Abstract
Leptin resistance is a feature of obesity that poses a significant therapeutic challenge. Any treatment that is effective to reduce body weight in obese patients must overcome or circumvent leptin resistance, which promotes the maintenance of excess body fat in obese individuals. Ciliary neurotrophic factor (CNTF) is unique in its ability to reduce food intake and body weight in obese, leptin-resistant humans and rodents. Although attempts to use CNTF as an obesity therapy failed due to the development of neutralizing antibodies to the drug, efforts to understand mechanisms for CNTF's anorectic effects provide an opportunity to develop new drugs for leptin-resistant individuals. CNTF and leptin share several structural, anatomic, and signaling properties, but it is not understood whether or how the two cytokines might interact to affect energy balance. Here, we conditionally deleted the CNTF receptor (CNTFR) subunit, CNTFRα, in cells expressing leptin receptors. We found that CNTFR signaling in leptin-responsive neurons is not required for endogenous maintenance of energy balance and is not required for the anorectic response to exogenous administration of a CNTF agonist. These results indicate that despite anatomical overlap for CNTF and leptin action, CNTF appears to act within a distinct neuronal population to elicit its potent anorectic effect.
Energy homeostasis is normally tightly regulated. However, the consumption of high-fat foods, which make up a large part of the typical Western diet, can lead to dysregulation of homeostatic mechanisms and weight gain (1). An important aspect of this dysregulation is leptin resistance. Secreted in proportion to white adipose tissue stores, leptin circulates at higher levels in the plasma of obese individuals (2). Leptin receptors (LepR) are found in many regions of the central nervous system including the arcuate nucleus (ARC) where increased leptin signaling can reduce food intake (3). Exposure to a high-fat diet (HFD) results in reduced ability of leptin to constrain additional food intake (4, 5). Several mechanisms, including increased suppressor of cytokine signaling (SOCS)-3 signaling (6), increased protein tyrosine phosphatase (PTP)1B signaling (7), and attenuated mammalian target of rapamycin (mTOR) signaling (8), have been proposed to underlie leptin resistance.
Ciliary neurotrophic factor (CNTF) is a cytokine in the IL-6 family that shares several structural and functional features with leptin. Based on reports that CNTF protects motor neurons in animal models, CNTF was initially used as a treatment for amylotrophic lateral sclerosis (9). Although this treatment was found to be ineffective, it was observed that CNTF treatments also elicited weight loss in obese human patients and that this weight loss was sustained after discontinuation of the drug (10). The ability of CNTF to reduce food intake has been hypothesized to be the result of leptin-like actions in the hypothalamus. Activation of either LepR or CNTF receptors (CNTFR) in the hypothalamus leads to the phosphorylation and activation of local signal transduction activator of transcription 3 (STAT3) (11, 12) as well as of mTOR (8). Both cytokines also reduce AMP-activated kinase phosphorylation (13, 14). Additionally, CNTFR and leptin receptors have overlapping distributions in the hypothalamus (15), including the paraventricular nucleus (16–18) and ARC (16).
Despite several common functional and mechanistic effects of CNTF and leptin, a critical distinction between the two is the unique ability for exogenous CNTF administration to elicit weight loss in HFD-induced obese and leptin-resistant (db/db) mice (11, 15). This suggests that the anorectic effects of CNTF and leptin may be due to distinct mechanisms of action. Postulated mechanisms for CNTF's action in obese animals include hypothalamic neurogenesis (19), mTOR signaling (8), and AMP-activated kinase signaling (14). However, it is unclear what determines the ability of CNTF to activate these mechanisms despite resistance of these same pathways to the actions of leptin. One possibility is that CNTFR activation elicits downstream events that are common to signaling through both the CNTF and leptin receptors. If this were the case, then CNTF might activate such pathways even in the face of leptin resistance. To date, no studies have determined whether CNTF's actions rely upon CNTFR signaling specifically within leptin-responsive neurons.
Given common anatomic and molecular pathways activated by CNTF and leptin, it is critical to define the functional and anatomic relationship between these two cytokines. Here, we used conditional knockout (KO) technology to ask whether CNTF signaling in leptin-responsive neurons contributes to its anorectic actions. We hypothesized that CNTF acts primarily through CNTFR-mediated signaling in leptin-responsive neurons to elicit body weight loss and reduction in food intake. The CNTFR consists of three subunits: CNTFR subunit α (CNTFRα), gp130, and the leukemia inhibitory factor receptor β (20). Among type 1 cytokine (IL-6-like) receptors, CNTFRα is unique to the CNTFR (21). To test our hypothesis, we generated mice lacking CNTFRα in cells expressing leptin receptors (LepR-cre-CNTFRfl/fl, or KO). We demonstrate here that although the deletion of CNTFRα in leptin receptor-expressing cells attenuates local phospho-STAT3 (pSTAT3) activation in response to exogenously delivered recombinant CNTF (Axokine, CNTFAxo), the deletion does not result in increased food intake or body weight and does not impair the anorexigenic response to CNTFAxo.
Materials and Methods
Animals and genotyping
Mice were housed at the University of Cincinnati at the Metabolic Diseases Institute under controlled conditions (12-h light, 12-h dark cycle, 50–60% humidity, 25 C with free access to water and food except where noted). All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
For this study, animals expressing cre-recombinase (cre) driven by the leptin receptor promotor (LepR-cre) were crossed with animals expressing a loxP-flanked CNTFRα allele (CNTFR). Both the cre (22, 23) and loxP (24) lines have been characterized extensively before these studies. Breeding-pair animals were homozygous for the LepR-cre allele and heterozygous for the loxP-flanked CNTFR allele (CNTFR+/fl). For genotyping, genomic DNA was isolated from tail biopsies by digesting in proteinase K overnight, followed by potassium acetate incubation, ethanol precipitation, and resuspension in TE buffer. Genotyping was accomplished using primers for cre (forward, 5′-TAT ATC AGG CGC GCG GT-3′, and reverse, 5′-AAA TCC GCT CGA CCA GT-3′) or for CNTFRα (forward, 5′-GTT CCT GCC TCC ATT GAG CAG-3′, and reverse, 5′-GAG CGG CAG CTG AGC ACA G-3′). Presence of the wild-type vs. loxP-containing CNTFRα gene allele was determined by band length on the resulting PCR product. All mice included in the experimental cohort were homozygous for cre (LepR-cre) and homozygous either for the wild-type CNTFRα (CNTFR+/+) or for the CNTFRα allele flanked by loxP sites (CNTFRfl/fl). To breed our colony to homozygosity for cre, we initially used a set of three primers (5′-GCA AAA AAA GTA GTT AAC CTA TTC CTC TTC-3′, 5′-GCC CTC ATT AAT CTA GTA ATG TAG ATG G-3′, and 5′-CCT CTC CAC CCA AGC GGC CGG AGA ACC-3′) to detect the presence of the cre-expressing and wild-type alleles.
For anatomical characterization of CNTF and leptin signaling in the ARC, we used mice that express a green fluorescent protein (GFP) reporter gene in leptin receptor-expressing cells (LepR-Cre+/+; GFP-stop-loxP+/−). These mice have been previously characterized (22, 23). Genotyping was performed for the GFP-expressing allele (forward primer, 5′-CAA GGG CGA GGA GCT GTT CAC-3′, and reverse primer, 5′-CGT CCT TGA AGA AGA TGG TGC G-3′) and for the cre allele (as described above).
Food intake and body weight measurements
Control (CON) (LepR-cre-CNTFR+/+) and KO (LepR-cre-CNTFRfl/fl) mice were weaned onto a chow diet. At 9 wk of life, animals were either continued on chow (n = 2–3 females and n = 3–4 males for each genotype) or switched to HFD (Research Diets, New Brunswick, NJ; D12451: 45% fat, 4.73 kcal/g; n = 6–8 females, and n = 3–8 males for each genotype). Body weight and food intake were measured on a weekly basis. Fat and lean tissue mass was measured at 19 wk using nuclear magnetic resonance (Echo MRI; Echo Medical Systems, Houston, TX). Food intake was assessed after an ip injection of CNTFAxo on wk 14–15, after 5–6 wk of HFD exposure, using a within-subjects design (n = 12–14). Briefly, animals were fasted and placed in clean cages 4 h before injection of 0.6 mg/kg CNTFAxo. HFD was returned at the time of injection, which coincided with the onset of the dark phase of the light-dark cycle in the animal facility. Food intake was measured after 1, 2, 4, and 24 h, and body weight was measured 24 h after the injection.
Glucose tolerance study
Clearance of an ip glucose load was assayed in 16-wk-old CON and KO mice, after 7 wk of HFD exposure. Animals were fasted and placed in clean cages 4 h before the injection of 1 g/kg glucose, using a solution of 10% dextrose in water. Plasma glucose was measured in blood collected from the tip of the tail before glucose injection and 15, 30, 45, 60, and 120 min after glucose injection, using an Accu-Chek Advantage glucometer and test strips (Roche Diagnostics, Indianapolis, IN).
Immunohistochemistry
For pSTAT3-GFP immunohistochemistry, animals were injected ip with 0.5 mg/kg CNTF or 5 mg/kg leptin and euthanized 45 min later via perfusion with ice-cold saline and 4% paraformaldehyde (PFA) in 0.1 m PBS. Mouse brains were removed and fixed in 4% PFA for 24 h at 4 C. After fixation, brains were immersed in 30% sucrose overnight. The 30-μm sections were taken using a sliding microtome and, after incubation in 0.3% glycine and permeabilization with 20% sodium dodecyl sulfate, were blocked in 3% horse serum in 0.3% Triton X-100 in PBS. Sections were stained for pSTAT3 by incubation overnight at room temperature in 1:500 rabbit anti-pSTAT3 (Cell Signaling Technologies, Danvers, MA; 9131L) in blocking solution, followed by goat antirabbit Alexa-568 (1:200; Invitrogen, Grand Island, NY). To stain for GFP expressed by the reporter gene, sections were next incubated in chicken anti-GFP antibody (1:1000; Abcam, Cambridge, MA) overnight at room temperature, followed by goat antichicken Alexa-488.
To examine the effects of the CNTFRα gene disruption on CNTFR signaling in the hypothalamus, mice were injected ip with either 0.6 or 0.3 mg/kg CNTFAxo and euthanized 45 min later via transcardial perfusion with ice-cold saline and 4% PFA in 0.1 m phosphate buffer. Brains were removed and fixed in 4% PFA overnight at 4 C and then transferred to 30% sucrose for at least 48 h. Twenty-micrometer coronal sections from throughout the hypothalamus were cut on a cryostat. Every seventh section was immunohistochemically processed for pSTAT3 as previously described (25) with the exceptions that the sections were incubated free floating and the primary antibody was visualized with a 594 Alexafluor-conjugated secondary antibody. Images were captured with a Nikon DXM1200 digital camera and analyzed by counting labeled nuclei within the region of the ARC. Throughout the procedure from injection to data analysis, the mice and tissue were processed as CON-KO age-matched pairs while blind to genotype.
Statistical analysis
All data are expressed as mean ± sem. Immunoreactivity for pSTAT3 was analyzed, and statistical significance was determined via unpaired, two-tailed t test. A two-way ANOVA (variables: drug dose and genotype) was used to compare CNTFAxo doses on the basis of pSTAT3 immunoreactivity in CON vs. KO mice. Body weight, food intake, and plasma glucose data were analyzed via two-way ANOVA (variables: treatment and time) with a Bonferonni post hoc test, where appropriate. Nuclear magnetic resonance data were analyzed using unpaired, two-tailed t tests. For all experiments, significance was predefined as P < 0.05.
Results
Exogenous CNTFAxo induces pSTAT3 activation in leptin receptor-expressing neurons of ARC and paraventricular nucleus
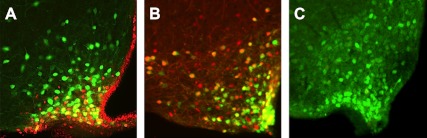
To demonstrate that CNTFAxo and leptin activate an overlapping subset of neurons in the hypothalamus, we administered each drug via ip injection to mice expressing GFP in leptin receptor-positive neurons (LepR-Cre+/+; GFP-stop-loxP+/−). As expected, we found that both CNTF and leptin were able to elicit STAT3 phosphorylation within GFP-positive cells (Fig. 1). However, both CNTFAxo and leptin injections were also associated with pSTAT3 in cells not expressing GFP. The induction of pSTAT3 in non-GFP neurons is consistent with the underreporting of cre activity by the GFP-stop-loxP+/− allele used in these animals. Because CNTFAxo induced pSTAT3 in a population of leptin receptor-positive neurons, however, at least some leptin receptor-positive neurons are direct targets for CNTF.
Fig. 1.
CNTF induces phosphorylation of STAT3 in neurons expressing leptin receptors. A, Intraperitoneal injection of 0.5 mg/kg CNTF elicits pSTAT3 expression (shown in red) in ARC neurons. This includes leptin receptor-expressing neurons (GFP-positive neurons, depicted in green). B, Leptin (5 mg/kg ip) elicits phosphorylation of STAT3 (red) in ARC neurons. Leptin receptor-containing neurons are GFP positive, depicted in green. C, PBS injection does not elicit phosphorylation of pSTAT3 in leptin-responsive neurons (depicted in green).
pSTAT3 signaling is reduced in ARC after deletion of CNTFRα in neurons expressing leptin receptors
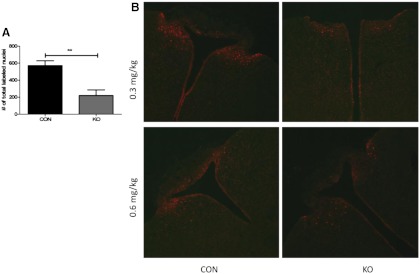
To disrupt CNTFR function, CNTFRα-floxed mice were crossed with mice expressing cre in leptin receptor-expressing cells. To verify the disruption of CNTFR function in our KO mice, we quantified pSTAT3 staining after CNTFAxo injection in KO vs. CON mice at 30–31 wk of age. We observed a large, consistent decrease in the number of responding cells in the region of the ARC in all KO mice injected with either 0.6 or 0.3 mg/kg. Quantification revealed that this decrease was statistically significant (Fig. 2, P = 0.0038 by t test, n = 5 pair) and represented a 61.54 ± 10.0% decrease in the number of responding cells in KO mice compared with identically treated, littermate controls injected, processed, and analyzed in parallel. The size of the effect did not differ at 0.6 vs. 0.3 mg/kg (effect of genotype, P < 0.0001; effect of drug, P = 0.0028; interaction of genotype × drug, P = 0.5875; n = 2–3 pairs per drug dose). These outcomes are consistent with a reduced number of functional CNTFR in the ARC as a result of the cre-driven CNTFRα gene excision. These results also suggest that at least approximately 60% of CNTFRα-containing ARC neurons also express leptin receptor.
Fig. 2.
Phosphorylation of STAT3 in ARC neurons is reduced by deletion of CNTFR in leptin-responsive cells. A, KO mice exhibit reduced pSTAT3 expression after ip CNTFAxo as compared with CON mice (P = 0.0038). **, P < 0.01. B, Representative images from KO and CON animals after injection of 0.3 or 0.6 mg/kg CNTFAxo.
Energy balance is unaffected by deletion of CNTFR in leptin-responsive cells
To test the hypothesis that endogenous CNTF requires action in leptin receptor-expressing neurons to affect energy balance, we measured weekly food intake and body weight in C57/B6 mice with conditionally deleted CNTFRα in leptin receptor-expressing cells (KO) vs. control mice expressing cre only (CON). Because we hypothesized that loss of CNTF signaling in leptin-resistant animals might predispose these animals to further obesity, we recorded food intake and body weight in HFD-fed mice. We found no differences in either body weight (Fig. 3, A and B) or food intake (Fig. 3, C and D) between HFD-fed CON and KO mice. We also detected no significant differences in body weight or cumulative food intake between chow-fed KO and CON mice (Table 1). CNTFRα gene excision also had no effect on body composition in HFD-fed animals, measured as absolute fat mass (Fig. 3, E and F) and absolute lean mass (Fig. 3, G and H). Male CON mice had a greater percent lean mass than female KO mice (P < 0.05). To address whether endogenous CNTF signaling in leptin-responsive cells might affect glucose homeostasis, we performed a 2-h glucose tolerance test in HFD-fed CON and KO. No differences in either peak glucose levels or in the rate of glucose disappearance were detected in HFD-fed CON vs. KO mice (Fig. 3, I and J).
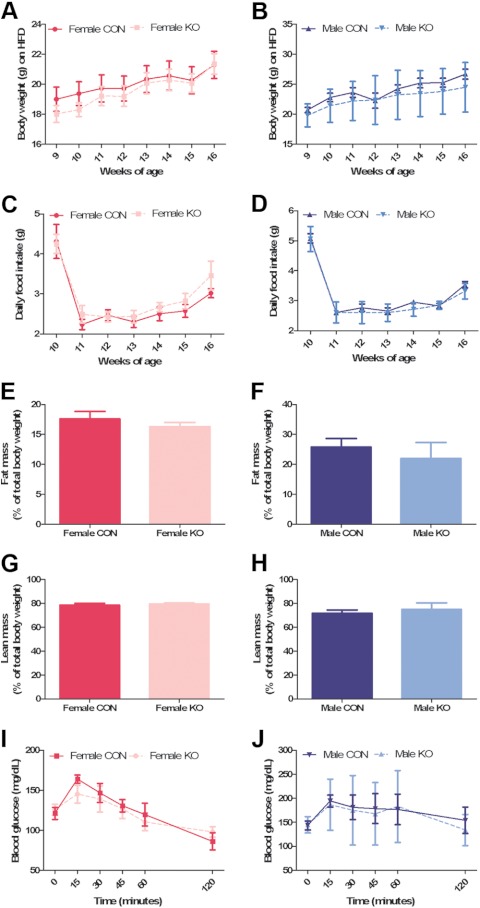
Fig. 3.
Energy balance is unaffected by deletion of CNTFR in leptin-responsive cells. A and B, Body weight in HFD-fed mice is unaffected by genotype in both female (A) and male (B) mice (interaction of age and genotype for female mice, P = 0.3742, and for male mice, P = 0.8630); C and D, daily HFD intake was unaffected by genotype in either female (C, interaction of genotype and age, P = 0.7270) or male (D, interaction of genotype and age, P = 0.7891); E and F, body fat mass is unaffected by the deletion of CNTFR in HFD-fed female (E, P = 0.3475) and male (F, P = 0.5158) mice; G and H, lean body mass is also unchanged in KO mice as compared with CON mice [females (G), P = 0.4695; males (H), P = 0.5475]; I and J, the disruption of CNTFR signaling in leptin-responsive neurons does not alter glucose homeostasis in HFD-fed mice (interaction of genotype and time, 0.3922 for female mice (I) and 0.9841 for male mice (J)].
Table 1.
Cumulative food intake is unaffected by genotype in chow-fed mice
| Female |
Male |
|||
|---|---|---|---|---|
| CON | KO | CON | KO | |
| Mean ± sem | 27.38 ± 0.6494 | 34.44 ± 2.474 | 31.03 ± 1.712 | 27.90 ± 0.6324 |
Animals were studied from 10–17 wk of age. P < 0.05 for all comparisons. By one-way ANOVA, P = 0.0709.
CNTF signaling in leptin-responsive neurons is not required for the anorectic response to exogenous CNTF agonist in HFD-fed mice
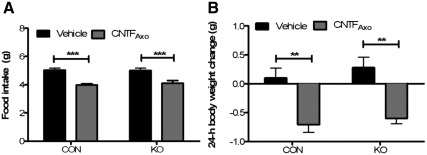
To test the hypothesis that an anorectic response to exogenous CNTF in HFD-fed animals requires CNTF signaling in leptin receptor-expressing neurons, we measured food intake and body weight in HFD-fed KO and CON mice after ip injection of CNTFAxo. There was a statistically significant reduction in food intake in both KO and CON mice 24 h after CNTFAxo (Fig. 4A). The response did not differ in magnitude between the two groups. Body weight was also decreased in both groups after CNTFAxo injection and was not differentially affected by genotype (Fig. 4B).
Fig. 4.
CNTF signaling in leptin-responsive neurons is not required for the anorectic response to CNTF in HFD-fed mice. A, Disruption of CNTFR signaling in leptin-responsive neurons does not affect the anorectic response to CNTFAxo in HFD-fed mice: effect of CNTFAxo on 24-h food intake, P < 0.0001 in CON mice, P < 0.001 in KO mice; interaction of genotype and treatment, P = 0.6289. ***, P < 0.001. B, The 24-h body weight change after CNTFAxo treatment is unaltered by genotype: effect of CNTFAxo, P < 0.01 in both CON and KO mice; interaction of genotype and treatment, P = 0.8218. **, P < 0.01; ***, P < 0.001.
Discussion
Chronic administration of CNTF has been reported to correct obesity in leptin-resistant mice (11). This is despite considerable overlap in both the signaling (8, 11–14, 26 and the anatomic location (15) of CNTF and leptin receptors. It is unclear which mechanisms underlie the disparate efficacy of CNTF vs. leptin in HFD-induced obese animals. Our experiments were designed to ask whether signaling within leptin-responsive cells might mediate CNTF's efficacy in leptin-resistant animals.
First, we found that administration of the CNTF agonist CNTFAxo induced pSTAT3 activity in ARC neurons that express the leptin receptor as determined by crossing LepR-cre mice with GFP reporter mice. Such an outcome indicates that there are CNTFR that colocalize with leptin receptors in the ARC, making a direct interaction between the signaling pathways of both receptors a possibility. However, CNTFAxo injections induced pSTAT3 in many cells not expressing GFP. Additionally, a few cells demonstrated leptin-induced pSTAT3 in non-GFP neurons, consistent with the underreporting of cre activity by the GFP-stop-loxP+/− allele used in these animals. Because CNTFAxo induced pSTAT3 in a population of leptin receptor-positive neurons, however, at least some leptin receptor-positive neurons are direct targets for CNTF. Because KO animals exhibited an approximately 60% decrease in CNTF-stimulated ARC pSTAT3, at least 60% of ARC CNTFRα neurons must also express leptin receptor to permit CNTFR inactivation by LepR-cre.
We then crossed the same LepR-cre mice with mice expressing loxP-flanked CNTFRα. The result was mice with a greatly reduced response to CNTFAxo as measured by pSTAT3 in the ARC (Fig. 2). These mice did not differ from control mice on the basis of body weight, body composition, or glucose tolerance (Fig. 3). Furthermore, these mice responded to CNTFAxo by reducing food intake, without any attenuation of the effect as compared with control mice (Fig. 4). Together, these data argue that the anorectic response to a CNTF agonist does not require CNTFR signaling within an overlapping population of CNTF- and leptin-responsive neurons. Additionally, because these animals did not exhibit an obese phenotype at baseline, these data show that CNTFR signaling in this leptin- and CNTF-responsive neuronal population is also not required for any effect of endogenous CNTF on energy balance. Our data are, of course, not free from limitations. Although deletion of CNTFRα in LepR-expressing cells results in a significant reduction in functional CNTFR, we did not quantify the proportion of LepR-containing neurons affected by the deletion. This quantification would require breeding to obtain mice containing two copies of the loxP-flanked CNTFRα allele, along with the LepR-cre construct and the leptin receptor-GFP reporter, which would have severely limited the sample size for this study. Moreover, the leptin receptor-GFP reporter, like all such reporters, may not faithfully identify all leptin receptor-expressing cells. However, we argue that a greater than 50% loss of function within the ARC would be expected to have at least some effect on body weight and food intake in our animals if CNTFR on leptin-responsive neurons were the critical site of action mediating the anorectic effects of exogenously administered CNTF agonists.
Our results suggest that CNTF and leptin might act on different neuronal populations to influence feeding behavior. One potential explanation is that CNTF and leptin do use similar signaling pathways but target different populations of neurons. Consistent with this hypothesis, unlike leptin, CNTF is effective to promote negative energy balance in obese melanocortin-4 receptor (MC4R)-null animals who lack melanocortin signaling (27). Thus activation of CNTF-responsive neurons must affect unique second-order neurons to bypass leptin resistance, rather than to improve sensitivity to endogenous leptin. This is despite the fact that CNTF can elicit pSTAT3 signaling in leptin-responsive neurons (Fig. 1), implying that only a subset of CNTF-responsive neurons must be important for CNTF's anorectic properties.
The anorectic response to CNTF appears to require neither MC4R activation (27) nor signaling within leptin-responsive neurons to reduce food intake and body weight (Figs. 3 and 4). On the other hand, deletion of gp130 in proopiomelanocortin (POMC) neurons reduces the anorectic response to intracerebroventricular CNTF (28), suggesting that at least a subset of POMC neurons must be required for this response. Recently, CNTF has been found to elicit POMC expression in isolated hypothalamic nuclear fractions (29). Because all POMC neurons do not express leptin receptors (30), it is possible that a population of POMC neurons exists that is required for CNTF's anorectic actions but that does not respond to leptin.
If chronically elevated leptin levels are responsible for leptin resistance, then it is possible that leptin-resistant neurons remain healthy in HFD-induced obese animals and therefore remain responsive to CNTF. For example, leptin signaling can elicit the expression of SOCS-3 (6) and PTP1B (31, 32), which promote leptin resistance by inhibiting Janus kinase-STAT (JAK-STAT) signaling. Increased expression of SOCS-3 and/or PTP1B in hyperleptinemic animals might be expected to also suppress CNTF signaling. However, this is not consistent with the fact that CNTFAxo is equally effective in leptin-resistant animals. It is also not consistent with our finding that the disruption of CNTFR signaling in HFD animals did not lead to significant changes in food intake, body weight, or body fat.
Our observations are consistent with previous evidence suggesting that endogenous CNTFR signaling may not be critical for energy balance. Although embryonic deletion of CNTFRα is lethal within hours of birth (33), the CNTFR subunit gp130 has been conditionally deleted in POMC neurons without any observed changes to energy balance (28). This finding combined with the current data points to the conclusion that endogenous CNTF action is not required for baseline energy homeostasis. Despite this, agonists have potent effects on food intake and body weight in rodents and humans (10, 11, 15). This leaves us in the unenviable position of not knowing what populations of CNTFR are most crucial for the potent actions of these agonists. Given the need to develop more tools to help curb obesity, understanding how these CNTF agonists interact with the known circuitry that regulates energy balance remains an important research goal.
Acknowledgments
These studies were supported by a National Institutes of Health (NIH) grant to R.J.S. (DK54890). M.A.S. is supported by an NIH training grant (1 F30 DK 83870-0).
Disclosure Summary: R.J.S. discloses research grant funding from Ethicon Endo-surgery, NovoNordisk, and Pfizer. He also has received financial compensation for consulting and speaking for Ethicon Endo-surgery, NovoNordisk, and Pfizer. M.M. is a member of the scientific advisory board for Amylin Pharmaceuticals. M.A.S., A.J.M., N.L., C.M.P., A.H., J.S., and S.C.W. have no disclosures. Axokine used in these studies was provided by Regeneron Pharmaceuticals.
Footnotes
- ARC
- Arcuate nucleus
- CNTF
- ciliary neurotrophic factor
- CNTFR
- CNTF receptor
- CON
- control
- GFP
- green fluorescent protein
- HFD
- high-fat diet
- KO
- knockout
- LepR
- leptin receptor
- mTOR
- mammalian target of rapamycin
- pSTAT3
- phospho-STAT3
- PFA
- paraformaldehyde
- POMC
- proopiomelanocortin
- PTP
- protein tyrosine phosphatase
- SOCS
- suppressor of cytokine signaling
- STAT3
- signal transduction activator of transcription 3.
References
- 1. Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. 2004. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav 83:573–578 [DOI] [PubMed] [Google Scholar]
- 2. Maffei M, Halaas J, Rayussin E, Pratley RE, Lee GM, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. 1995. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- 3. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. 1996. Identification of hypothalmic targets of leptin action. J Clin Invest 98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. 2000. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scarpace PJ, Matheny M, Tümer N. 2001. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 104:1111–1117 [DOI] [PubMed] [Google Scholar]
- 6. Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. 1998. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1:619–625 [DOI] [PubMed] [Google Scholar]
- 7. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12:917–924 [DOI] [PubMed] [Google Scholar]
- 8. Cota D, Matter EK, Woods SC, Seeley RJ. 2008. The role of hypothalamic mTORC1 signaling in diet-induced obesity. J Neurosci 28:7202–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. 1996. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology 46:1244–1249 [DOI] [PubMed] [Google Scholar]
- 10. Ettinger MP, Littlejohn TW, Schwartz SL, Weiss SR, McIlwain HH, Heymsfield SB, Bray GA, Roberts WG, Heyman ER, Stambler N, Heshka S, Vicary C, Guler HP. 2003. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA 289:1826–1832 [DOI] [PubMed] [Google Scholar]
- 11. Lambert PD, Anderson KD, Sleeman MW, Wong V, Tan J, Hijarunguru A, Corcoran TL, Murray JD, Thabet KE, Yancopoulos GD, Wiegand SJ. 2001. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci USA 98:4652–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. 1996. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- 13. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. 2004. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 14. Steinberg GR, Watt MJ, Fam BC, Proietto J, Andrikopoulos S, Allen AM, Febbraio MA, Kemp BE. 2006. Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology 147:3906–3914 [DOI] [PubMed] [Google Scholar]
- 15. Gloaguen I, Costa P, Demartis A, Lazzaro D, Di Marco A, Graziani R, Paonessa G, Chen F, Rosenblum CI, Van der Ploeg LH, Cortese R, Ciliberto G, Laufer R. 1997. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc Natl Acad Sci USA 94:6456–6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kordower JH, Yaping C, Maclennan AJ. 1997. Ciliary neurotrophic factor receptor α-immunoreactivity in the monkey central nervous system. J Comp Neurol 377:365–380 [DOI] [PubMed] [Google Scholar]
- 17. MacLennan AJ, Vinson EN, Marks L, McLaurin DL, Pfeifer M, Lee N. 1996. Immunohistochemical localization of ciliary neurotrophic factor receptor alpha expression in the rat nervous system. J Neurosci 16:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee MY, Hofmann HD, Kirsch M. 1997. Expression of ciliary neurotrophic factor receptor-alpha messenger RNA in neonatal and adult rat brain: an in situ hybridization study. Neuroscience 77:233–246 [DOI] [PubMed] [Google Scholar]
- 19. Flier JS. 2006. Neuroscience. Regulating energy balance: the substrate strikes back. Science 312:861–864 [DOI] [PubMed] [Google Scholar]
- 20. De Serio A, Graziani R, Laufer R, Ciliberto G, Paonessa G. 1995. In vitro binding of ciliary neurotrophic factor to its receptors: evidence for the formation of an IL-6-type hexameric complex. J Mol Biol 254:795–800 [DOI] [PubMed] [Google Scholar]
- 21. Boulay JL, O'Shea JJ, Paul WE. 2003. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 19:159–163 [DOI] [PubMed] [Google Scholar]
- 22. Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr 2010. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci 30:5713–5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr 2009. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee N, Robitz R, Zurbrugg RJ, Karpman AM, Mahler AM, Cronier SA, Vesey R, Spearry RP, Zolotukhin S, Maclennan AJ. 2008. Conditional, genetic disruption of ciliary neurotrophic factor receptors reveals a role in adult motor neuron survival. Eur J Neurosci 27:2830–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee N, Neitzel KL, Devlin BK, MacLennan AJ. 2004. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol 474:535–545 [DOI] [PubMed] [Google Scholar]
- 26. Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. 2006. Hypothalamic mTOR signaling regulates food intake. Science 312:927–930 [DOI] [PubMed] [Google Scholar]
- 27. Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. 1999. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21:119–122 [DOI] [PubMed] [Google Scholar]
- 28. Janoschek R, Plum L, Koch L, Münzberg H, Diano S, Shanabrough M, Müller W, Horvath TL, Brüning JC. 2006. gp130 signaling in proopiomelanocortin neurons mediates the acute anorectic response to centrally applied ciliary neurotrophic factor. Proc Natl Acad Sci USA 103:10707–10712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Couvreur O, Aubourg A, Crépin D, Degrouard J, Gertler A, Taouis M, Vacher CM. 2012. The anorexigenic cytokine ciliary neurotrophic factor stimulates POMC gene expression via receptors localized in the nucleus of arcuate neurons. Am J Physiol Endocrinol Metab 302:E458–E467 [DOI] [PubMed] [Google Scholar]
- 30. Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. 1999. Leptin receptor long form splice variant protein expression in neuron cell bodies of the brain and colocalization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem 47:353–362 [DOI] [PubMed] [Google Scholar]
- 31. Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. 2008. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283:14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2:497–503 [DOI] [PubMed] [Google Scholar]
- 33. DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, Ip NY, Yancopoulos GD. 1995. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 83:313–322 [DOI] [PubMed] [Google Scholar]