Abstract
The anti–herpes simplex virus type 1 and anti–herpes simplex virus type 2 effects of apple pomace, a by-product from the cider-processing industry, were investigated. The mechanisms of antiviral action were assessed using a battery of experiments targeting sequential steps in the viral replication cycle. The anti-herpetic mechanisms of apple pomaces included the inhibition of virus attachment to the cell surface and the arrest of virus entry and uncoating. Quercitrin and procyanidin B2 were found to play a crucial role in the antiviral activity.
Key Words: apple pomace, antiviral, HSV, polyphenols
Introduction
Apple pomace is a by-product of the cider-processing industry representing about 35% of the original fruit. The disposal of pomace has an added cost to the beverage industry, especially in regions with an ancient and vast tradition in cider consumption associated to high production rates, which is the case in Asturias where about 20,000 tons of pomace is generated every year. The use of apple pomace as compost without some kind of pretreatment may not continue to be acceptable due to evident environmental concerns. Furthermore, this product is a poor animal food because of its low protein content and its season-restricted availability. For these reasons, the exploration of potential alternative uses for this waste is needed.1
In recent years, the nutritional value of apple pomaces has been demonstrated and these by-products have been regarded as a food additive due to their high content in antioxidant polyphenols and dietary fiber.2,3 The beneficial effects of apple pomaces on health include the improvement of lipid metabolism; the decrease in lipid peroxidation4; the cholesterol lowering; the prevention, reduction, and treatment of diverticular and coronary heart diseases; the prevention of asthma and other chronic diseases; and the inhibition of cancer cell proliferation.5
Herpes simplex virus type 1 (HSV-1) and 2 (HSV-2) are enveloped viruses with large, linear, double-stranded DNA genomes. These viruses are major human pathogens responsible for long-term latent infections with periods of recurring viral replication associated to a broad spectrum of diseases. The herpes-associated clinical symptoms vary from mild self-limiting skin vesicular lesions to severe manifestations such as encephalitis, conjunctivitis, pneumonia, and hepatitis.6,7 The lack of effective vaccines, the moderate-to-high toxicity of the available anti-herpes compounds, and the appearance of resistant viral strains, especially among immunocompromised patients, highlight the need for new drugs.
In a previous study, we have reported the strong anti-herpetic and antioxidant effects of apple pomace crude extracts1 but data concerning the mechanisms underlying the antiviral effects and the putative responsible phytochemicals are still missing. The pomace used on our previous work was made from a blend of distinct apple varieties (namely poly-varietal pomace). The differences between Asturian apple varieties regarding their phenolic profiles as well as their antioxidant activities have been well established.8 In this study, we describe the anti–HSV-1 and anti–HSV-2 activity of pomace produced from single-cultivar apples (mono-varietal pomace) and investigated their putative anti-herpetic mode of action through a comprehensive series of mechanistic assays.
Materials and Methods
Reagents
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and commercial standards of protocatechuic acid, cafeic acid, chlorogenic acid, trans-cinnamic acid, phloridzin, hyperin, quercitrin, isoquercitrin, and procyanidin B2 were purchased from Sigma-Aldrich.
Apple pomace and extraction procedure
Three different single-cultivar apple varieties were used separately to make the pomaces analyzed throughout this work: Meana, De la Riega, and Carrió, referred to as M, DR, and C, respectively. The pomaces were dried, grounded, extracted with 70% acetone, and lyophilized as previously reported.8
Viruses and cells
HSV-1 and HSV-2 clinical isolates were obtained from the Virology Service of Asturias University Central Hospital (Asturias, Spain) and propagated in Vero cells (ECACC No. 84113001). Virus titrations were performed using both the endpoint dilution method first reported by Reed and Muench9 and by plaque assay. Vero cells were propagated in Dulbecco's Modified Eagle's medium (DMEM) (Gibco BRL) supplemented with 10% fetal bovine serum (Sigma) and maintained in DMEM without bovine serum.
Cytotoxicity assay
Increasing concentrations of the test sample were added to confluent Vero cells in 96-well plates, with a replicate number of six wells per concentration. After 72-h incubation, the MTT solution (0.5 mg/mL) was added and the plates were incubated for 3 h. The formazan precipitate was dissolved with 2-propanol and the OD570nm was measured using a μQuant Spectrophotometer (Biotek Instruments) with a reference wavelength of 620 nm. The resulting cell viability was calculated as first described by Mosmann.10
Antiviral activity assays
Ninety-six–well plates containing confluent cell monolayers were preincubated for one hour with increasing noncytotoxic concentrations of the test sample (100 μL/well of 25–1600 μg/mL solutions, with a replicate number of six wells per test concentration) and HSV-1 or HSV-2 (10 TCID50) was added to each well. The plates were incubated at 37°C in a 5% CO2 humidified atmosphere and observed daily for cytopathic effect (CPE) using a light microscope. When CPE was observed in all virus control wells, the percentage of wells with CPE was determined for each treatment concentration, as has been described.11–13 Acyclovir (ACV) at concentration varying from 0.1 to 10 μg/mL served as a positive control.
Time of addition assay
The anti-HSV effect of apple pomace extracts when added to cell cultures at different moments during the course of virus infection and replication was investigated by means of a modified plaque assay.14 The analyzed sample (3× EC50) was added at different time intervals with respect to the infection with 200 pfu of either HSV-1 or HSV-2. Each extract was added to cell cultures 1 h before infection, simultaneously with the infective virus and 2, 4, 8, or 14 h after infection. After 1-h adsorption, the virus inocula were removed and the cells were washed with phosphate-buffered saline (PBS). The extract was re-added to the wells in which the additions were done 1 h before infection and simultaneously with the infective virus. The rest of wells in the plate were replenished with fresh DMEM and the pomace extract was added at the corresponding time points. After the last addition of the extract, the culture medium was replaced by a 1.5% methylcellulose overlay containing an identical concentration of the test extract. The plates were further incubated at 37°C in a 5% CO2 atmosphere and observed daily using an inverted light microscope, until the appearance of viral lysis plaques in the mock-treated (virus control) wells. After 72–96 h of incubation, the cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet in 20% methanol. For each time of addition the percentage of inhibition (%I) was calculated as follows: (1−number of viral plaques in treated wells/number of viral plaques in virus control) × 100.
Adsorption inhibition assay
The effects of apple pomace extracts on virus attachment to cells were investigated as follows: 12-well plates containing confluent Vero cell monolayers were incubated for 1 h at 4°C. Increasing noncytotoxic concentrations of the test extract (500 μL/well of either 50, 100, 300, 500, or 700 μg/mL solutions) were added in duplicate and 200 pfu of HSV-1 or HSV-2 were immediately added. The plates were further incubated at 4°C for 3 h to allow virus adsorption. Afterward, the medium was removed, the cell monolayers were gently washed with DMEM without bovine serum, a 1.5% methylcellulose overlay was added to each well, and the plates were finally incubated at 37°C in a 5% CO2 atmosphere for 96 h or until complete development of viral lysis plaques in mock-treated (virus control) wells. The percentage of inhibition (%I) was calculated as previously indicated in the time of addition method.
Virucidal assay
The virus suspensions were mixed with identical volumes of the test extract, or the equivalent quantity of culture medium (negative control) and then incubated at room temperature (25°C) for 30 min. The residual virus infectious titers were further determined, as previously reported.15
Penetration inhibition assay
The effects of apple pomaces on HSV-1 and HSV-2 cell entry were assessed using a previously described assay16 with minor modifications. The experiment was performed in 12-well plates using a virus inoculum of 200 pfu/well and the same test concentrations used in the adsorption inhibition assay (500 μL/well of 50, 100, 300, 500, or 700 μg/mL solutions).
Real-time (quantitative) polymerase chain reaction
To investigate the effect of apple pomaces on viral DNA synthesis, a quantitative polymerase chain reaction (qPCR) experiment was conducted as follows: 24-well plates containing confluent Vero cells were preincubated for 1 h at 4°C. Each well was then infected at MOI=0.5 with HSV-1 or HSV-2 and the plates were maintained at 4°C to synchronize infection. Following 1-h adsorption at 4°C the inocula were removed, the cells were washed with PBS, the wells replenished with fresh medium, and the plates were further incubated at 37°C for 2 h to allow virus entry and uncoating. Afterward, the medium was removed and the pomace extracts (3× EC50) were added (3 h post-infection). At 6, 8, 12, 24, 36, and 50 h post-infection the supernatants were removed and the cell monolayers were scraped and used to extract DNA (QIAAmp DNA Mini Kit, QIAgen). The viral DNA was quantified by real-time PCR using a Lightcycler 2.0 equipment (Roche). The specific oligonucleotides GCCGTGTGACACTATCGTCCATA (forward primer) and TTGTTATACCCCTCCTCCTCGTAA (reverse primer) were used to amplify an 87 bp-fragment within HSV-1 US5 (envelope glycoprotein J, gJ) gene (see GenBank sequence NC_001806 for further details). Ten-fold dilutions of a plasmid containing a 222-bp fragment including the just-mentioned HSV-1 target sequence were used as standards for quantitation. For HSV-2, the following specific primers were employed: AGACGTGCGGGTCGTACACG (forward primer) and GGCGCGGTCCCAGATCGGCA (reverse primer), which amplify a 101-bp fragment within the HSV-2 US4 (envelope glycoprotein G, gG) gene (see GenBank sequence NC_001798). Ten-fold dilutions of a plasmid containing a 196-bp fragment including the just-mentioned HSV-2 target sequence were used as standards for quantitation. An identical procedure was followed with untreated infected cells (negative) and ACV-treated infected cell (positive) controls. ACV (3 μg/mL) served as positive control for virus inhibition.
Statistics
Statistical analyses were performed with the Statistica 6.1 software. The differences in antiviral activity were analyzed using Student unpaired t-tests. Values of P<.05 were considered indicative of statistical differences. The concentration of the sample reducing cell viability by 50% (mean cytotoxic concentration, CC50) and that reducing viral-induced CPE by 50% (mean effective concentration, EC50) was calculated by regression analysis using the dose–response curves generated from the experimental data. A selectivity index (SI) was calculated for each extract and virus, by dividing the CC50 by the EC50 value.
Results and Discussion
The cytotoxic effects of apple pomace extracts on Vero cell cultures were evaluated using the MTT colorimetric assay, based on the formazan production by mitochondrial enzymes in viable cells. The presence of apple pomace extracts showed to be rather innocuous for the cells up to 10 mg/mL approximately. No pronounced morphological changes were observed in Vero cells at the CC50 value by microscope examination of monolayers (data not shown). The acetone extracts from single-cultivar apple pomaces used in this work were two- to three-fold less toxic than the acetone extract obtained from a poly-varietal pomace.1
The inhibitory effects of apple pomace extracts on in vitro HSV-1 and HSV-2 replication have previously been reported for a poly-varietal pomace.1 In this work, the anti-herpetic effects of single-cultivar pomaces were investigated. SI values >2 were considered as indicative of specific antiviral activity, as has been previously suggested.17,18 The pomaces analyzed strongly inhibited viral-induced CPE at noncytotoxic concentrations, with the exception of the pomace C against HSV-1, whose EC50 value could not be determined at the concentrations tested (Table 1). However, the putative existence of anti–HSV-1 activity of pomace C at greater concentrations cannot be ruled out. The inhibition recorded was concentration dependent for all apple pomace, since a gradual reduction in the percentage of CPE was observed to be associated with increasing concentration of the extracts (data not shown). The SI values found for apple pomaces ranged from 12.5 to 28.1.
Table 1.
Anti–Herpes Simplex Virus Type 1 and Anti–Herpes Simplex Virus Type 2 Activity of Apple Pomace Extracts and Standards of Identified Metabolites
| |
|
Antiviral activity |
|
||
|---|---|---|---|---|---|
| Sample | Cytotoxicity CC50 (μg/mL) | Anti–HSV-1 EC50 (μg/mL) | SI | Anti–HSV-2 EC50 (μg/mL) | SI |
| Pomace M | 11,827±1852 | 946.9±19.1 | 12.5 | 757.0±60.8 | 15.6 |
| Pomace DR | 15,251±3503 | 1012±186 | 15.1 | 541.8±59.1 | 28.1 |
| Pomace C | 9466±260 | >1600 | <5.92 | 337.4±25.2 | 28.0 |
| Procyanidin B2 | 323.6±33.2 | 33.23±1.33 | 9.74 | 24.22±1.2 | 13.4 |
| Quercitrin | 1211±17.3 | 560.9±31.6 | 2.16 | 514.3±34.2 | 2.35 |
| Isoquercitrin | 453.4±69.7 | >300 | <2 | >300 | <2 |
| Chlorogenic acid | 273.9±14.6 | >200 | <2 | >200 | <2 |
| Cinnamic acid | 550.3±12.7 | >400 | <2 | >400 | <2 |
| Protocatechuic acid | 328.2±60.3 | >200 | <2 | >200 | <2 |
| Cafeic acid | 80.36±7.75 | >50 | <2 | >50 | <2 |
| Phloridzin | >1000 | >500 | N/D | >500 | N/D |
| Hyperin | >1000 | >500 | N/D | >500 | N/D |
| ACV | 1454±120 | 0.9±0.01 | 1615 | 0.8±0.12 | 1817 |
Data are presented as the mean values from three independent experiments with their standard deviations. ACV served as positive control.
CC50, mean cytotoxic concentration; HSV, herpes simplex virus type; EC50, mean effective concentration; SI, selectivity index; M, Meana; DR, De la Riega; C, Carrió; N/D, not determined; ACV, acyclovir.
To investigate the mode of action of apple pomace extracts, a battery of mechanistic experiments were performed including a time-of-addition assay, adsorption inhibition experiments, virucidal assays, viral penetration inhibition assays, and a qPCR-based DNA synthesis inhibition experiment. As observed in the time-of-addition assays (Fig. 1), inhibition values close to 100% were recorded for both tested samples for HSV-1 and all three tested samples for HSV-2, if added 1 h before or during virus inoculation. The inhibitory effects decreased to 20%–60% if added 2 h post-inoculation, and even lower (2%–20%) if added 4 h post-inoculation, suggesting that the apple pomaces mainly targeted early viral replication events, that is, adsorption, penetration, and/or viral uncoating.
FIG. 1.
Effect of the time of addition of apple pomace extracts on HSV-1 (A) and HSV-2 (B) inhibition, as measured by percentage of lysis plaques developed compared with mock-treated virus controls. M, Meana; DR, De la Riega; C, Carrió; HSV, herpes simplex virus type.
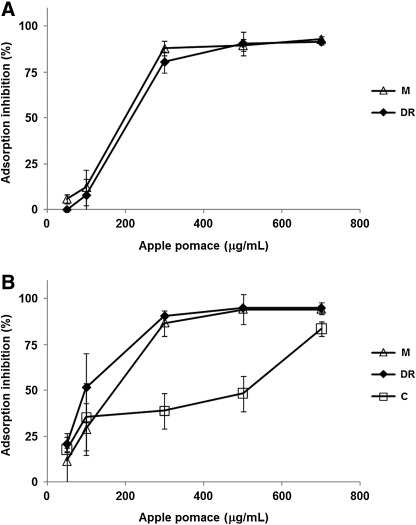
The apple pomace extracts added during virus adsorption protected Vero cells to different extents from HSV-1 and HSV-2 infection, in a concentration-dependent manner (Fig. 2). At 300 μg/mL (30-fold lower than the CC50 value of C, the most toxic pomace) the pomace extracts inhibited virus adsorption by more than 75%, except for pomace C extract, whose strongest HSV-2 adsorption inhibition was recorded at the highest tested concentration (700 μg/mL).
FIG. 2.
Inhibitory effects of apple pomace extracts on HSV-1 (A) and HSV-2 (B) cell attachment.
Phenolic compounds are known to prevent virus attachment to the cell surface by two main mechanisms: denaturation of viral envelope molecules leading to irreversible inactivation of virions, with the consequent decrease in infectivity (namely virucidal activity) and/or by interacting with cell surface receptors required for virus attachment.19,20 To investigate the ability of apple pomace to directly inactive extracellular virions, a virucidal assay was carried out. After incubating HSV-1 or HSV-2 suspensions with apple pomace extracts at concentrations ranging from 500 to 5000 μg/mL (∼1–15-fold greater than the EC50 values), the decrease in virus infectious titers was about 0.3 log10 or lower. It is generally accepted that a good virucidal agent must be able to reduce virus titers by 2 log10; that is equivalent to inactivating 99% of the virions in the preparation.21 The lack of virucidal activity suggested that the observed adsorption inhibition (Fig. 2) exerted by apple pomaces would probably take place through an interaction with cell surface receptors prior to (or during) adsorption, rather than acting on extracellular virions as described for the majority of antiviral compounds found in plants.19,20
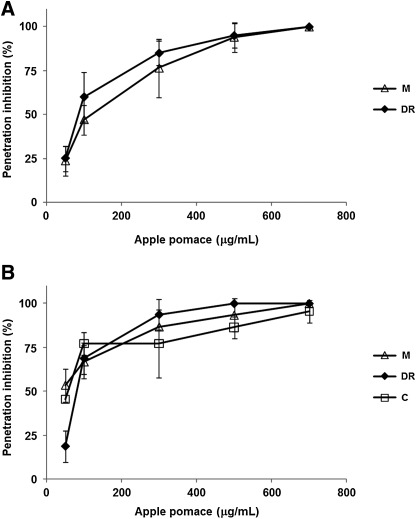
A strong concentration-dependent inhibition was also recorded when the apple pomace extracts were added to the infected cells during virus entry and uncoating. At a concentration of 300 μg/mL all pomace extracts blocked virus penetration/uncoating by more than 50% (Fig. 3).
FIG. 3.
Inhibitory effects of apple pomace extracts on HSV-1 (A) and HSV-2 (B) entry into Vero cells.
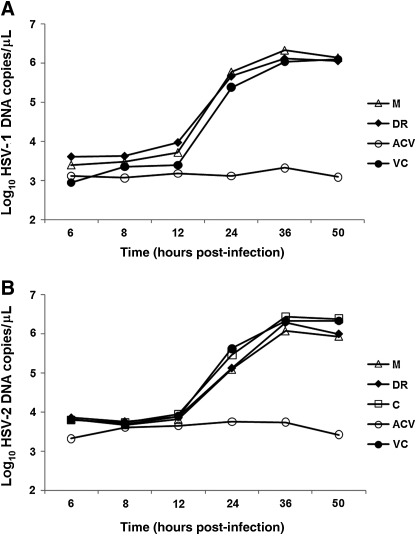
It is well documented that HSV DNA synthesis starts shortly after penetration within the infected cells (3 h post-inoculation).22,23 A real-time PCR assay was used to evaluate the inhibitory activity of pomaces on DNA synthesis over time. HSV-1 and HSV-2 intracellular DNA levels found in pomace-treated cells (Fig. 4) showed a kinetic behavior similar to that of untreated virus controls. Thus, apple pomaces had no inhibitory effects on HSV-DNA synthesis. ACV-treated controls showed viral DNA amounts similar to the DNA concentration in the inoculum throughout the time course of the experiment (Fig. 4).
FIG. 4.
Time course of HSV-1 (A) and HSV-2 (B) DNA yields, in the presence or absence of apple pomace extracts, determined by real-time polymerase chain reaction. VC, untreated (negative) virus control. Acyclovir (ACV, 3 μg/mL) served as positive control.
The phytochemical profiles of the Asturian apple varieties used in this work have previously been reported.8 They contained polyphenols including phenolic acids (protocatechuic, chlorogenic, cafeic, and cinnamic acids), flavan-3-ols and procyanidins (epicatechin, procyanidin B2, trimer C1, and tetramer), dihydrochalcones (phloridzin and phloretin-2-xyloglucoside), and flavonols (hyperin, reynoutrin, quercitrin, isoquercitrin, and avicularin).8 In an attempt to understand the relationships between the chemical composition of apple pomaces and their antiviral effects, we evaluated the anti-herpetic activity of several commercial standards of compounds (Table 1) previously identified in these extracts. Only procyanidin B2 and the flavonol quercitrin showed significant anti-herpes virus activity (Table 1), in agreement with previous studies.20,24 In contrast, no antiviral activity was found for protocatechuic acid, cinnamic acid, chlorogenic acid, isoquercitrin, hyperin, or phloridzin (the most abundant compound in these apple pomaces) (SI<2). Our results clearly differ from those of Chiang et al.25 who reported anti–HSV-1 and anti–HSV-2 activity for caffeic and chlorogenic acids in BCC-1/KMC cells. In our hands, using Vero cells, caffeic acid was the most toxic compound and no effect on viral-induced CPE was observed up to 50 μg/mL. Supporting this finding, Schnitzler et al.26 reported no inhibitory effects for caffeic acid on HSV-1 propagation. This compound was unable to protect Vero cells from HSV-1 infection when the cells or virions were separately pretreated and it was also unable to block viral DNA replication.26 Data concerning a putative anti-herpetic action of phloridzin, chlorogenic acid, and hyperin are scarce in the scientific literature. Although anti-herpetic effects for isoquercitrin have been reported,27 no activity for this metabolite was found in our study model. Furthermore, the lack of anti-herpes activity of cinnamic and protocatechuic acids has previously been reported.28
The antiviral activity found for apple pomaces suggests their potential use as a therapeutic candidate for the treatment of herpetic diseases. Apple pomaces could be included in topical formulations to treat vesicular skin lesions or to prevent sexually acquired HSV infections, which strongly predispose to human immunodeficiency virus acquisition. This approach may represent an environment-friendly alternative for the revalorization of this residue, generated in excessive amounts by the cider industry in Asturias and many other cider-producing regions. The proven harmlessness of apple pomaces together with their high contents in antioxidant metabolites also supports their potential to be included in nutraceutical and dietary supplements or as a food-enrichment additive.
Acknowledgments
The authors are very grateful to A. Rojas Álvarez (University of Havana, Cuba) for statistical advice. The work at F.P.'s laboratory was funded by AECID PCI grant D/030639/10. Financial support (grant: UNOV-10-BECDOC) given to Á.L.Á. by the University of Oviedo is acknowledged.
Author Disclosure Statement
No ethical issues or conflict of interest exists concerning this work.
References
- 1.Suárez B. Álvarez AL. Diñeiro Y. del Barrio G. Picinelli A. Parra F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010;120:339–342. [Google Scholar]
- 2.Masooi FA. Sharma B. Chauhan GS. Use of apple pomace as a source of dietary fiber in cakes. Plant Foods Hum Nutr. 2002;57:121–128. doi: 10.1023/a:1015264032164. [DOI] [PubMed] [Google Scholar]
- 3.Figuerola F. Hurtado M. Estévez AM. Chiffelle I. Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. [Google Scholar]
- 4.Leontowicz H. Gorinstein S. Lojek A. Leontowicz M. Ciž M. Soliva-Fortuny R. Park YS. Jung ST. Trakhtenberg S. Martin-Belloso O. Comparative content of some bioactive compounds in apples, peaches and pears and their influence on lipids and antioxidant capacity in rats. J Nutr Biochem. 2002;13:603–610. doi: 10.1016/s0955-2863(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 5.Boyer J. Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitley RJ. Kimberlin DW. Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:97–109. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 7.Whitley RJ. Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 8.Diñeiro Y. Suárez B. Picinelli A. Phenolic and antioxidant composition of by-products from the cider industry: apple pomace. Food Chem. 2009;117:731–738. [Google Scholar]
- 9.Reed LJ. Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Al Jabri AA. Wigg MD. Oxford JS. Initial in vitro screening of drug candidates for their potential antiviral activities. In: Mahy BWJ, editor; Kangro HO, editor. Virology Methods Manual. Academic Press; London: 1996. pp. 293–307. [Google Scholar]
- 12.Cann A. Antiviral testing. In: Cann A, editor. Virus Culture: A Practical Approach. Oxford University Press; New York: 1999. pp. 201–219. [Google Scholar]
- 13.del Barrio G. Spengler I. García T. Roque A. Álvarez AL. Calderón JS. Parra F. Antiviral activity of Ageratina havanensis and major chemical compounds from the most active fraction. Braz J Pharmacogn. 2011;21:915–920. [Google Scholar]
- 14.Álvarez AL. Habtemariam S. Juan-Badaturuge M. Jackson C. Parra F. In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: an approach to their mechanisms of action. Phytother Res. 2011;25:296–301. doi: 10.1002/ptr.3382. [DOI] [PubMed] [Google Scholar]
- 15.Álvarez AL. del Barrio G. Kourí V. Martínez PA. Suárez B. Parra F. In vitro anti-herpetic activity of an aqueous extract from the plant Phyllanthus orbicularis. Phytomedicine. 2009;16:960–966. doi: 10.1016/j.phymed.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Gescher K. Hensel A. Hafezi W. Derksen A. Kuhn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. 2011;89:9–18. doi: 10.1016/j.antiviral.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya Y. Shimizu M. Hiyama Y. Itoh K. Hashimoto Y. Nakayama M. Horie T. Morita N. Antiviral activity of natural occurring flavonoids in vitro. Chem Pharm Bull. 1985;33:3881–3886. doi: 10.1248/cpb.33.3881. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq E. Antivirals for the treatment of herpesvirus infections. J Antimicrob Chemother. 1993;32(Suppl A):121–132. doi: 10.1093/jac/32.suppl_a.121. [DOI] [PubMed] [Google Scholar]
- 19.Sydiskis RJ. Owen DG. Lohr JL. Rosler KH. Blomster RN. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother. 1991;35:2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bruyne T. Pieters L. Witvrouw M. De Clercq E. Vanden Berghe D. Vlietinck AJ. Biological evaluation of proanthocyanidin dimers and related polyphenols. J Nat Prod. 1999;62:954–958. doi: 10.1021/np980481o. [DOI] [PubMed] [Google Scholar]
- 21.Hu JM. Hsiung GD. Evaluation of new antiviral agents: I. In vitro perspectives. Antiviral Res. 1989;11:217–232. doi: 10.1016/0166-3542(89)90032-6. [DOI] [PubMed] [Google Scholar]
- 22.Phelan A. Dunlop J. Patel AH. Stow ND. Clements JB. Nuclear sites of herpes simplex virus type 1 DNA replication and transcription colocalize at early times postinfection and are largely distinct from RNA processing factors. J Virol. 1997;71:1124–1132. doi: 10.1128/jvi.71.2.1124-1132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh J. Fraser NW. Temporal association of the herpes simplex virus genome with histone proteins during a lytic infection. J Virol. 2008;82:3530–3537. doi: 10.1128/JVI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mucsi I. Gyulai Z. Beladi I. Combined effects of flavonoids and acyclovir against herpes viruses in cell cultures. Acta Microbiol Hung. 1992;39:137–147. [PubMed] [Google Scholar]
- 25.Chiang LC. Chiang W. Chang MY. Ng LT. Lin CC. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 2002;55:53–62. doi: 10.1016/s0166-3542(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 26.Schnitzler P. Neuner A. Nolkemper S. Zundel C. Nowack H. Sensch KH. Reichling J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. 2010;24(Suppl 1):S20–S28. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 27.Abou-Karam M. Shier WT. Isolation and characterization of an antiviral flavonoid from Waldsteinia fragarioides. J Nat Prod. 1992;55:1525–1527. doi: 10.1021/np50088a022. [DOI] [PubMed] [Google Scholar]
- 28.Kane CJ. Menna JH. Sung CC. Yeh YC. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci Rep. 1988;8:95–102. doi: 10.1007/BF01128976. [DOI] [PubMed] [Google Scholar]