Abstract
Activated hepatic stellate cells (HSCs) play a key role in hepatic fibrogenesis, and inhibition of HSC activation may prevent liver fibrosis. Acetaldehyde, the most deleterious metabolite of alcohol, triggers HSC activation in alcoholic liver injury. In the present study, we investigated the protective effect of sodium ferulate (SF), a sodium salt of ferulic acid that is rich in fruits and vegetables, on acetaldehyde-stimulated HSC activation using precision-cut liver slices (PCLSs). Rat PCLSs were co-incubated with 350 μM acetaldehyde and different concentrations of SF. Hepatotoxicity was assessed by measuring enzyme leakage and malondialdehyde content in tissue. α-Smooth muscle actin, transforming growth factor-β1, and hydroxyproline were determined to assess the activation of HSCs. In addition, matrix metalloproteinase (MMP)-1 and the tissue inhibitor of metalloproteinase (TIMP-1) were determined to evaluate collagen degradation. SF prominently prevented the enzyme leakage in acetaldehyde-treated slices and also inhibited HSC activation and collagen production stimulated by acetaldehyde. In addition, SF increased MMP-1 expression and decreased TIMP-1 expression. These results showed that SF protected PCLSs from acetaldehyde-stimulated HSC activation and liver injury, which may be associated with the attenuation of oxidative injury and acceleration of collagen degradation.
Key Words: Angelica sinensis, ethanol-induced, hepatoprotection
Introduction
Hepatic stellate cells (HSCs) are nonparenchymal cells located in perisinusoidal space. In the normal and quiescent state, HSCs are mainly responsible for depositing retinoids. However, the cells are activated and transdifferentiate into myofibroblast-like cells during liver injury.1 Activated HSCs plays a key role in hepatic fibrogenesis caused by various etiological factors, including xenobiotic-induced toxicity (e.g., by drugs or alcohol), viral infection, and certain genetic diseases.2,3 Therefore, inhibiting the activation of HSCs may prevent the progression of liver fibrosis.
Acute and heavy intake of alcohol can induce liver injury, including early fibrogenic changes, marked fat accumulation in hepatocytes, and distortion of the normal sinusoidal architecture.4 Acetaldehyde is known as the most deleterious metabolite of alcohol. It triggers many intricate reactions, including excessive lipid oxidation of polyunsaturated membrane lipids and activation of HSCs.5,6 Acetaldehyde exposure increases the synthesis of collagen I while decreasing its degradation in HSCs, resulting in high expression and accumulation of collagen in liver. Svegliati-Baroni et al.7 reported the early fibrogenic actions of acetaldehyde (up to approximately 6 h) and suggested that early acetaldehyde-dependent events induce the late fibrogenic responses.
Ferulic acid (3-methoxy-4-hydroxycinnamic acid) is widely distributed in plants and constitutes a bioactive ingredient of many staple foods, such as grain bran, fruits, and vegetables. Sodium ferulate (3-methoxy-4-hydroxycinnamate sodium) (SF), a sodium salt of ferulic acid, also an active component from Angelica sinensis and Cimicifuga heracleifolia, is clinically used for cardiovascular and cerebrovascular diseases in China.8 It has been shown that SF has a broad spectrum of biological activities, such as anti-inflammatory, antioxidative, and antimutagenic effects.9–12 Our previous studies have demonstrated the antihepatotoxic effect of SF against various hepatotoxins, including prednisolone, carbon tetrachloride, bromobenzene, paracetamol, tripterygium glycosides, and ethanol, in vivo.13–15 However, little is known about the effect of SF on HSC activation.
Many studies have indicated the crucial role of oxidative stress, especially lipid peroxidation products, in the early activation of HSCs.16–18 Considering the antioxidative effect of SF, we used an in vitro model—precision-cut liver slices (PCLSs) treated with acetaldehyde—to observe the effect of inhibition of SF on HSC activation in this study.
Materials and Methods
Animals
Specific pathogen-free male Wistar rats with body weights of 250±20 g were obtained from the Experimental Center of the Medical Scientific Academy of Hubei (China). The experiments were conducted according to the National Research Council's guidelines, and the certificate number of the animal breeder is SCXK (E) 2003-0004. Animals were caged individually under a controlled environment at a constant temperature (21±2°C) and relative humidity (50±10%) with a 12-h light/dark cycle. The rats had free access to standard rat chow and water and were acclimatized to the surroundings for 1 week before the experiments.
Chemicals
Dulbeccco's modified Eagle's medium and newborn calf serum were from Gibco Co. (Paisley, Scotland, United Kingdom). Reduced glutathione (GSH) and 1-chloro-2,4-dinitrobenzene were purchased from Sigma Co. (Poole, Dorset, United Kingdom). SF was purchased from Southwest Medicine Co. (Chongqing, China), and it was freshly prepared with Dulbeccco's modified Eagle's medium just before use. Acetaldehyde was obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). Lactate dehydrogenase and hydroxyproline (HYP) kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The transforming growth factor (TGF)-β1 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Shanghai Sengxiong Co. (Shanghai). The streptavidin–peroxidase conjugate kit was produced by Zhongshan Jinqiao Biotechnology Co. (Beijing, China). Goat anti-actin was from Boshide Bioengineering Co. (Wuhan, China). Goat anti-matrix metalloproteinase (MMP)-1 and goat anti-tissue inhibitor of metalloproteinase (TIMP-1) were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The chromogenic agent (3,3′-diaminobenzidine) was from Hyclone (Logan, UT, USA).
Liver slice preparation
Animals were sacrificed, and the livers were excised and immediately placed in oxygenated, ice-cold Krebs–Henseleit solution. Cylindrical liver tissue cores (8 mm in diameter) were prepared with a hand-operated tissue coring press. PCLSs with a thickness of 250±10 μm were prepared from the tissue cores using a modified Vibratome (Xiangshan Precision Instruments Co., Zhejiang, China). Tissue slices were preincubated with 1 mL of Dulbecco's modified Eagle's medium (pH 7.2) containing 10% (vol/vol) newborn calf serum, 2.5 μg/mL amphotericin, 3.7 mg/mL NaHCO3, 50 μg/mL streptomycin, and 100 IU/mL penicillin in a 12-core culture plate using a gyratory shaker (90 revolution/min) for 1 h at 37°C under an atmosphere of 5% CO2/95% O2. The medium was discarded after preincubation for 1 h, and the slices were kept in an incubator for the following work.
PCLS treatment
After preincubation, the slices were incubated with acetaldehyde (350 μM) in Dulbeccco's modified Eagle's medium containing the same components as above, 0.1% dimethyl sulfoxide, and different concentrations of SF (0.5, 1, and 2 mM) for 6 h at 37°C. The clinically recommended dose of SF of 100 mg/day, added to 10% dextrose and administered by intravenous infusion, was given to the acetaldehyde groups. In brief, five groups of liver slices were used, including a normal control group, an acetaldehyde control group, and acetaldehyde groups treated with different concentrations of SF.
At the end of the incubation, the culture medium was collected for further determination. The liver slices were homogenized and centrifuged at 200 g for 10 min, and then the aliquot supernatant was centrifuged at 9000 g for another 20 min at 4°C to collect liver S9. Liver slices were fixed with 10% (vol/vol) neutral formalin for histological observation.
Biochemical and ELISA analysis
Lactate dehydrogenase and HYP activities in medium and liver S9 were measured as described previously.19,20 The extent of membrane lipid peroxidation was determined by measuring malondialdehyde content in the tissue as described previously.21 ELISA was used for determining TGF-β1 level in medium as described.22
Glutathione S-transferase activity was measured as described but with minor modifications.23 In brief, the reaction of 1 mM 1-chloro-2,4-dinitrobenzene with 1 mM GSH in the liver S9 was monitored spectrophotometrically by recording the increase in absorbance at 340 nm (ɛ340=9.6 mM/cm). A correction was made for the spontaneous reaction between GSH and 1-chloro-2,4-dinitrobenzene in the absence of enzyme.
The protein content of the liver slices was determined by the method of Lowry et al.24 using bovine serum albumin as a standard.
Immunohistochemistry assay
PCLSs were formalin-fixed and paraffin-embedded, and then the PCLS sections (5 μm thick) were processed for immunohistochemistry using a streptavidin–peroxidase conjugate kit. Sections were deparaffinized, hydrated, and then pretreated for antigen retrieval in a microwave oven for approximately 10 min using the antigen unmasking solution. To quench the endogenous peroxidase activity, the sections were incubated with 3% H2O2 for 15 min. Nonspecific binding was blocked with 5% normal rabbit serum for 30 min at room temperature. The sections were incubated with goat anti-actin antibody (diluted 1:100) in a humidified chamber at 37°C for 2 h, then rinsed three times in phosphate-buffered saline for 5 min each, and incubated with biotinylated rabbit-anti goat IgG as the secondary antibody for 30 min at room temperature. After rinsing with phosphate-buffered saline, the sections were incubated with peroxidase-conjugated streptavidin for 30 min. α-Smooth muscle actin (α-SMA) protein was localized by the development of substrate–chromogen mixture. The sections were counterstained by hematoxylin, dehydrated, and mounted immediately with gum. Phosphate-buffered saline was substituted for goat anti-actin antibody as a negative staining control. The signal was visualized by light microscopy (Axiostar Plus, Carl Zeiss, Oberkochen, Germany) and analyzed by measuring the optical density of positive staining using the HMIAS-2000 photo imaging system (Champion Medical Imaging Co., Wuhan). Quantitative stereology was performed in triplicate, with five fields in each slide, at a magnification of ×400. The positive rate was calculated as a percentage: (positive area/total area)×100.
Western blot analysis
After treatment, the medium was collected for western blot analysis. Samples were precipitated and resuspended in loading buffer. Electrophoresis was performed in sodium dodecyl sulfate–polyacrylamide gel, and the proteins were transferred to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) at 40 V over a 10-h period. The membrane was blocked in 5% nonfat milk in Tris-buffered saline containing Tween at room temperature for 2 h and then incubated with primary antibodies against MMP-1, TIMP-1, or glyceraldehyde dehydrogenase for 2 h. Target protein was subsequently detected using rabbit anti-goat immunoglobulin G conjugated with horseradish peroxidase in conjunction with the chromogenic agent.25
Statistics
The experimental results were expressed as mean±SD values. The Statistical Package for Social Sciences (SPSS, Inc., Chicago, IL, USA) was used for data analysis. One-way analysis of variance was used for comparison of the means of several groups, and Fisher's discriminant analysis was performed to test the differences in proportions of categorical variables among groups. The level of statistical significance was set at P<.05.
Results
Protective effect of SF on enzyme leakage and lipid peroxidation
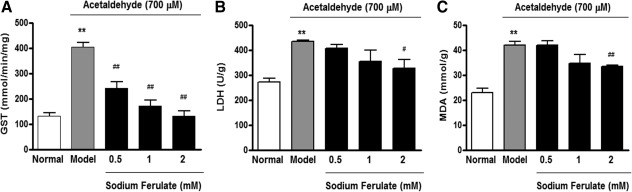
As shown in Figure 1, compared with the normal control group, 6-h acetaldehyde treatment caused an increased leakage of glutathione S-transferase and lactate dehydrogenase (P<.01) in the medium, but this leakage could be prevented by SF in a dose-dependent manner. Meanwhile, the content of tissue malondialdehyde was increased by 1.6-fold (P<.01) in acetaldehyde-treated liver slices compared with the normal control, whereas it was reduced notably after treated with 2 mM SF.
FIG. 1.
Effect of sodium ferulate on leakage of (A) glutathione S-transferase (GST), (B) lactate dehydrogenase (LDH), and (C) malondialdehyde (MDA) content of precision-cut rat liver slices. The liver slices were treated for 6 h with acetaldehyde (700 μM) only (▪) or with acetaldehyde (700 μM) plus different concentrations of SF (▪). Data are mean±SD values (n=3–4). **P<.01 vs. the normal group; #P<.05, ##P<.01 vs. the acetaldehyde control (model) group.
Effect of SF on histological damage and α-SMA expression
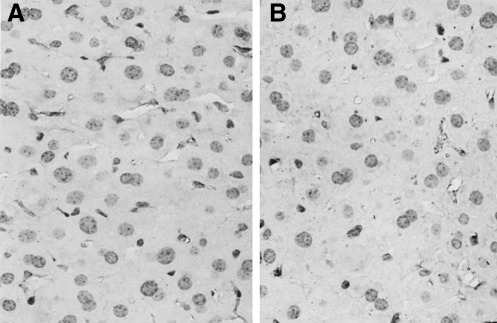
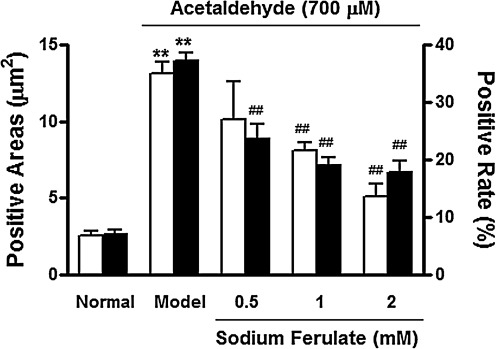
There was little α-SMA expression in normal liver slices in the present study, which was consistent with our previous finding.26 After incubation with acetaldehyde for 6 h, strong positive staining for α-SMA was detected in the Disse space (Fig. 2A). In contrast, when acetaldehyde-treated liver slices were co-incubated with 2 mM SF, α-SMA-positive cells were much fewer (Fig. 2C). The α-SMA-positive cell areas and positive rate were assayed by image analysis. The acetaldehyde-treated liver slices showed the most positive areas and highest positive rate, whereas the SF-treated group exhibited fewer positive areas, coupled with a reduction in the α-SMA-positive cell rate (Fig. 3).
FIG. 2.
Effect of sodium ferulate on acetaldehyde-induced α-smooth muscle actin expression in precision-cut rat liver slices: (A) acetaldehyde (350 μM) only–treated liver slice, (B) acetaldehyde plus 0.5 mM sodium ferulate–treated liver slice, and (C) acetaldehyde plus 2 mM sodium ferulate–treated liver slice. Magnification ×400. The α-smooth muscle actin expression was determined by histological and immunohistochemical analyses.
FIG. 3.
Effect of sodium ferulate on positive areas (□) and positive rate (▪) of α-smooth muscle actin expression in precision-cut rat liver slices. The α-smooth muscle actin expressions were determined by immunohistochemical analyses. The liver slices were treated for 6 h with acetaldehyde (350 μM) only (model) or with acetaldehyde (350 μM) plus different concentrations of sodium ferulate. Data are mean±SD values (n=5). **P<.01 vs. the normal group; ##P<.01 vs. the acetaldehyde control (model) group.
Effect of SF on TGF-β1 secretion and HYP content
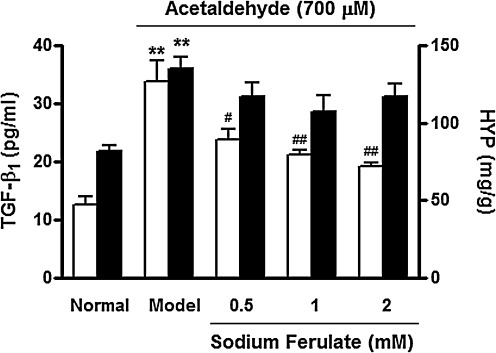
TGF-β1 was deemed to be a crucial cytokine for HSC activation and hepatic fibrosis. In this study, SF inhibited the secretion of TGF-β1 in acetaldehyde-treated liver slices in a dose-dependent manner (Fig. 4).
FIG. 4.
Effect of sodium ferulate on transforming growth factor-β1 (TGF-β1) secretion (□) and hydroxyproline (HYP) content (▪) of precision-cut rat liver slices. The liver slices were treated for 6 h with acetaldehyde (350 μM) only (model) or with acetaldehyde (350 μM) plus different concentrations of sodium ferulate. Data are mean±SD values (n=5). **P<.01 vs. the normal group; #P<.05, ##P<.01 vs. the acetaldehyde control (model) group.
Figure 4 also shows that HYP content was increased in acetaldehyde-treated liver slices compared with the normal control (P<.01), and SF showed a moderate protective effect on acetaldehyde-induced elevation of HYP level.
Effect of SF on MMP-1 and TIMP-1 expression
MMP-1 and TIMP-1 expressions in medium are shown in Figure 5. Compared with the normal control, the expression of TIMP-1 protein was increased, whereas MMP-1 protein was reduced after acetaldehyde treatment. After incubation with SF (2 mM), the expression of MMP-1 was enhanced slightly, whereas the expression of TIMP-1 was decreased notably.
FIG. 5.
Expression of matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinase (TIMP-1) by Western blot in medium of precision-cut rat liver slices incubated with acetaldehyde and sodium ferulate (SF). Model was the slice treated with 350 μM acetaldehyde only; SF represented the slice treated with 2 mM SF and 350 μM acetaldehyde. GAPDH, glyceraldehyde dehydrogenase.
Discussion
The PCLS has been increasingly used for studying hepatotoxicity and evaluating hepatotoxins in vitro. Using PCLSs protects the tissue architecture from protease-induced damage during cell isolation.27,28 Although some previous studies used isolated animal HSCs for evaluating the fibrogenic effect of acetaldehyde,29,30 the process of HSC activation is quite complicated, and other liver cell types (e.g., Kupffer cells) are also involved, especially in the initiation phase. Liver slices are a microcosm of the intact liver, consisting of highly organized cellular communities in which different cell types have mutual contact with each other.31 Therefore, we established a model of acetaldehyde-stimulated HSC activation in vitro using the PCLS in this study.
Results from different laboratories suggest that both ethanol and its principal metabolic product, acetaldehyde, are stimulators of HSC activation.32,33 We observed α-SMA expression in ethanol-treated PCLSs but did not see significant alteration (data not shown), which may be attributed to the limited incubation time as well as the chronic and indirect effects of ethanol on HSC activation. However, in this study, immunohistochemistry studies showed that HSCs were activated in acetaldehyde-treated liver slices, which is consistent with previous reports that acetaldehyde acted as a directly stimulator for activation of HSCs and up-regulation of collagen-related genes,5,34 and this activation could be reversed by SF.
TGF-β1 stimulates the production of extracellular matrix molecules of the HSCs via an autocrine loop and induces their phenotypic differentiation.35 In this study, the TGF-β1 level was decreased when the slices were co-incubated with SF, indicating that SF blocked the process of HSC activation. We also investigated the oxidative damage and lipid peroxidation of PCLSs treated with acetaldehyde and found that SF could inhibit the damage by blocking the formation of malondialdehyde and leakage of cytoplasmic enzymes (glutathione S-transferase and lactate dehydrogenase).
The production of several extracellular matrix components is stimulated by acetaldehyde after HSC activation. In our study, the level of HYP, a main component of collagen, was increased compared with that of the control group. Although it was not obvious in the image results that collagen formation had occurred, HYP augmentation did indicate that acetaldehyde-treated liver slices had a tendency to fibrosis, and HYP content was slightly decreased when the slices were co-treated with SF. MMP-1 is the main protease responsible for degradation of type I collagen, the principal collagen in fibrotic liver. TIMPs are endogenous MMP inhibitors, and sustained TIMP-1 expression is a key factor resulting in the progression of fibrosis.36–38 In this study, when liver slices were exposed to 350 μM acetaldehyde for 6 h, TIMP-1 expression was reinforced over that of the control group. However, TIMP-1 expression was decreased and MMP-1 content was increased after SF treatment, suggesting that SF could degrade collagen indirectly by regulating the activities of MMP-1 and TIMP-1.
In addition, our previous work indicated that SF decreased the activity of cytochrome P4502E1 (CYP2E1),39 an enzyme that contributes to ethanol metabolism following excessive or long-term alcohol ingestion.40 It has been reported that damaged hepatocytes release reactive oxygen species during alcohol metabolism via CYP2E1, which increases collagen production in HSCs.41 Simultaneously, CYP2E1 may be induced by alcohol and then accelerate the generation of oxidative products. In addition, the extraordinary capacity of CYP2E1 to convert many xenobiotics to highly toxic metabolites increases the vulnerability of alcoholic liver. Previous reports have demonstrated the protection of CYP2E1 inhibitors against HSC activation and suggested that CYP2E1 inhibitors may serve as useful agents for prevention and treatment of heavy drinking associated hepatotoxicity.41,42 Therefore, both our previous and present studies have indicated that SF may ameliorate alcoholic liver disease by suppressing the noxious ethanol metabolic pathway and by inhibiting activation of HSCs.
Acknowledgments
This work was supported by grants (numbers 30830112, 81072709, and 30901835) from the National Nature Sciences Foundation of China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gutierrez-Reyes G. Gutierrez-Ruiz MC. Kershenobich D. Liver fibrosis and chronic viral hepatitis. Arch Med Res. 2007;38:644–651. doi: 10.1016/j.arcmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R. Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1659. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves HL. Burt AD. Wood S. Day CP. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677–683. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenwel P. Acetaldehyde-mediated collagen regulation in hepatic stellate cells. Alcohol Clin Exp Res. 1999;23:930–933. [PubMed] [Google Scholar]
- 6.Novitskiy G. Traore K. Wang L. Trush MA. Mezey E. Effects of ethanol and acetaldehyde on reactive oxygen species production in rat hepatic stellate cells. Alcohol Clin Exp Res. 2006;30:1429–1435. doi: 10.1111/j.1530-0277.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Svegliati-Baroni G. Inagaki Y. Rincon-Sanchez AR, et al. Early response of alpha2 (I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology. 2005;42:343–352. doi: 10.1002/hep.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang BH. Ou-Yang JP. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc Drug Rev. 2005;23:161–172. doi: 10.1111/j.1527-3466.2005.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirabayashi T. Ochiai H. Sakai S. Nakajima K. Terasawa K. Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin-8 production in response to influenza virus infections in vitro and in vivo. Planta Med. 1995;61:221–226. doi: 10.1055/s-2006-958060. [DOI] [PubMed] [Google Scholar]
- 10.Van der Watt E. Pretorius JC. Purification and identification of active antibacterial components in Carpobrotus edulis L. J Ethnopharmacol. 2001;76:87–91. doi: 10.1016/s0378-8741(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 11.Rice Evans CA. Miller NJ. Paganga G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;22:761–769. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata K. Yamamoto T. Hara A, et al. Modifying effects of ferulic acid on azoxymethane induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu DF. Peng Rx. Wang H. [Sodium ferulate alleviates prednisolone induced liver toxicity in mice] Yao Xue Xue Bao. 1995;30:801–805. (In Chinese.) [PubMed] [Google Scholar]
- 14.Wang H. Peng RX. [Sodium ferulate alleviated paracetamol-induced liver toxicity in mice] Zhongguo Yao Li Xue Bao. 1994;15:81–83. (In Chinese.) [PubMed] [Google Scholar]
- 15.Wang H. Peng RX. Wang RK. Kong R. [Antagonizing effect of sodium ferulate on the changes of hepatic antioxidative function induced by ethanol in mice] Yao Xue Xue Bao. 1997;32:511–514. (In Chinese.) [PubMed] [Google Scholar]
- 16.Hou Y. Shimizu I. Lu G, et al. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059–1065. doi: 10.1006/bbrc.2001.5479. [DOI] [PubMed] [Google Scholar]
- 17.Zamara E. Novo E. Marra F, et al. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS. Buck M. Houglum K. Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wroblewski F. Ladue JS. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1995;90:210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- 20.Jamall IS. Finelli VN. Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 21.Mihara M. Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683–693. doi: 10.1042/BJ20020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ping J. Wang H. Huang M. Liu ZS. Genetic analysis of glutathione S-transferase A1 polymorphism in the Chinese population and the influence of genotype on enzymatic properties. Toxicol Sci. 2006;89:438–443. doi: 10.1093/toxsci/kfj037. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH. Rosebrough NJ. Farr AL. Randall RJ. Protein measurement with the Folin phend reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Young DA. Lakey RL. Pennington CJ, et al. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther. 2005;7:R503–R512. doi: 10.1186/ar1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y. Wu XQ. Zhang C, et al. Effect of indole-3-carbinol on ethanol-induced liver injury and acetaldehyde-stimulated hepatic stellate cells activation using precision-cut rat liver slices. Clin Exp Pharmacol Physiol. 2010;37:1107–1113. doi: 10.1111/j.1440-1681.2010.05450.x. [DOI] [PubMed] [Google Scholar]
- 27.Lerche-Langrand C. Toutain HJ. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology. 2000;153:221–253. doi: 10.1016/s0300-483x(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AE. Fisher RL. Organ slices for the evaluation of human drug toxicity. Chem Biol Interact. 2004;150:87–96. doi: 10.1016/j.cbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Itatsu T. Oide H. Watanabe S. Tateyama M. Ochi R. Sato N. Alcohol stimulates the expression of L-type voltage-operated Ca2+ channels in hepatic stellate cells. Biochem Biophys Res Commun. 1998;251:533–537. doi: 10.1006/bbrc.1998.9458. [DOI] [PubMed] [Google Scholar]
- 30.Konishi M. Kato S. Kajihara M. Cederbaum A. Ishii H. Ethanol upregulates pro-fibrogenic connective tissue growth factor (CTGF) gene expression in HepG2 cells via cytochrome P450 2E1-mediated ethanol oxidation. Hepatol Res. 2004;28:102–108. [Google Scholar]
- 31.Parrish AR. Gandolfi AJ. Brendel K. Precision-cut tissue slices: applications in pharmacology and toxicology. Life Sci. 1995;57:1887–1901. doi: 10.1016/0024-3205(95)02176-j. [DOI] [PubMed] [Google Scholar]
- 32.Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- 33.Siegmund SV. Brenner DA. Molecular pathogenesis of alcohol-induced hepatic fibrosis. Alcohol Clin Exp Res. 2003;29:S102–S109. doi: 10.1097/01.alc.0000189275.97419.58. [DOI] [PubMed] [Google Scholar]
- 34.Anania FA. Womack L. Jiang M. Saxena NK. Aldehydes potentiate α2(I) collagen gene activity by JNK in hepatic stellate cells. Free Radic Biol Med. 2001;30:846–857. doi: 10.1016/s0891-5849(01)00470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellerbrand C. Stefanovic B. Giordano F. Burchardt ER. Brenner DA. The role of TGF beta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 36.Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43–54. doi: 10.1016/s1357-2725(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 37.Koks CA. Groothuis PG. Slaats P. Dunselman GA. de Goeij AE. Evers JL. Matrix metalloproteinases and their tissue inhibitors in antegradely shed menstruum and peritoneal fluid. Fertil Steril. 2000;73:604–612. doi: 10.1016/s0015-0282(99)00566-x. [DOI] [PubMed] [Google Scholar]
- 38.Takahara T. Zhang LP. Yata Y, et al. Modulation of matrix metalloproteinase-9 in hepatic stellate cells by three-dimensional type I collagen: its activation and signaling pathway. Hepatol Res. 2003;26:318–326. doi: 10.1016/s1386-6346(03)00169-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C. Wang H. Guo Y. Protection of sodium ferulate on ethanol-induced hepatotoxicity in precision liver-cut slices of rats and its mechanism. Med J Wuhan University. 2005;26:477–480. [Google Scholar]
- 40.Ramchandani VA. Bosron WF. Li TK. Research advances in ethanol metabolism. Pathol Biol. 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 41.Nieto N. Friedman SL. Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 42.Lieber CS. Alcoholic liver injury pathogenesis and therapy in 2001. Pathol Biol (Paris) 2001;49:738–752. doi: 10.1016/s0369-8114(01)00239-5. [DOI] [PubMed] [Google Scholar]