Abstract
The acute phase response (APR) produces marked alterations in lipid and carbohydrate metabolism including decreasing plasma ketone levels. Fibroblast growth factor 21 (FGF21) is a recently discovered hormone that regulates lipid and glucose metabolism and stimulates ketogenesis. Here we demonstrate that lipopolysaccharide (LPS), zymosan, and turpentine, which induce the APR, increase serum FGF21 levels 2-fold. Although LPS, zymosan, and turpentine decrease the hepatic expression of FGF21, they increase FGF21 expression in adipose tissue and muscle, suggesting that extrahepatic tissues account for the increase in serum FGF21. After LPS administration, the characteristic decrease in plasma ketone levels is accentuated in FGF21−/− mice, but this is not due to differences in expression of carnitine palmitoyltransferase 1α or hydroxymethyglutaryl-CoA synthase 2 in liver, because LPS induces similar decreases in the expression of these genes in FGF21−/− and control mice. However, in FGF21−/− mice, the ability of LPS to increase plasma free fatty acid levels is blunted. This failure to increase plasma free fatty acid could contribute to the accentuated decrease in plasma ketone levels because the transport of fatty acids from adipose tissue to liver provides the substrate for ketogenesis. Treatment with exogenous FGF21 reduced the number of animals that die and the rapidity of death after LPS administration in leptin-deficient ob/ob mice and to a lesser extent in control mice. FGF21 also protected from the toxic effects of cecal ligation and puncture-induced sepsis. Thus, FGF21 is a positive APR protein that protects animals from the toxic effects of LPS and sepsis.
Infection, inflammation, trauma, and malignancy induce the acute phase response (APR), which is characterized by changes in hepatic protein synthesis resulting in alterations in the levels of specific plasma proteins (1, 2). Plasma proteins that increase during the APR are positive acute phase proteins (e.g. C-reactive protein and serum amyloid A), whereas plasma proteins that decrease are negative APR proteins (e.g. albumin and apolipoprotein A1). Most of these changes in hepatic protein synthesis are mediated by alterations in gene transcription (1, 2).
In addition to altering hepatic protein production, the APR also alters the synthesis of specific proteins in many extrahepatic tissues, which have profound effects on lipid and carbohydrate metabolism (3, 4). An increase in serum triglyceride levels due to both increased very low density lipoprotein production and decreased clearance of triglyceride-rich lipoproteins is one characteristic change in lipid metabolism induced by the APR (3). Serum glucose levels typically increase with the APR due to insulin resistance (4, 5). However, with severe sepsis hypoglycemia can occur due to the increased uptake of glucose by macrophages coupled with a decrease in hepatic gluconeogenesis (4, 5).
The changes that are induced during the APR are thought to be beneficial to the host (1, 2). These changes have been shown to neutralize invading microorganisms, minimize the extent of tissue damage, participate in the local immune response and tissue regeneration, and replenish the proteins that are used during the inflammatory process (1, 2). For example, the increase in serum very low density lipoprotein may play a role in neutralizing microbial-derived toxins, such as endotoxin and lipoteichoic acid, as well as providing an energy source for tissues involved in host defense and tissue repair (3).
Fibroblast growth factor 21 (FGF21) is a recently discovered hormone that regulates lipid and glucose metabolism (6, 7). Specifically, an increase in FGF21 leads to decreases in serum triglyceride and glucose levels (8–11). The decrease in serum triglyceride levels may be due to decreased hepatic lipogenesis and an increased hepatic fatty acid oxidation with increased ketone production (8, 12, 13). Several of the enzymes required for fatty acid oxidation and ketone body formation are increased by FGF21, including carnitine palmitoyltransferase 1α (CPT-1α), acyl-Coenzyme A oxidase (ACO), and hydroxymethylglutaryl-CoA synthase 2 (HMGCS2) (12, 13). Recent studies have shown that FGF21 stimulates peroxisome proliferator-activated receptor γ (PPAR) coactivator 1α and that the FGF21-induced increase in PGC-1 α mediates some of the changes in metabolism (14).
Serum FGF21 levels are increased by fasting, fibrate treatment, obesity, and type 2 diabetes (12, 15–19). FGF21 expression in the liver is regulated by PPARα activation and treatment with PPARα activators increase FGF21 mRNA levels, whereas conversely hepatic FGF21 expression is markedly decreased in PPARα-deficient mice (12, 13, 20). Additionally, fasting and feeding a ketogenic diet, which increase hepatic PPARα levels, also are accompanied by an increase in FGF21 mRNA in the liver, an effect that is markedly attenuated in PPARα-deficient mice (12, 13, 20). Finally, PPARγ activators stimulate FGF21 expression and FGF21 coreceptor, βKlotho, in adipose tissue (21–23), whereas FGF21 is able to regulate the expression of both PPARα and PPARγ (8).
During the APR, there is a decrease in fatty acid oxidation in the liver and in the enzymes responsible for fatty acid oxidation with an associated decrease in hepatic ketogenesis and plasma ketone levels (3). Decreases in the expression of PPARα, PGC-1α and β, and other transcription factors and cofactors that increase fatty acid oxidation occur during the APR (24, 25). Additionally, there is an increase in hepatic lipogenesis (3). These APR-induced changes in hepatic lipid metabolism are opposite of what is induced by increases in FGF21, leading us to hypothesize that FGF21 may be down-regulated during the APR and contribute to the alterations in hepatic metabolism. Therefore we asked whether FGF21 is regulated during the APR and if so, whether FGF21 plays a beneficial role in host defense. Rather, we found that serum FGF21 levels increased and that FGF21 protects from LPS-induced toxicity.
Materials and Methods
Materials
Lipopolysaccharide (LPS), Escherichia coli 55:B5, was obtained from Difco Laboratories (Detroit, MI) (and freshly diluted to the desired concentration in pyrogen-free 0.9% saline. For the FGF21 protection studies LPS (E. coli type 0111:B4, 3 × 106 endotoxin units/mg), and human albumin were purchased from Sigma (St. Louis, MO). Turpentine was purchased from BDH Laboratories (Dubai, UAE). Zymosan A and Tri-Reagent were purchased from Sigma. iScript cDNA synthesis kit was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA). LightCycler 480 SYBR Green I Master was purchased from Roche Diagnostics (Indianapolis, IN).
Animals
Eight-week-old female C57BL/6 mice and PPARα-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). For the FGF21 protection studies female ob/ob and C57BL/6 mice were purchased from Harlan UK (Belton Loughborough, UK). FGF21 knockout (KO) mice were purchased from Taconic Farms, Inc. (Hudson, NY). Animals were maintained in a normal light-cycle room and were provided with rodent chow and water ad libitum. Anesthesia was induced with isoflurane inhalation. Animals were injected with saline, LPS (5 mg/kg ip), zymosan A (80 mg/kg ip) or Oil of Turpentine (100 μl sc in left hind leg), and food was removed for the duration of the experiment from both control and treated animals after injection because these stimuli induce anorexia in rodents. These doses of LPS, zymosan, and turpentine were previously shown to induce the APR in mice, but are far below the lethal dose (26). Blood and tissues samples were removed at the time indicated in the text after treatment. All experiments were performed according to protocols approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center or the Animal Use and Care Committee of Eli Lilly and Co.
Expression and purification of recombinant FGF21
A pET30a vector was used to express human FGF21 in the E. coli strain BL21 (DE3) (Novagen, Madison, WI; EMD Biosciences, Inc., San Diego, CA). FGF21 product accumulated in the insoluble fraction. Inclusion bodies were prepared by standard centrifugation method. We solubilized inclusion bodies by bringing granule pellets to 10 times the original volume in 50 mm Tris-HCl (pH 9.0)-7 m urea and homogenized the material. The protein mixture was adjusted to pH 11, stirred for 1 h, readjusted to pH 9.0, and loaded onto a Q Sepharose Fast Flow (Amersham Biosciences, Piscataway, NJ). Anion-exchange (AEX) chromatography was done in 50 mm Tris-HCl (pH 9.0), 7 m urea, 1 mm dithiothreitol with a 0–400 mm NaCl gradient elution. The eluted AEX pool was treated with 10 mm DTT for 2 h at room temperature and diluted 10-fold with 10 mm cysteine/7 m urea. The protein was refolded by dialysis against 20 mm glycine (pH 9.0) for 48 h at 4 C. Further purification was carried out with reversed-phase HPLC (RP-HPLC) performed with a Grace Vydac C18 column run in H20/0.1% trifluoroacetic acid/acetonitrile mobile phase with a 0–50% acetonitrile gradient; size-exclusion chromatography on Superdex 75 (Amersham Biosciences) in PBS, pH 7.4; and AEX chromatography on MonoQ (Amersham Biosciences) in 50 mm Tris (pH 8.0) with 0–300 mm NaCl gradient. The final FGF21 pool was dialyzed into PBS (pH 7.4), sterile filtered, and stored at −80 C. The FGF21 used in these experiments had levels of endotoxin ≤ 0.2 EU/mg. Data on the purity and in vitro activity of FGF21 are provided in Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from 50–100 mg of snap-frozen liver, pancreas, leg muscle, and adipose tissue from the periuterine-urinary bladder area using Tri-Reagent. RNA was quantified by measuring absorption at 260 nm. First-strand cDNA was synthesized from 1 μg of total RNA with random hexamer primers using iScript cDNA Synthesis Kit. The RT-PCR contained, in a final volume of 20 μl, 20 ng of reversed transcribed total RNA, 450 nm forward and reverse primers, and 10 μl of 2 × LightCycler 480 SYBR Green I Master. Primers sequences used are FGF21 forward (F), 5′-CTGGGGGTCTACCAAGCATA-3′; FGF21 reverse (R), 5′-CACCCAGGATTTGAATGACC-3′; HMGCS2 F, 5′-ATCAACTCCCTGTGCCTGAC-3′; HMGCS2 R, 5′-GCAATGTCACCACAGACCAC-3′; glucose transporter (GLUT)1 F, 5′-GGATCTCTCTGGAGCACAGG-3′; GLUT1 R, 5′-TCCTCCTGGACTTCACTGCT-3′; CPT-1α F, 5′-GCACTGCAGCTCGCACATTACAA-3′; CPT-1α R, 5′-CTCAGACAGTACCTCCTTCAGGAAA-3′. The relative amounts of mRNA were calculated using the comparative CT method (ΔΔCT). 36B4 mRNA was used as the invariant control for all experiments.
Serum FGF21 measurements
Serum was obtained at the times indicated in the text and FGF21 was assessed by specific RIA (Phoenix Pharmaceuticals, Inc., Burlingame, CA) according to manufacturer's instructions [intraassay coefficient of variation (CV) = 5–7%; interassay CV = 12–15%]. FGF21 was measured in duplicate by a mouse-specific ELISA (Biovendor, Modrice, Czech Republic) according to the manufacturer's protocol (intraassay CV = 8.4%; interassay CV = 8.7%).
Tissue FGF21 measurements
Total protein extracts from liver, muscle, and adipose tissues were homogenized in radioimmune precipitation assay buffer. Crude homogenates were centrifuged at 12,000 × g for 10 min. The resulting supernatants were used to measure FGF21 protein levels by ELISA kit (Millipore Corp., Billerica, MA) following manufacturer's instructions.
LPS challenge
Mice were acclimated for 1 wk and used at 8–11 wk of age. Mice were housed in a room that maintained constant temperature and humidity and were subjected to one 12-h light, 12-h dark cycle per day. Female ob/ob mice received special diet Purina 5008 rodent chow and water ad libitum. Body weights and blood glucose values were obtained 1 d before LPS challenge and used to randomize the animals into similar groups for the experiments. Mice were injected ip with 0.1 ml of E. coli LPS at doses ranging from 6–27.5 mg/kg in PBS. Human albumin (Sigma) or human recombinant FGF21 protein (50 or 30 μg) was injected two or three times daily sc in a volume of 0.1 ml of PBS beginning at 1 h after LPS and continued out to d 3 or d 7. Mice were monitored for survival two to three times daily, and the endpoint was 54 or 168 h.
Cecal ligation and puncture (CLP) procedure
Body weights and blood glucose values were obtained 1 d before CLP surgery and used to randomize the animals into similar groups. Mice were anesthetized with a Nembutal bolus (45 mg/kg, ip) and then shaved, scrubbed with betadine, and swabbed with 70% isopropyl alcohol. The cecum was exposed aseptically through a 1-cm incision of the lower abdomen, tightly ligated with a 4–0 silk suture below the ileocecal valve without causing bowel obstruction, and punctured through-and-through once with a 25-gauge-diameter needle. The ligated and perforated cecum was replaced in the peritoneal cavity, and surgical incision was closed with 4–0 silk sutures and stainless steel wound clips (Becton Dickinson and Co., Sparks, MD). All the mice received 1 ml of prewarmed (37 C) normal saline sc for fluid resuscitation and were placed on a heating pad until they recovered from anesthesia. Mice were injected with 50 μg of FGF21 recombinant protein or human albumin sc two times daily for 4 d beginning at 2 h after CLP surgery. The mice were monitored four times per day for 4 d.
Measurement of blood glucose, plasma free fatty acids, and plasma ketones
Blood (30–50 μl) from the tail vein was directly applied to a Precision PCx blood glucose sensor electrode strip for blood glucose determination with a MediSense Precision PCx glucometer (Abbott Laboratories, North Chicago, IL). Blood glucose was determined 1 d before experimentation. The lower limit of detection was 20 mg/dl, and the upper limit of detection was 600 mg/dl; thus, those values were assigned when values exceeded these limits.
Blood was obtained at the end of the experiments and plasma was isolated. Ketones and free fatty acids were measured using kits provided by WAKO Diagnostics (Richmond, VA).
Statistical analysis
Data are expressed as the mean ± se of experiments from four to seven animals. The difference between experimental groups was analyzed using the Student's t test. Data for survival curves were analyzed by a Kaplan Meier analysis and a Wilcoxon test with JMP software (SAS Institute, Inc., Cary, NC).
Results
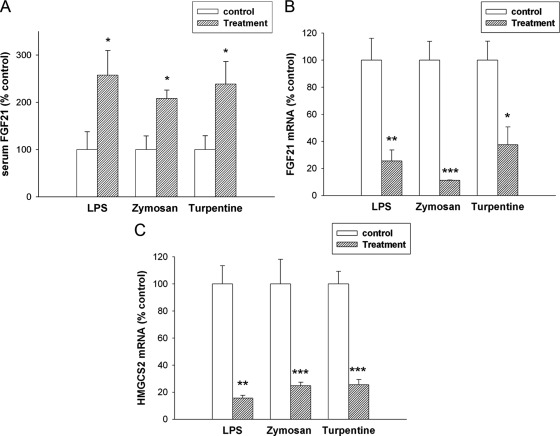
We initially determined the effect of the administration of LPS, a TLR4 activator and model of gram-negative infections (27), on serum FGF21 levels. At 16 h after LPS administration there was a 2.5-fold increase in serum FGF21 levels measured by ELISA (Fig. 1A). A similar increase was observed when serum FGF21 levels were measured by RIA (0.46 ng/ml ± 0.022 for control; 0.74 ng/ml ± 0.077 for LPS; P < 0.01; Supplemental Fig. 2). Of note, the increase in FGF21 after LPS administration was a relatively late effect, and no difference in FGF21 serum levels between LPS-treated and controls was observed at 4 h after LPS (Supplemental Fig. 2). We next determined the effect of other treatments that induce an APR on serum FGF21 levels. The administration of either zymosan, a TLR2 activator and model of fungal infections (27), or turpentine, which causes sterile abscesses, resulted in an increase in serum FGF21 levels (Fig. 1A). Thus, a variety of stimulants that induce the APR lead to an increase in serum FGF21 levels, despite a decrease in ketogenesis.
Fig. 1.
Effect of APR stimuli on serum FGF21 levels and mRNA levels in mouse liver. Mice were injected with saline, LPS (5 mg/kg, ip), zymosan A (80 mg/kg, ip), or turpentine (100 μl/mouse, sc), and the animals were euthanized at 16 h. A, Serum FGF21 levels were measured by ELISA as described in Materials and Methods. B, Total RNA was isolated from liver tissue, cDNA was synthesized with reverse transcriptase, and quantitative real-time PCR was performed as described in Materials and Methods. FGF21 mRNA levels were measured. C, HMGCS2 mRNA levels were measured. Quantitative PCR data were normalized to 36B4 mRNA for all experiments. Data (mean ± se, n = 4) are expressed as a percentage of controls administered saline. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
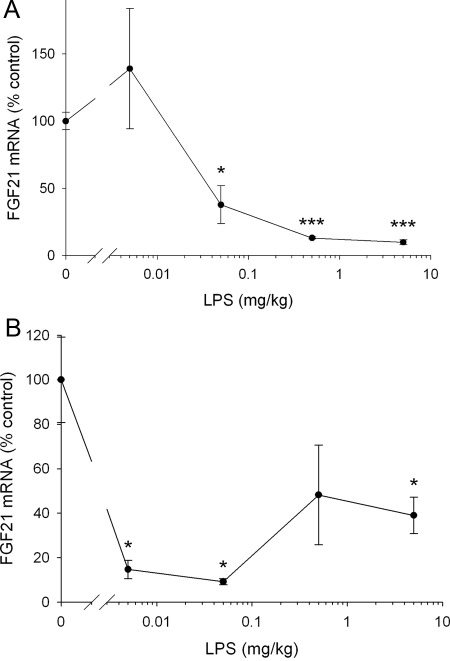
We next determined the effect of LPS on FGF21 mRNA levels in the liver, one of the major sites of FGF21 production and of the fatty acid metabolism changes in the APR. As shown in Fig. 1B, despite the increase in serum levels of FGF21, LPS treatment surprisingly resulted in a marked reduction in hepatic FGF21 mRNA levels at 16 h after administration (60% decrease). Hepatic FGF21 mRNA levels were also decreased at 4 h after LPS administration (Fig. 2A). Moreover, the decrease in FGF21 expression in the liver induced by LPS was a very sensitive response with marked decreases in FGF21 mRNA seen after very low doses of LPS at both 4 and 16 h (Fig. 2, A and B). In concordance with the decrease in FGF21 mRNA levels, we also observed a decrease in FGF21 protein levels in the liver 16 after LPS treatment (Control 19.8 ± 2.0 vs. LPS 15.9 ± 0.5 pg/ml × μg protein; P = 0.052). Similarly, zymosan and turpentine administration also resulted in a decrease in hepatic FGF21 mRNA levels (Fig. 1B). Additionally, the reductions in FGF21 mRNA levels induced by LPS, zymosan, or turpentine treatment were accompanied by decreases in the hepatic mRNA levels of HMGCS2, a target gene of FGF21 and an important enzyme in ketone body synthesis, which decreases in the APR (Fig. 1C) (28).
Fig. 2.
Effect of LPS on FGF21 mRNA in mouse liver. Mice were injected with saline or LPS at indicated doses and animals were euthanized. Total RNA was isolated from liver tissue, cDNA was synthesized with reverse transcriptase, and quantitative real-time PCR was performed as described in Materials and Methods. A, Animals were euthanized 4 h after LPS administration and FGF21 mRNA levels were measured. B, Animals were euthanized 16 h after LPS administration and FGF21 mRNA levels were measured. Quantitative PCR data were normalized to 36B4 mRNA for all experiments. Data (mean ± se, n = 4) are expressed as a percentage of controls administered saline. *, P < 0.05; ***, P < 0.001.
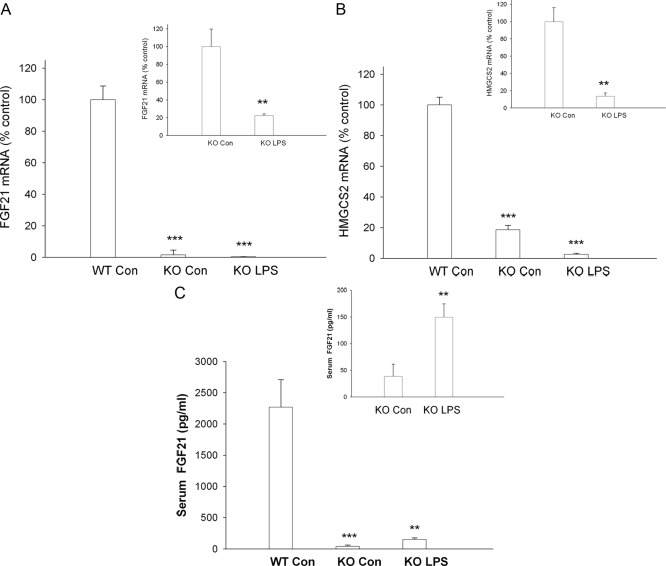
As discussed in the introduction, FGF21 expression in the liver is markedly stimulated by PPARα signaling (12, 13, 20), and PPARα is decreased in the APR (24, 25). We therefore determined the effect of LPS treatment at 16 h on FGF21 mRNA levels in the liver of PPARα KO mice. Basal FGF21 mRNA levels were markedly decreased in PPARα KO mice (Fig. 3A), an observation similar to that reported by other investigators (12, 13, 20). However, despite this marked decrease in basal FGF21 expression in PPARα KO mice, LPS treatment results in a further reduction in hepatic FGF21 mRNA levels (Fig. 3A, inset), indicating that LPS can decrease FGF21 expression via a PPARα-independent mechanism. Not surprisingly, basal HMGCS2 mRNA levels were also markedly decreased in PPARα KO mice (Fig. 3B). Similar to our observations with FGF21, LPS administration resulted in a further decrease in HMGCS2 mRNA levels in PPARα KO mice (Fig. 3B, inset). Additionally, serum FGF21 levels were markedly decreased in PPARα-KO mice (Fig. 3C). However, similar to the results described above in wild-type mice, serum FGF21 levels also increase in PPARα KO mice after LPS administration (Fig. 3C, inset).
Fig. 3.
Effect of LPS on serum FGF21 and mRNA levels in mouse liver. Wild-type (WT) and PPARα-deficient mice were injected with either saline [con (control)] or LPS (5 mg/kg), and the animals were euthanized at 16 h after injection. Total RNA was isolated from liver tissue, cDNA was synthesized with reverse transcriptase, and quantitative real-time PCR was performed as described in Materials and Methods. A, FGF21 mRNA levels were measured. B, HMGCS2 mRNA levels were measured. Quantitative PCR data were normalized to 36B4 mRNA for all experiments. Quantitative PCR data (mean ± se, n = 4) are expressed as a percentage of controls administered saline. C, Serum FGF21 levels were measured by ELISA as described in Materials and Methods. Data (mean ± se, n = 5). **, P < 0.01; ***, P < 0.001 vs. control.
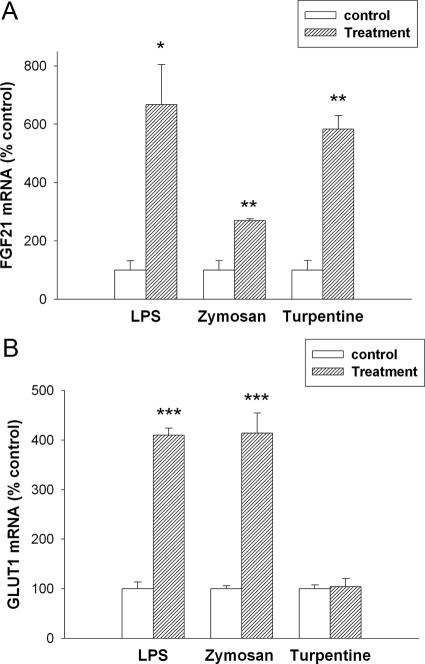
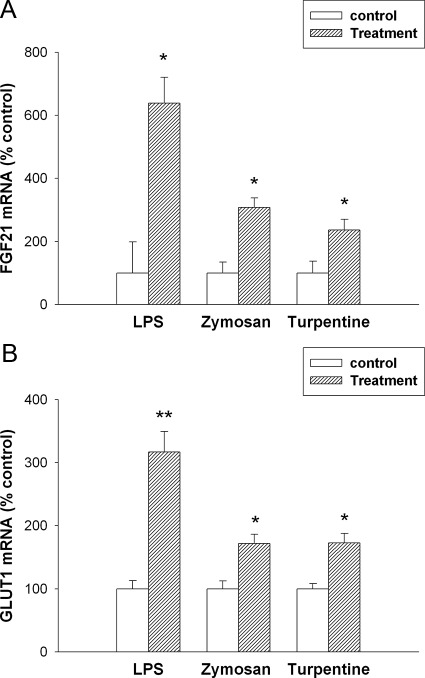
Because of the discordance between the effect of LPS, zymosan, and turpentine treatment on serum FGF21 levels and on FGF21 expression in liver, we therefore determined the effect of the APR on FGF21 expression in other tissues. Although liver is thought to be an important site of FGF21 production, it is now well recognized that FGF21 is produced in other tissues (19, 21, 29–32). Likewise, the APR is recognized to occur in extrahepatic tissues as well (33). LPS administration had no effect on FGF21 mRNA levels in the pancreas (data not shown). Consistent with the changes in FGF21 serum levels, LPS administration resulted in marked increases in FGF21 mRNA levels in adipose tissue at 16 h (Fig. 4A). However, FGF21 protein levels were similar in adipose tissue from control and LPS-treated animals (Control 62.0 ± 8.5 vs. LPS 65.1 ± 6.8 pg/ml × μg protein). The absence of an increase in FGF21 protein could be due to the secretion of the FGF21 into the circulation. Of note is that adipose tissue on a protein basis has a higher concentration of FGF21 compared with liver tissue. Likewise, in parallel with the findings in serum, no change in FGF21 mRNA levels was seen at 4 h after LPS administration (data not show). We have reported that FGF21 increases glucose uptake in adipose tissue by increasing the expression of GLUT1 (9, 23). LPS induced an increase in GLUT1 mRNA in adipose tissue (Fig. 4B). As shown in Fig. 4, A and B, zymosan treatment also increased both FGF21 and GLUT1 expression in adipose tissue. In contrast, turpentine treatment increased FGF21 expression, but for unknown reasons did not increase GLUT1 mRNA levels.
Fig. 4.
Effect of APR stimuli on mRNA levels in mouse adipose tissue. Mice were injected with saline, LPS (5 mg/kg, ip), zymosan A (80 mg/kg, ip), or turpentine (100 μl/mouse, sc), and the animals were euthanized at 16 h. Total RNA was isolated from adipose tissue, cDNA was synthesized with reverse transcriptase, and quantitative real-time PCR was performed as described in Materials and Methods. A, FGF21 mRNA levels were measured. B, GLUT1 mRNA levels were measured. Quantitative PCR data were normalized to 36B4 mRNA for all experiments. Data (mean ± se, n = 4) are expressed as a percentage of controls administered saline. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In addition to adipose tissue, recent studies have suggested that muscle may also be an important site of FGF21 production (29). Similar to our observation in adipose tissue, LPS treatment resulted in increases in muscle FGF21 and GLUT1 mRNA levels at 16 h (Fig. 5, A and B) and also consistent with the changes in serum, no change was observed at 4 h (data not shown). Analogous to adipose tissue, we did not observe an increase in FGF21 protein levels in muscle (Control 4.0 ± 0.5 vs. LPS 3.6 ± 0.3 pg/ml × μg protein). Additionally, both zymosan and turpentine treatment also increase FGF21 and GLUT1 mRNA levels in muscle tissue (Fig. 5, A and B). Thus, whereas treatments that induce the APR do not increase FGF21 expression in liver, they consistently induce FGF21 expression in both muscle and adipose tissue, suggesting that these tissues contribute to the LPS-induced increase in serum FGF21 levels. It should be noted that the increase in serum FGF21 levels and FGF21 expression in muscle and adipose tissue is a relatively late effect occurring at 16 h but not at 4 h after LPS treatment.
Fig. 5.
Effect of APR stimuli on mRNA levels in mouse muscle tissue. Mice were injected with saline, LPS (5 mg/kg, ip), zymosan A (80 mg/kg, ip), or turpentine (100 μl/mouse, sc), and the animals were euthanized at 16 h. Total RNA was isolated from muscle tissue, cDNA was synthesized with reverse transcriptase, and quantitative real-time PCR was performed as described in Materials and Methods. A, FGF21 mRNA levels were measured. B, GLUT1 mRNA levels were measured. Quantitative PCR data were normalized to 36B4 mRNA for all experiments. Data (mean ± se, n = 4) are expressed as a percentage of controls administered saline. *, P < 0.05; **, P < 0.01.
One could speculate that local changes in FGF21 expression were regulating local gene expression. To address this question, we next examined the effect of LPS administration on gene expression in the liver, muscle, and adipose tissue of wild-type and FGF21 KO mice. Much to our surprise, LPS administration decreased HMGCS2 and CPT-1α expression to a similar extent in the liver of wild-type and FGF21 KO mice (data not shown). Moreover, in muscle and adipose tissue, the LPS-induced increase in GLUT1 mRNA levels was similar in wild-type and FGF21 KO mice. These results indicate that the alterations in the expression of these genes after LPS treatment are not dependent on changes in FGF21 expression.
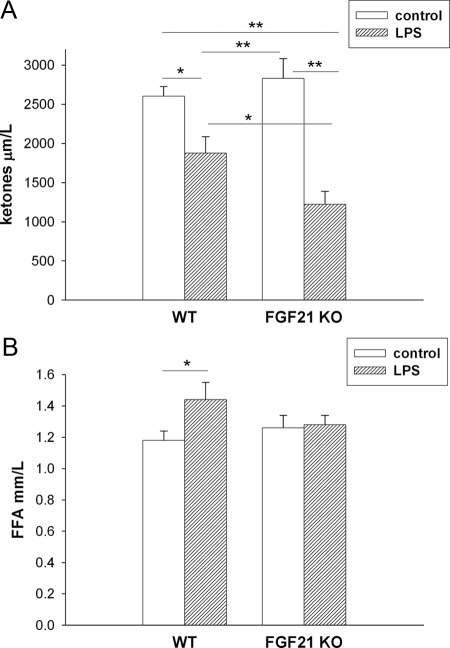
However, the decrease in plasma ketone levels induced by LPS administration is enhanced further in FGF21 KO mice (Fig. 6A). One key factor determining the rate of ketone body formation by the liver is the delivery of fatty acids from adipose tissue. As shown in Fig. 6B, LPS treatment, as expected, increases plasma free fatty acids in control mice, an effect that was not observed in the FGF21 KO mice (Fig. 6B).
Fig. 6.
Effect of LPS in FGF21 KO mice. Wild-type (WT) and FGF21 KO mice were injected with LPS (5 mg/kg) and the animals were euthanized at 16 h. Plasma ketone (A) and free fatty acid (FFA) expressed as mean ± se (n = 7). *, P < 0.05; **, P < 0.01.
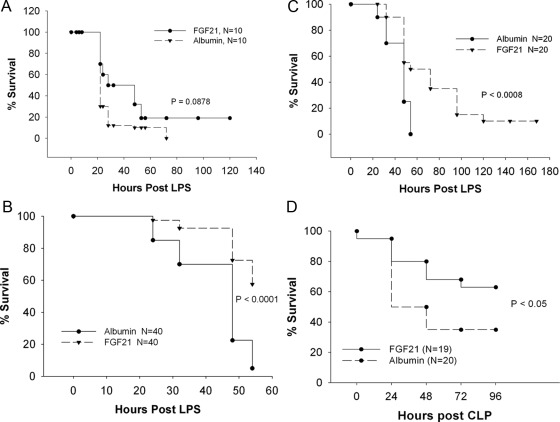
We next determined whether administering exogenous FGF21 would reduce the toxicity of LPS. Initial experiments looked at the time course and dose response of LPS from E. coli in C57BL/6 mice. Doses of LPS from 22.5–47.5 mg/kg resulted in death at 24 h. The lowest dose of 22.5 mg/kg resulted in 40% mortality, whereas the maximal dose of 47.5 mg/kg resulted in 100% mortality at 54 h after challenge with an LD50 of about 27.5 mg/kg. Based on these results, subsequent experiments used a dose of 42.5 mg/kg, which represented an LD90 dose. Treatment of C57BL/6 mice with 30 μg of FGF21 protein three times daily beginning at 1 h after LPS injection resulted in an improvement in survival compared with animals administered albumin that was not quite statistically significant (Fig. 7A; P = 0.0878 vs. albumin).
Fig. 7.
Effect of FGF21 on mouse survival. A, Survival curves of female C57Bl/6 mice subjected to LPS challenge and then treated with 30 μg of either recombinant human FGF21 protein or human albumin protein three times daily for 7 d starting at 1 h after LPS injection. B, Survival curves of female ob/ob mice receiving 50 μg of either recombinant human FGF21 or human albumin protein two to three times daily starting at 1 h after LPS injection. The data show survival up to 54 h after LPS challenge and are a composite of four independent experiments, each with similar results. C, Survival curves of female ob/ob mice receiving 50 μg of either recombinant FGF21 or human albumin protein two to three times daily for 7 d beginning at 1 h after LPS challenge. The data are a composite of two independent experiments each with similar results. D, Survival curves of female ob/ob mice subjected to CLP surgery and then treated with 50 μg of either recombinant human FGF21 protein or human albumin protein two times daily for 4 d starting at 2 h after CLP.
We have previously shown that ob/ob mice are more sensitive to the toxic effects of LPS (34). Compared with control mice, serum FGF21 levels were increased in ob/ob mice (1.02 ng/ml ± 0.23 for control; 3.67 ng/ml ± 0.61 for ob/ob; P < 0.01). However, similar to control mice, LPS treatment resulted in an increase in serum FGF21 levels (Supplemental Fig. 3). Therefore we next studied the effect of exogenous FGF21 administration on LPS-induced toxicity in ob/ob mice. Initial experiments examined the time course and dose dependence of LPS-induced death in female ob/ob mice. In these experiments, E. coli LPS was injected ip at doses from 6–27.5 mg/kg, and mice were dosed twice daily sc with 50 μg of human albumin as a negative control protein. Death was detected as early as 24 h after the maximal dose of LPS used (27.5 mg/kg) and about 90% of the mice were dead after 54 h. There was no mortality at 6 mg/kg and the LD50 was about 8 mg/kg. Based on these results, a dose of 27.5 mg/kg was used for subsequent experiments.
To determine what effect FGF21 had on the survival of LPS-challenged ob/ob mice, we conducted four independent experiments in which mice were injected with LPS, then dosed two to three times daily with 50 μg of either human albumin or recombinant human FGF21 protein beginning at 1 h after LPS injection. This dose of FGF21 was chosen based on previous studies in our laboratory, which showed that this dose could significantly lower blood glucose in female ob/ob mice (8). The results from these experiments are shown in Table 1 and Fig. 7, B and C. The first two experiments were 54 h in duration, and the results from these experiments demonstrated that FGF21 treatment resulted in a significant improvement in short-term survival from a lethal LPS challenge (Fig. 7B). We then extended the duration of experiments 3 and 4 to 7 d to determine whether longer-term survival was also improved with FGF21 treatment. These experiments also demonstrated a significant improvement in both short-term and longer-term survival after lethal endotoxemia (Fig. 7C).
Table 1.
Effects of FGF21 on survival in LPS challenged ob/ob female mice
| Expt. no. | Group | n Value | Average BW | Average BG | % Survival @ 54 h | % Survival @ 168 h | Kaplan-Meier P value vs. albumin |
|---|---|---|---|---|---|---|---|
| 1 | Albumin | 10 | 36 ± 1.4 | 215 ± 78 | 10 | nd | |
| FGF21 | 10 | 36 ± 1.7 | 199 ± 68 | 50 | nd | 0.0561 | |
| 2 | Albumin | 10 | 40.4 ± 2.2 | 246 ± 75 | 0 | nd | |
| FGF21 | 10 | 41.2 ± 2.8 | 247 ± 68 | 80 | nd | <0.001 | |
| 3 | Albumin | 10 | 42.3 ± 2.4 | 281 ± 72 | 0 | 0 | |
| FGF21 | 10 | 42.3 ± 2.4 | 280 ± 66 | 50 | 0 | 0.0085 | |
| 4 | Albumin | 10 | 44.7 ± 3.8 | 239 ± 88 | 10 | 0 | |
| FGF21 | 10 | 43.3 ± 1.5 | 241 ± 46 | 50 | 20 | 0.0178 |
Data shown are the means and ses for body weight (BW) and blood glucose (BG) before LPS challenge. Expt, Experiment; %, percent; nd, not determined.
To determine whether FGF21 treatment could improve survival in an animal model of peritonitis and sepsis, we subjected ob/ob mice to a cecal ligation and puncture procedure (CLP) and treated the mice with exogenous FGF21 or albumin beginning 2 h after CLP. Previous observations in our laboratory had determined that we could induce about 70% mortality over 4 d in female ob/ob mice with a 25-Gauge diameter needle. Treatment of ob/ob mice after CLP surgery with FGF21 resulted in a significant improvement in survival compared with the control albumin-treated mice (Fig. 7D; P < 0.05). Together these studies demonstrate that FGF21 administration protects from both LPS- and bacterial sepsis-induced death.
Discussion
A major finding of the present study is that FGF21 is a positive acute phase protein. The administration of LPS, zymosan, or turpentine, all of which induce the APR, result in an approximately 2-fold increase in serum FGF21 levels. Of interest, this increase in serum FGF21 level is seen at 16 h after LPS administration, but not at 4 h, indicating that FGF21 increases relatively late during the APR.
An increase in hepatic protein synthesis accounts for the increase in many positive acute phase proteins (1, 2), and the liver was thought to be the major site of FGF21 production. Surprisingly, administration of LPS, zymosan, or turpentine all decreased FGF21 expression in the liver, indicating that the liver cannot be the source of the increase in serum FGF21 during the APR. In contrast, the administration of LPS, zymosan, and turpentine stimulated FGF21 expression in adipose tissue and muscle, suggesting that extrahepatic tissues likely play an important role in the APR-induced increase in serum FGF21.
This discordant expression of FGF21 in liver and extrahepatic tissues during the APR is unusual, because in most instances the APR leads to similar direction of change in expression in different tissues. For example, we have shown that expression of serum amyloid A increases, whereas the expression of apolipoprotein E decreases in both in liver and extrahepatic tissues after LPS treatment (33). The mechanisms that account for the discordant effect of the APR on FGF21 expression are unknown. Unfortunately, we have been unable to stimulate FGF21 expression in 3T3-L1 adipocytes with cytokines or LPS. Similarly we have also been unable to inhibit FGF21 expression in Hep3B cells, a human hepatoma cell line, with cytokines. Additionally, LPS or TNF treatment did not alter FGF21 expression in RAW cells (a murine macrophage cell line) or endothelial cells (human umbilical vein endothelial cells). In the absence of in vitro models, it will be difficult to elucidate the molecular mechanisms that account for the discordant regulation in liver and extrahepatic tissues.
One could speculate that some effects of FGF21 may be local. For example, decreased hepatic expression of FGF21 could result in decreased FGF21 activity within the liver mostly affecting hepatic metabolism. Hepatic expression of HMGCS2, a gene regulated by FGF21, decreased in the liver during the APR. Conversely, in muscle and adipose tissue, the increase in FGF21 expression might not only contribute to increased serum FGF21, but might directly induce increased FGF21 activity within these tissues. In muscle and adipose tissue the expression of GLUT1, a gene induced by FGF21, is increased by LPS treatment. However, LPS administration decreased HMGCS2 expression in the liver and increased GLUT1 expression in muscle and adipose tissue of wild-type and FGF21 KO mice to a similar extent. These results indicate that the alterations in the expression of HMGCS2 and GLUT1 after LPS treatment are not dependent on changes in FGF21 expression. It is possible that LPS regulation of the expression of other genes in these tissues may be mediated by FGF21 levels.
Studies have shown that administration of FGF21 or over expression of FGF21 in the liver of transgenic animals causes an increase in hepatic fatty acid oxidation and ketone production (9–11, 13). Conversely, mice in which hepatic FGF21 expression has been knocked down by adenovirus expression of an small interfering RNA have decreased hepatic fatty acid oxidation, decreased ketone production, and fatty livers (12). Additionally, several of the enzymes required for fatty acid oxidation and ketone body formation are decreased in the liver of these hepatic FGF21-deficient mice including CPT-1α, ACO, and HMGCS2 (12). During the APR, hepatic fatty acid oxidation and ketone body production are decreased leading to reductions in serum ketone levels (3, 35–40). Of note, previous studies have shown that CPT-1α and ACO expression in the liver are decreased during the APR, and the present study shows that HMGCS2 expression is also decreased (24, 25). In the present study, we demonstrate that after LPS administration, the decrease in plasma ketone levels is accentuated in the FGF21 KO mice compared with wild-type mice. However, this is not due to differences in the expression of CPT-1α or HMGCS2 in the liver, because the LPS-induced decrease is similar in FGF21 KO and control mice. Hotta et al. (41) previously reported that fasting induced the expression of CPT-1a and HMGCS2 to a similar degree in wild-type and FGF21 KO mice, which in conjunction with our results, suggests that regulation of these genes in the liver is not dominantly dependent on alterations in the FGF21 levels.
One study has shown that FGF21 stimulates adipose tissue lipolysis, whereas other studies have shown an antilipolytic effect of FGF21 (13, 41–43). Additionally, in FGF21 KO mice lipolysis has been reported to be decreased in the fasting state (41). After LPS administration, lipolysis increases and there is an increase in plasma free fatty acid (FFA) levels (3). In the FGF21 KO mice, the ability of LPS to increase plasma FFA is blunted. This failure to increase plasma FFA could contribute to the accentuated decrease in plasma ketone levels because it is well recognized that the transport of fatty acids from adipose tissue to liver provides the substrate for the synthesis of ketone bodies (44, 45). The mechanism by which the absence of FGF21 blunts the ability of LPS to increase plasma FFA is unknown and will require further studies.
As previously reported by others, basal FGF21 expression in the liver is markedly suppressed in PPARα KO mice (12, 13, 20). These data suggest that the decrease in PPARα signaling that occurs during the APR (24, 25) could contribute to the decrease in hepatic FGF21 expression observed after LPS, zymosan, and turpentine treatment. However, LPS treatment resulted in a further reduction in hepatic FGF21 mRNA levels in PPARα KO mice, suggesting that LPS treatment decreases FGF21 expression via other mechanisms in addition to decreasing PPARα-signaling pathways. PPARγ activation has been shown to increase FGF21 expression in adipose tissue (21, 22), but this is unlikely to account for the increase seen during the APR because previous studies have shown that the APR reduces adipose tissue PPARγ and retinoid X receptor, an obligate heterodimer that is required for PPARγ transcriptional activity (46).
Another major observation in this paper is that treatment of mice with exogenous FGF21 reduces the toxicity of LPS. The number of animals that die and the rapidity of their death after LPS administration are both reduced by FGF21 treatment. This is most clearly observed in ob/ob mice, which have an increased sensitivity to LPS-induced death (34). Additionally, FGF21 also protected mice from the toxic effects of cecal ligation and puncture, a model of bacterial peritonitis and sepsis. Of note is that recent studies have shown that FGF21 also reduces the toxicity that occurs with cerulein-induced pancreatitis (47). The mechanisms that account for the protection induced by FGF21 are unknown, and we observed no difference in the increase in serum levels of TNF, a key cytokine that mediates the toxicity of LPS and sepsis (48), in LPS-treated mice administered FGF21 and controls (data not shown). These results suggest that further studies in other sepsis models should evaluate the potential beneficial effects of FGF21 during sepsis. One can speculate that the increased serum FGF21 levels that occur as part of the APR play an important role in protecting animals from toxicity.
In conclusion, the present study demonstrates that during the APR there is a rapid decrease in FGF21 expression in the liver and a late increase in expression in muscle and adipose tissue accompanied by a late increase in serum FGF21 levels. The increased expression of FGF21 in muscle and adipose tissue could contribute to the increase in serum FGF21, adipose tissue lipolysis, and ketone production that occur during inflammation. Finally, the administration of exogenous FGF21 protects animals from the toxic effects of LPS and bacterial sepsis, suggesting that the increase in serum FGF21 during inflammation may be a protective response.
Supplementary Material
Acknowledgments
We thank Christine Cheng (Eli Lilly and Company) for performing FGF21 ELISA on mouse serum and tissue samples.
This work was supported by grants from the Research Service of the Department of Veterans Affairs and by National Institutes of Health Grants 5 RO1 AR049932, 2 RO1 HD29706, and the Albert L. and Janet A. Shultz Supporting Foundation.
Disclosure Summary: K.R.F., C.G., J.K.S., S.M.P., Z.W.C., and A.M. have nothing to declare. J.G.H., A.G., M.C., T.Z., H.B., and A.K. are employed by Eli Lilly and Company.
Footnotes
- ACO
- Acyl-coenzyme A oxidase
- AEX
- anion-exchange
- APR
- acute phase response
- CLP
- cecal ligation and puncture
- CPT-1α
- carnitine palmitoyltransferase 1α
- CV
- coefficient of variation
- FFA
- free fatty acid
- FGF21
- fibroblast growth factor 21
- GLUT
- glucose transporter
- HMGCS2
- hydroxymethylglutaryl-CoA synthase 2
- KO
- knockout
- PPARα
- peroxisome proliferator-activated receptor α.
References
- 1. Baumann H, Gauldie J. 1994. The acute phase response. Immunol Today 15:74–80 [DOI] [PubMed] [Google Scholar]
- 2. Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454 [DOI] [PubMed] [Google Scholar]
- 3. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45:1169–1196 [DOI] [PubMed] [Google Scholar]
- 4. Spitzer JJ. 1995. Bacterial endotoxin effects on carbohydrate utilization and transport. Biochem Soc Trans 23:998–1002 [DOI] [PubMed] [Google Scholar]
- 5. Wolfe RR. 1997. Substrate utilization/insulin resistance in sepsis/trauma. Baillieres Clin Endocrinol Metab 11:645–657 [DOI] [PubMed] [Google Scholar]
- 6. Kharitonenkov A, Larsen P. 2011. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 22:81–86 [DOI] [PubMed] [Google Scholar]
- 7. Kliewer SA, Mangelsdorf DJ. 2010. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 91:254S–257S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 9. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. 2005. FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. 2007. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 [DOI] [PubMed] [Google Scholar]
- 11. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. 2009. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- 13. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. 2007. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- 14. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. 2009. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106:10853–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. 2009. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. 2008. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 116:65–68 [DOI] [PubMed] [Google Scholar]
- 17. Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. 2009. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 94:3594–3601 [DOI] [PubMed] [Google Scholar]
- 18. Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. 2008. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab 8:169–174 [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. 2008. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 20. Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. 2007. PPARα is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 360:437–440 [DOI] [PubMed] [Google Scholar]
- 21. Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. 2008. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor γ and altered metabolic states. Mol Pharmacol 74:403–412 [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Qiang L, Farmer SR. 2008. Identification of a domain within peroxisome proliferator-activated receptor γ regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 28:188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. 2007. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARγ signaling. J Cell Physiol 210:1–6 [DOI] [PubMed] [Google Scholar]
- 24. Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. 2000. The acute phase response is associated with retinoid X receptor repression in rodent liver. J Biol Chem 275:16390–16399 [DOI] [PubMed] [Google Scholar]
- 25. Kim MS, Sweeney TR, Shigenaga JK, Chui LG, Moser A, Grunfeld C, Feingold KR. 2007. Tumor necrosis factor and interleukin 1 decrease RXRα, PPARα, PPARγ, LXRα, and the coactivators SRC-1, PGC-1α, and PGC-1β in liver cells. Metabolism 56:267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feingold KR, Wang Y, Moser A, Shigenaga JK, Grunfeld C. 2008. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res 49:2179–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gay NJ, Gangloff M. 2007. Structure and function of Toll receptors and their ligands. Annu Rev Biochem 76:141–165 [DOI] [PubMed] [Google Scholar]
- 28. Hegardt FG. 1999. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J 338:569–582 [PMC free article] [PubMed] [Google Scholar]
- 29. Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. 2008. FGF21 is an Akt-regulated myokine. FEBS Lett 582:3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishimura T, Nakatake Y, Konishi M, Itoh N. 2000. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492:203–206 [DOI] [PubMed] [Google Scholar]
- 31. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. 2011. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med 17:736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. 2006. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478 [DOI] [PubMed] [Google Scholar]
- 33. Hardardóttir I, Sipe J, Moser AH, Fielding CJ, Feingold KR, Grünfeld C. 1997. LPS and cytokines regulate extra hepatic mRNA levels of apolipoproteins during the acute phase response in Syrian hamsters. Biochim Biophys Acta 1344:210–220 [DOI] [PubMed] [Google Scholar]
- 34. Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. 1999. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol 276:R136–R142 [DOI] [PubMed] [Google Scholar]
- 35. Gitomer WL, Miller BC, Cottam GL. 1995. In vivo effects of lipopolysaccharide on hepatic free-NAD(P)(+)-linked redox states and cytosolic phosphorylation potential in 48-hour-fasted rats. Metabolism 44:1170–1174 [DOI] [PubMed] [Google Scholar]
- 36. Kilpatrick LE, Polin RA, Douglas SD, Corkey BE. 1989. Hepatic metabolic alterations in rats treated with low-dose endotoxin and aspirin: an animal model of Reye's syndrome. Metabolism 38:73–77 [DOI] [PubMed] [Google Scholar]
- 37. Lanza-Jacoby S, Rosato E, Braccia G, Tabares A. 1990. Altered ketone body metabolism during gram-negative sepsis in the rat. Metabolism 39:1151–1157 [DOI] [PubMed] [Google Scholar]
- 38. Memon RA, Feingold KR, Moser AH, Doerrler W, Adi S, Dinarello CA, Grunfeld C. 1992. Differential effects of interleukin-1 and tumor necrosis factor on ketogenesis. Am J Physiol 263:E301–E309 [DOI] [PubMed] [Google Scholar]
- 39. Takeyama N, Itoh Y, Kitazawa Y, Tanaka T. 1990. Altered hepatic mitochondrial fatty acid oxidation and ketogenesis in endotoxic rats. Am J Physiol 259:E498–E505 [DOI] [PubMed] [Google Scholar]
- 40. Takeyama N, Takagi D, Matsuo N, Kitazawa Y, Tanaka T. 1989. Altered hepatic fatty acid metabolism in endotoxicosis: effect of L-carnitine on survival. Am J Physiol 256:E31–E38 [DOI] [PubMed] [Google Scholar]
- 41. Hotta Y, Nakamura H, Konishi M, Murata Y, Takagi H, Matsumura S, Inoue K, Fushiki T, Itoh N. 2009. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology 150:4625–4633 [DOI] [PubMed] [Google Scholar]
- 42. Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. 2008. FGF21 attenuates lipolysis in human adipocytes—a possible link to improved insulin sensitivity. FEBS Lett 582:1725–1730 [DOI] [PubMed] [Google Scholar]
- 43. Li X, Ge H, Weiszmann J, Hecht R, Li YS, Véniant MM, Xu J, Wu X, Lindberg R, Li Y. 2009. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett 583:3230–3234 [DOI] [PubMed] [Google Scholar]
- 44. Beylot M. 1996. Regulation of in vivo ketogenesis: role of free fatty acids and control by epinephrine, thyroid hormones, insulin and glucagon. Diabetes Metab 22:299–304 [PubMed] [Google Scholar]
- 45. Foster DW, McGarry JD. 1983. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med 309:159–169 [DOI] [PubMed] [Google Scholar]
- 46. Lu B, Moser AH, Shigenaga JK, Feingold KR, Grunfeld C. 2006. Type II nuclear hormone receptors, coactivator, and target gene repression in adipose tissue in the acute-phase response. J Lipid Res 47:2179–2190 [DOI] [PubMed] [Google Scholar]
- 47. Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Köester A, Pin CL. 2009. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology 137:1795–1804 [DOI] [PubMed] [Google Scholar]
- 48. Tracey KJ. 1991. Tumor necrosis factor (cachectin) in the biology of septic shock syndrome. Circ Shock 35:123–128 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.