culture. Breast Cancer Res Treat 113:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Young MV, Schwartz GG, Wang L, Jamieson DP, Whitlatch LW, Flanagan JN, Lokeshwar BL, Holick MF, Chen TC. 2004. The prostate 25-hydroxyvitamin D-1α-hydroxylase is not influenced by parathyroid hormone and calcium: implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis 25:967–971 [DOI] [PubMed] [Google Scholar]
- 65. Gross C, Stamey T, Hancock S, Feldman D. 1998. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J Urol 159:2035–2039; discussion 2039–2040 [DOI] [PubMed] [Google Scholar]