Abstract
During fetal lung development, cells within the mesenchyme differentiate into vascular endothelia. This process of vasculogenesis gives rise to the cells that will eventually form the alveolar capillary bed. The cellular mechanisms regulating lung vasculogenesis are poorly understood, partly due to the lack of experimental systems that model this process. Here, we have developed and characterized a novel fetal mouse lung cell model of mesenchymal to endothelial differentiation. Using mesenchymal cells from the lungs of embryonal day 15 Immortomice, we show that endothelial growth media containing fibroblast growth factor-2 and vascular endothelial growth factor can stimulate formation of vascular endothelial cells in culture. These newly formed endothelial cells retain plasticity, as removing endothelial growth media causes loss of vascular markers and renewed formation of α-smooth muscle actin positive stress fibers. Cells with the highest Flk-1 expression differentiated into endothelia more efficiently. Individual mesenchymal cell clones had varied ability to acquire an endothelial phenotype. These fetal lung mesenchymal cells were multipotent, capable of differentiating into not only vascular endothelia, but also osteogenic and chondrongenic cell lineages. Our data establish a cell culture model for mesenchymal to endothelial differentiation that could prove useful for future mechanistic studies in the process of vasculogenesis both during normal development and in the pathogenesis of pulmonary vascular disease.
Introduction
The lung first arises as an extension from the ventral surface of the foregut endoderm. A series of branching and elongation events sequentially forms the trachea, bronchi, and then bronchioles. Later in development, terminal airways eventually produce the mature alveolar structures that allow efficient gas exchange with the bloodstream [1]. Throughout this process, cells within the surrounding mesenchyme tightly regulate lung formation. Mesenchymal cells produce many of the growth factors that control spatiotemporal events during airway morphogenesis. Cells within the developing lung mesenchyme also differentiate into multiple cell types, including the mesothelium that lines the surface of the lung, smooth muscle cells surrounding large airways, and myofibroblasts that both form alveolar septa and provide mechanical, elastic strength to mature alveoli. In addition, fetal lung mesenchymal cells give rise to both pericytes and endothelia of the lung vasculature [2].
The lung vasculature develops by several distinct mechanisms. As the airway structures initially begin to form, blood vessels elongate and branch in parallel to the conducting airways. These new blood vessels sprout from larger existing vessels via angiogenesis [3,4]. However, the vascular structures that will eventually form the alveolar capillary bed first originate de novo from within the mesenchyme, before being connected to the vascular circulation [5,6]. Congregations of endothelial cells form an immature plexus that remodels to form more mature vessels which eventually connect to the pulmonary blood supply [7]. As the lung further grows and matures, new vessels form by angiogenesis, undergo remodeling, and locate to the tips of alveolar septa [8]. Formation of this alveolar capillary bed is required for extrauterine survival, and largely determines viability of extremely preterm infants.
Multiple growth factors regulate pulmonary vasculature development. Among these, vascular endothelial growth factor (VEGF) is expressed first by lung mesenchymal cells early in fetal development and then later by airway and alveolar epithelia [5,9,10]. Expression of VEGF by epithelial cells may recruit alveolar capillaries from the lung interstitium to the basement membrane beneath the alveolar epithelia [11]. Close approximation of vessels to alveoli may, therefore, minimize the potential barrier for gas exchange. Other factors also contribute to lung vascular formation. The lung mesothelium produces fibroblast growth factor (FGF)-9, which works with SHH to maintain mesenchymal VEGF expression and normal vascular formation. FGF-9 is required for normal endothelial cell number, possibly by stimulating mesenchymal cell proliferation and by promoting epithelial expression of vascular growth factors [9]. Both epithelial and mesenchymal cells in the developing lung express FGF-2, a pro-angiogenic growth factor that may work in cooperation with VEGF [12]. The roles that FGF-2 and VEGF play in differentiation of mesenchymal cells into vascular endothelia are not clear.
Despite the obvious importance of vascular development in the fetal lung, a few models exist for studying the molecular mechanisms involved in alveolar capillary formation. Many questions especially surround mesenchymal to endothelial differentiation. Previous studies have described transformed MFLM cells, which express both mesenchymal and endothelial markers [13]. These cells may represent endothelial precursor cells, as they are capable of forming vessels and incorporating into chimeric endothelia in vivo. Multiple studies have also isolated colonies of endothelia precursors from both fetal and adult lung tissue [14,15]. However, these have started with cells expressing endothelial markers or endothelial precursors rather than demonstrating de novo endothelial differentiation from a mesenchymal cell population. We, therefore, set out to establish an experimental model of fetal lung mesenchymal to endothelial differentiation to learn more about how endothelial vasculogenesis occurs. For these studies, we isolated primary fetal lung mesenchymal cells from Immortomice, which express a temperature-sensitive SV40 T antigen. The cells were expanded and cultured in the presence of factors that promote endothelial cell growth. Here, we present results demonstrating that these fetal mouse lung mesenchymal cells are capable of differentiating into vascular endothelial cells. These cells could prove helpful for studying the molecular events regulating formation of blood vessels during development and differentiation of mesenchymal subpopulations during organogenesis.
Materials and Methods
Animals and cell culture
All animal procedures were performed with approval of the Institutional Animal Care and Use Committee at the Vanderbilt University Medical Center. Immortomice expressing the temperature-sensitive early region SV40 mutant tsA58 allele downstream of the H-2Kb promoter were purchased from Charles River [16]. Timed matings were performed with the day of plug discovery defined as embryonal day 0 (E0). Pregnant females were euthanized at E15, and fetal lung tissue was dissected free of surrounding structures. To isolate lung mesenchymal cells, E15 lungs were minced into 1 mm3 cubes and placed on plastic dishes covered by a minimal amount of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The dishes were maintained at 37°C in a humidified environment of 95% air and 5% CO2. After 5–7 days, the lung pieces were removed under a dissecting microscope, leaving behind mesenchymal cells that had grown out onto the plastic dish. These cells were passaged once at 37°C and then shifted to 33°C in media containing interferon (IFN)-γ. Before study, cells were placed in IFN-γ-free media, cultured at 37°C for at least 48 h, and passaged at least once. To assess tube formation, cells cultured in either DMEM or endothelial growth media 2 (EGM-2) were plated onto wells coated with Matrigel (BD Biosciences). Images of tube formation were captured 18 h after plating on Matrigel.
To promote endothelial differentiation, mesenchymal cells were cultured on gelatin-coated plastic dishes in EGM-2 (Lonza). Control cells were maintained in DMEM with 10% FBS. To test for reversibility, parallel plates of cells were first cultured for 7 days in EGM-2. Cells were then switched back to DMEM with 10% FBS or continually cultured in EGM-2. To assess tube formation, cells cultured in either DMEM or EGM-2 were plated onto wells coated with Matrigel (BD Biosciences). Images of tube formation were captured 18 h after plating on Matrigel. Individual mesenchymal cell clones were isolated by serial dilution and initially grown in 96-well plates. Twelve separate clones were initially characterized for their ability to express Flk-1 and VE-cadherin after 7 days of culture in EGM-2. Bone marrow-derived mesenchymal stem cells from C57/Bl6 mice were purchased from Invitrogen. To promote mesenchymal stem cell differentiation, both fetal lung mesenchymal cells and bone marrow-derived mesenchymal cells were cultured in StemPro (Invitrogen) osteogenic, chondrogenic, or adipogenic differentiation media. Control cells were cultured in basal StemPro media.
RNA isolation and real-time polymerase chain reaction
Total RNA was isolated by using TRIzol Reagent (Invitrogen). First-strand cDNA was generated by using MMLV Reverse Transcriptase (SuperScript II; Invitrogen) and oligo-dT primers. Real-time polymerase chain reaction (PCR) primers were designed by using Beacon Designer software. Selected primer pairs spanned exon-intron boundaries and recognized sequences at the 3’ region of each gene. PCR efficiency and melting temperature analysis was performed for each separate set of primers. Real-time PCR was performed by using SYBR Green and an iQ5 thermocycler (Bio-Rad). Expression of each gene was compared with GAPDH and expressed as fold change by using the 2-ΔΔCT method. Differences in expression between groups were compared by one-way ANOVA and Tukeys post-hoc analysis. All values were presented as the mean±SEM.
Immunoblotting
Cell lysates were prepared from confluent culture dishes (60 mm) in lysis buffer containing 50 mM Tris·HCl (pH 7.5), 1% sodium dodecyl sulfate (SDS), and protease inhibitors. Protein samples (25 μg) were denatured in SDS-PAGE sample buffer, separated by SDS-polyacrylamide gel electrophoresis (PAGE), and electrophoretically transferred to polyvinylidene difluoride (Immobilon-P; Millipore). Membranes were blocked overnight at 4°C in 5% nonfat dried milk and 0.1% polyoxyethylenesorbitan monolaurate (Tween 20). The membranes were incubated at room temperature for 1 h with polyclonal rabbit anti-SV40 T Ag (Ab-1; Santa Cruz, sc-20800) and then developed with horseradish peroxidase-conjugated sheep anti-rabbit IgG and enhanced chemiluminescence (Amersham). Membranes were similarly probed with an antibody against β-actin (Sigma) as a loading control. Protein bands were visualized by using standard autoradiography film (Denville Scientific).
Immunostaining and microscopy
Cultured cells and frozen sections of E15 mouse lung tissue were fixed, processed, and immunolabeled by using standard techniques. For immunofluorescence, Alexa-conjugated secondary antibodies were used for visualization, and nuclei were labeled with either DAPI or TO-PRO-3. Alcian Blue and Oil Red O staining reagents were obtained from Sigma. Alizarin Red was purchased from Fluka. Each stain was used according to standard histochemical protocols. Confocal images were acquired by using either an Olympus FV1000 or a Leica SPE lazer scanning confocal microscope. Widefield fluorescence images and brightfield images of cultured fetal lung mesenchymal cells were captured by using an Olympus IX81 microscope equipped with a Hamamatsu Orca ER CCD monochrome camera and Slidebook software (Olympus). Color images of histochemical stains were obtained by using a Nikon TE800 and SPOT color CCD camera (Diagnostic Instruments). All microscopy images were saved in the Tagged Image File Format and imported into Photoshop (Adobe) for processing. Images for comparison were always identically processed.
FACS analysis
For FACS analysis, cultured fetal lung mesenchymal cells were washed with warm Hanks balanced salt solution (HBSS) and incubated for 1 min with 5 mL of cell dissociation solution (Accutase; Invitrogen). After cell detachment, 5 mL of ice-cold FACS Buffer (HBSS+1% FCS) was added, and the cells were collected by centrifugation. The cells were then labeled with antibodies against Flk-1 (APC conjugate, Avas12a1, eBioscience) or VE-cadherin (Cayman). Cells were filtered through a 100 μm basket filter and analyzed by using a BD LSRII (Becton Dickinson). Propidium Iodide exclusion staining measured cell viability. Sorting and collection of Flk-1 positive cells was performed by using a BD FACS Aria (Becton Dickinson). Cells incubated with nonimmune IgG served as controls. All raw data were analyzed and graphed by using FlowJo v8.8.2 software. Each experiment was performed at least 3 separate times.
Results
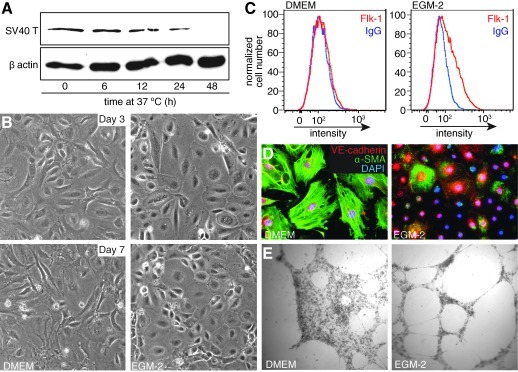
To better understand the cellular and molecular processes that occur during lung vasculogenesis, we established an experimental cell culture model of mesenchymal to endothelial differentiation. Fetal lung mesenchymal cells were isolated from dissected, minced pieces of E15 Immortomouse lungs and expanded at 33°C in the presence of IFN-γ. As previously reported, shifting cells to 37°C and removing IFN-γ from the media led to degradation of the SV40 T antigen as measured by immunoblot (Fig. 1A). The cells were then passaged and cultured at 37°C in DMEM with 10% fetal bovine serum (DMEM) or in endothelial growth media (EGM-2). After 3 days of culture, subpopulations of cells cultured in EGM-2 obtained a flat, cobblestoned morphology resembling vascular endothelia. This difference in cell shape was still observed at 7 days in culture (Fig. 1B). We further tested endothelial differentiation by using antibodies against the vascular endothelial markers Flk-1 and VE-cadherin. To measure mesenchymal phenotype, we also stained cells by using an antibody against α-smooth muscle actin (α-SMA). Mesenchymal cells cultured for 7 days in EGM-2 expressed the VEGF receptor Flk-1, whereas control cells remained negative (Fig. 1C). Control mesenchymal cells contained α-SMA-positive stress fibers and lacked cell-surface VE-cadherin expression. However, cells cultured for 7 days in EGM-2 developed cell-cell contacts containing VE-cadherin (Fig. 1D). In addition, the EGM-2 treated cells that expressed α-SMA did not form obvious stress fibers, but instead had a more diffuse actin distribution. Interestingly, some cells expressed both VE-cadherin and α-SMA, thus suggesting an intermediate mesenchymal-endothelial phenotype. Cells cultured in EGM-2 were also more likely to form cellular tubes in Matrigel (Fig. 1E), consistent with endothelial differentiation.
FIG. 1.
Endothelial growth media-2 (EGM-2) stimulates vascular endothelial differentiation of fetal lung mesenchymal cells. (A) Immunoblot for the SV40 Large T antigen shows degradation over time after culture at 37°C. β-actin included as loading control. (B) E15 cells were cultured in DMEM containing 10% fetal bovine serum (left panels) or endothelial growth media (EGM-2; right panels). Phase contrast micrographs of representative fields show that cells cultured in EGM-2 acquire a more flattened, cobblestone morphology. (C) At day 7, cells cultured in EGM-2 (right panel) had increased Flk-1 expression as measured by FACS. Control cells cultured in DMEM (left panel) did not express Flk-1 above background (labeled with nonimmune IgG). (D) Immunostaining of mesenchymal cells shows increased cell surface VE-cadherin in cells cultured for 7 days in EGM-2 and loss of α-SMA stress fibers. Staining for α-SMA in EGM-2 cultured cells was seen in a more cytoplasmic pattern. (E) Cells cultured for 7 days in DMEM or EGM-2 were then plated on Matrigel. Bright field images taken 18 h later show increased tube formation in EGM-2 cultured cells. DMEM, Dulbecco's modified Eagle's medium; α-SMA, α-smooth muscle actin.
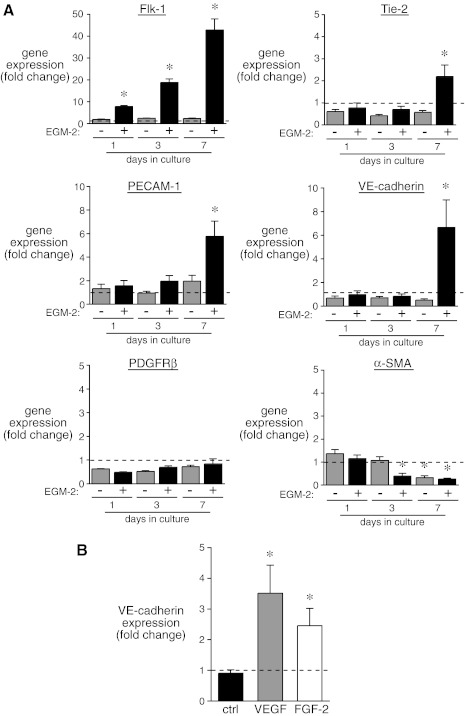
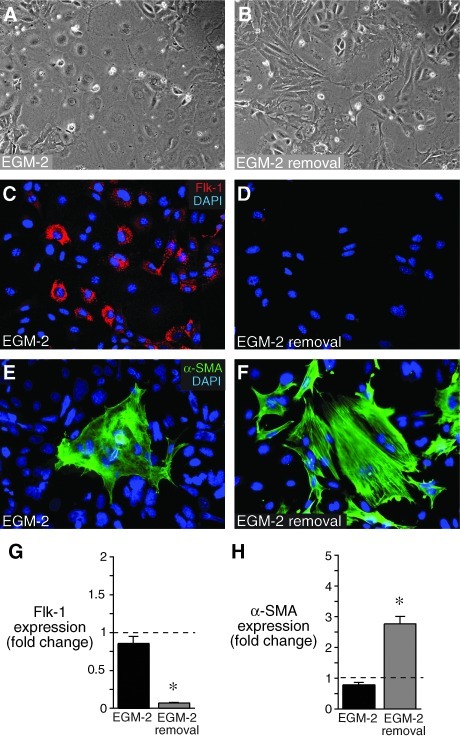
Mesenchymal cells cultured in EGM-2 also expressed endothelial markers as measured by real-time PCR. Flk-1 expression steadily increased over time compared with control cells cultured in DMEM (Fig. 2A). The additional endothelial markers Tie-2, PECAM-1, and VE-cadherin increased after 7 days of culture in EGM-2. Expression of the mesenchymal maker PDGFRβ did not change under either culture condition. α-SMA expression decreased over time after culture in both DMEM and EGM-2, with a more pronounced decrease after 3 days in EGM-2. To test whether the individual growth factor components in EGM-2 were sufficient to increase VE-cadherin expression, we individually added FGF-2, VEGF, EGF, IGF, retinoic acid, and hydrocortisone to cells cultured in basal media. Only VEGF and FGF-2 significantly increased VE-cadherin expression (Fig. 2B). Since there may well be synergy between the different components, we used complete EGM-2 media for subsequent experiments. To test whether endothelial differentiation was reversible, we cultured mesenchymal cells in EGM-2 for 7 days and then either replaced the EGM-2 with DMEM or continued to culture cells in EGM-2 for an additional 7 days. As seen in Fig. 3, EGM-2 removal caused a loss of endothelial phenotype. Cells returned to DMEM had a more elongated morphology (Fig. 3B), reduced Flk-1 expression (Fig. 3D, G), and increased α-SMA expression and stress fiber formation (Fig. 3F, H). These data show that mesenchymal cells cultured in EGM-2 do not irreversibly commit to the endothelial lineage, but instead retain plasticity.
FIG. 2.
Endothelial growth media increases vascular endothelial marker gene expression. (A) RNA was isolated from mesenchymal cells cultured for 1–7 days in DMEM or EGM-2. Gene expression was measured by real-time polymerase chain reaction and represented as fold change compared with cells at day 0 of the experiment (previously cultured only in DMEM; *P<0.05, n=9). (B) Purified VEGF and FGF-2 increased VE-cadherin expression when added to DMEM cultured cells for 7 days. (*P<0.05, n=6). FGF, fibroblast growth factor; VEGF, vascular endothelial growth factor.
FIG. 3.
Mesenchymal to endothelial differentiation is reversible. Fetal lung mesenchymal cells were cultured for 7 days in EGM-2, and then continued in EGM-2 for an additional 7 days (A, C, E) or switched to DMEM for 7 days (B, D, F). (A, B) Phase contrast images show loss of cobblestone morphology on EGM-2 removal. (C, D) Flk-1 expression was reduced in cells on EGM-2 removal. (E, F) EGM-2 removal led to increased formation of α-SMA positive stress fibers. (G, H) EGM-2 removal led to a reduction in Flk-1 expression and an increase in α-SMA expression compared with cells continually maintained in EGM-2 for 14 days in total. Values compared with samples cultured in EGM-2 for 7 days. (*P<0.05, n=4).
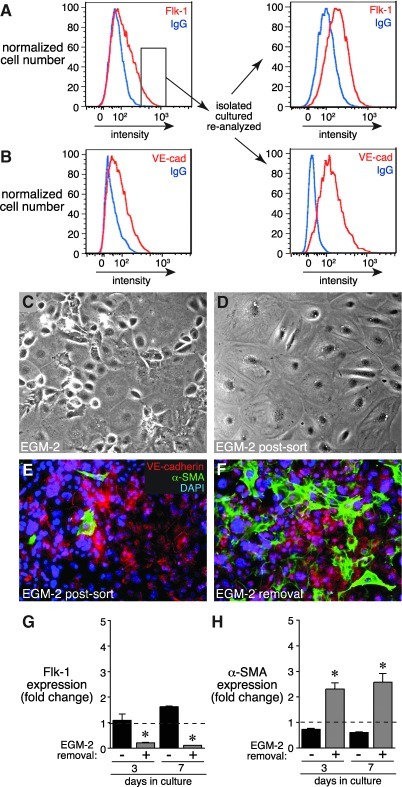
After 7 days of culture in EGM-2, ∼10%–20% of the cells expressed endothelial markers. In an attempt to increase the number of endothelial cells obtained in culture, we isolated and expanded the Flk-1 expressing subpopulation from an EGM-2 treated culture by cell sorting. When the highest Flk-1 expressing cells were continuously cultured in EGM-2 media (for a total of 14–21 days), we measured an increase in both Flk-1 and VE-cadherin expressing cells compared with non-sorted cells (Fig. 4A, B). Flk-1 sorted cells also appeared more homogenous, with most cells adopting flat, cuboidal endothelial morphology. These cells expressed VE-cadherin at the cell margins, and only a few cells expressed α-SMA (Fig. 4E). Interestingly, sorted cells retained plasticity, as removal of EGM-2 media again allowed expression of increased α-SMA-positive stress fibers (Fig. 4F, H) and reduced Flk-1 expression (Fig. 4G). These data suggest that cells with the highest level of Flk-1 expression are more likely to possess an endothelial phenotype, but still retain the ability to return to a mesenchymal cell phenotype when EGM-2 media is removed.
FIG. 4.
Mesenchymal cells expressing high levels of Flk-1 express VE-cadherin but still lose their endothelial phenotype on EGM-2 removal. (A) Mesenchymal cells were cultured in EGM-2 for 7 days and sorted based on Flk-1 expression. The cells with highest Flk-1 expression (indicated by small box inset) were isolated, expanded, and analyzed again by FACS. (B) VE-cadherin expression is shown in original cells (left panel) and in Flk-1 sorted cells after expansion (right panel). (C, D) Phase contrast images of cells before and after sorting based on Flk-1 expression. (E) The majority of Flk-1 sorted cells expressed cell surface VE-cadherin. Only a few cells expressed α-SMA. (F) On removal of EGM-2 media, more cells with α-SMA positive stress fibers were observed. (G, H) EGM-2 removal led to reduced Flk-1 expression and increased α-SMA expression in cells originally sorted based on Flk-1 expression (*P<0.05, n=6).
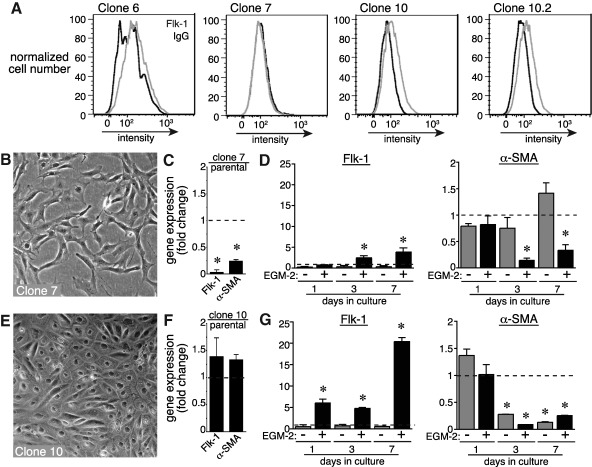
Individual mesenchymal cells or subpopulations of cells may have unique or distinct potential for differentiating into vascular endothelia. To test this, we isolated individual clones of fetal lung mesenchymal cells and measured Flk-1 expression after 7 days of culture in EGM-2 media. As seen in Fig. 5A, 3 representative clones demonstrated a range of Flk-1 expression after 7 days of culture in EGM-2. Clone 7 cells did not express a measurable increase in cell surface Flk-1 by FACS (Fig. 5A), and had spindle-like cell morphology even after 7 days of culture in EGM-2 (Fig. 5B). Clone 7 cells also expressed lower levels of Flk-1 and α-SMA compared with the original cell population (Fig. 5C). Interestingly, EGM-2 still increased Flk-1 gene expression and decreased α-SMA gene expression in these cells (Fig. 5D). In comparison, culturing clone 10 cells for 7 days in EGM-2 led to cell surface Flk-1 expression by FACS, a cobblestone morphology, similar baseline expression of Flk-1 and α-SMA compared with original cells, and strong Flk-1 induction with culture in EGM-2 (Fig. 5A, E–G). To ensure that these cells were truly clonal, a subclone from clone 10 (clone 10.2) was isolated and had similar increased Flk-1 expression after culture in EGM-2. Using clone 10.2, we tested whether endothelial differentiation was reversible even in cells derived from an individual mesenchymal cell clone.
FIG. 5.
Individual mesenchymal cell clones have varying endothelial potential. (A) Single clones of fetal lung mesenchymal cells were isolated and expanded. Clone 10.2 represents a distinct subclone obtained from clone 10. Each clone was cultured in EGM-2 for 7 days. Flk-1 expression was measured by FACS. Clone 10 appeared to express higher levels of Flk-1 in response to EGM-2. Similar results were seen with the subclone 10.2. (B) Culturing clone 7 cells for 7 days in EGM-2 did not induce classic cobblestone morphology. (C, D) Flk-1 and α-SMA expression in clone 7 cells was lower than original cells, but culturing clone 7 cells in EGM-2 did increase Flk-1 expression and inhibit α-SMA expression (*P<0.05, n=4). (E) EGM-2 stimulated endothelial cell morphology. (F, G) Flk-1 and α-SMA expression in clone 10 cells was comparable to original cells, and EGM-2 increased Flk-1 expression to higher levels than clone 7 cells. Clone 10 cells had lower α-SMA expression over time in both DMEM and EGM-2 (*P<0.05, n=4).
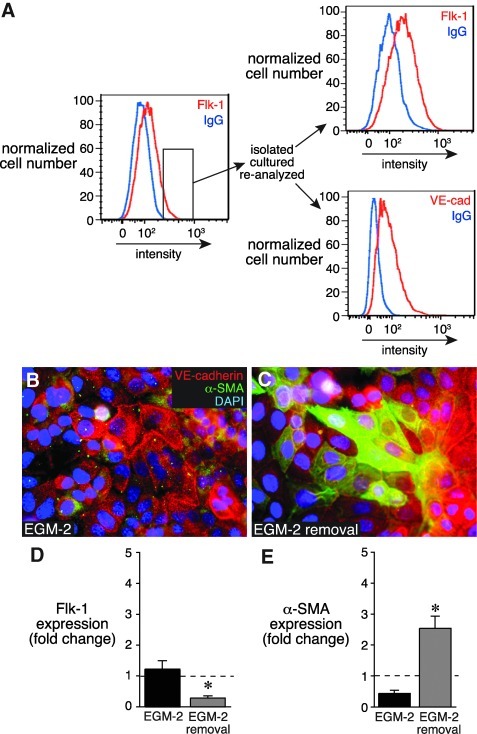
After 7 days of culture in EGM-2, clone 10.2 cells expressing the highest Flk-1 levels were isolated by FACS and expanded. Enrichment for Flk-1 and VE-cadherin expression is shown in Fig. 6A. A majority of 10.2 cells expressed cell surface VE-cadherin, whereas a few cells continued to express cytoplasmic α-SMA (Fig. 6B). On removal of EGM-2 media, α-SMA expression increased (Fig. 6C, E), and Flk-1 expression again decreased. These data demonstrated that fetal lung mesenchymal cells derived from a single cell clone display varied abilities to differentiate into endothelial cells. In addition, these clonal cells retain the plasticity required to revert back to a mesenchymal phenotype when EGM-2 is removed.
FIG. 6.
Clonally derived mesenchymal cells retain endothelial plasticity. (A) Clone 10.2 mesenchymal cells were cultured for 7 days in EGM-2. FACS was used to identify and isolate cells with the highest Flk-1 expression. Following expansion and continued culture in EGM-2, the sorted 10.2 cells continued to express both Flk-1 and VE-cadherin. (B) Immunostaining showed that most cells expressed cell surface VE-cadherin, but a few cells continued to express α-SMA. (C) EGM-2 removal increased α-SMA expression. (D, E) Removal of EGM-2 from sorted clone 10.2 cells caused lower Flk-1 expression and increased α-SMA expression compared with cells maintained in EGM-2. (*P<0.05, n=9).
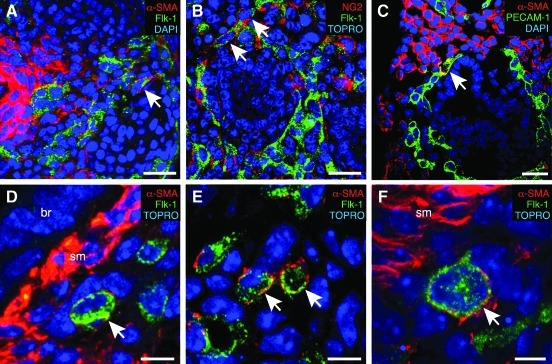
In cell culture, most cells express either mesenchymal or endothelial markers; however a fraction of cells express markers of both lineages. To test whether mesenchymal cells in the developing lung also express endothelial markers in vivo, we immunostained E15 fetal mouse lung sections by using antibodies that would label both mesenchymal cell populations and vascular endothelial cells. As seen in Fig. 7, most cells expressed either mesenchymal or endothelial markers, but an occasional cell expressed both α-SMA and Flk-1 (Fig. 7A), the pericytes marker NG2 and Flk-1 (Fig. 7B), or α-SMA and PECAM-1 (Fig. 7C). Higher magnification confocal images identified cells that appeared to express both Flk-1 and α-SMA (Fig. 7D–F). Conclusively identifying double positive cells in fetal lung sections was, therefore, difficult, but a small number of cells in the lung mesenchyme did appear to simultaneously express both mesenchymal and endothelial markers.
FIG. 7.
Rare co-localization of mesenchymal and endothelial markers in fetal mouse lung. E15 fetal mouse lung sections were immunostained using antibodies against α-SMA (A, C–F), NG2 (B), Flk-1 (A, D–F), or PECAM-1 (C). Images were then obtained by confocal microscopy. Arrows indicate individual cells that appear to express both an endothelial marker (Flk-1 or PECAM-1) and a mesenchymal marker (α-SMA, NG2). (scale bars in A–C, 25 μm). (D–F) Higher-magnification confocal images with cells positive for both α-SMA and Flk-1 (br=bronchial epithelium, sm=smooth muscle; scale bars 5 μm in D, E, 3 μm in F).
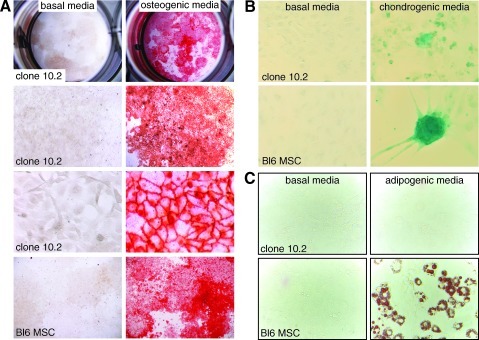
Cultured fetal lung mesenchymal cells appeared multipotent and plastic, properties found in bone marrow-derived mesenchymal stem cells and stromal progenitor cells. We further explored the ability of fetal lung mesenchymal cells to differentiate into other mesenchymal lineages. As shown in Fig. 8, clone 10.2 cells differentiated into osteocytes and chondrocytes when cultured in appropriate media. The Alcian Blue staining colonies seen in fetal lung mesenchymal cells were smaller in number compared with marrow-derived mesenchymal stem cells (Fig. 8B). When maintained in adiopogenic media, clone 10.2 cells did not develop lipid vesicles positive for Oil Red O. Therefore, although fetal lung mesenchymal cells may not be entirely multipotent, they are capable of differentiating into multiple mesenchymal cell lineages.
FIG. 8.
Fetal lung mesenchymal cells can differentiate into osteogenic and chondrogenic lineages. (A). Clone 10.2 fetal lung mesenchymal cells were cultured for 14 days in basal media (left panels) or chondrogenic media (right panels). Alizarian Red staining shows mineralization consistent with osteocyte differentiation. Bone marrow-derived mesenchymal stem cells are shown in the bottom panels. (B) Clone 10.2 fetal lung mesenchymal cells cultured for 21 days in chondrogenic media (right panels) form Acian Blue positive colonies. No staining was seen in cells cultured in basal media. Bone marrow-derived mesenchymal stem cells are shown in the bottom panels as a positive control. (C) Culturing clone 10.2 fetal lung mesenchymal cells in adipogenic media did not stimulate formation of Oil Red O positive adipocytes. Bone marrow-derived mesenchymal stem cells in bottom panels stained positive with Oil Red O when cultured in adipogenic media.
Discussion
We have isolated and characterized fetal mouse lung mesenchymal cells that can differentiate into endothelial cells in culture, providing an experimental model for studying the mechanisms of vasculogenesis. Although multiple factors likely regulate endothelial differentiation during vasculogenesis, VEGF and FGF-2 were each sufficient to promote expression of the endothelial marker VE-cadherin. Interestingly, mesenchymal to endothelial differentiation was reversible, thus suggesting cellular plasticity. Within mesenchymal cell cultures, individual cells appeared to have variable ability to differentiate into endothelial cells, likely representing a heterogeneous population of progenitor cells within the fetal mouse lung. Consistent with this idea, mesenchymal cells derived from single clones differentiated into endothelial cells with different efficiencies. These fetal lung mesenchymal cells were also multipotent, capable of differentiation into chondrogenic and osteogenic lineages. Our data demonstrate the dynamic, versatile capabilities of cells derived from the developing fetal lung mesenchyme.
This cell model will hopefully permit a more detailed analysis of the molecular steps occurring when blood vessels form de novo within developing lung tissue. Obviously, using cell lines to study blood vessel formation comes with an inherent set of caveats and potential limitations. We do not as yet know whether these cells form endothelial vessels in vivo or whether efficiency of endothelial differentiation changes with higher passage number. Even though the Immortomouse SV40 tsA58 antigen expression is reversible, expansion of transformed cell lines could lead to changes in cell behavior that differ from true primary cells. In addition, mimicking the relevant cellular environment required for vascular development may require additional trophic factors or specific extracellular matrices. However, the cells characterized here clearly adopt an endothelial phenotype and express multiple vascular endothelial markers.
Individual fetal lung mesenchymal cells may have unique potential for endothelial differentiation. Control cells cultured in DMEM did not express detectable Flk-1, but EGM-2 treated cells expressed varying levels of Flk-1. When isolated by FACS, cells with the highest Flk-1 expression appeared more likely to differentiate into endothelial cells. The reason that Flk-1 expression varies among cells in culture, even within clonally derived cells, is not clear, but this heterogeneity may explain why endothelial differentiation appears in subpopulations of cells. Perhaps cells with the highest Flk-1 expression and residing in VEGF-rich microenviroments are the cells most likely to differentiate into vascular endothelia. In fact, our cell culture data resemble vivo development where only a fraction of mesenchymal cells within the developing lung differentiate into vascular endothelia. This hypothesis is also supported by data showing that individual mesenchymal cell clones have varying expression of endothelial markers. Heterogeneous endothelial differentiation could also be due to asymmetric cell division or cell-cell interactions that differentially determine cell fate. Cells that begin to acquire an endothelial phenotype may inhibit endothelial differentiation in neighboring cells. A similar process occurs during differentiation of other cell lineages, including the segregation of endocrine and exocrine cells in the developing pancreas [17,18].
Our data suggest that fetal lung mesenchymal cells may undergo a mesenchymal to endothelial transdifferentiation process, as vascular endothelial markers are nearly undetectable in control cells. When mesenchymal cells in endothelial growth media acquire endothelial markers, they lose α-SMA containing stress fibers, consistent with loss of the myofibroblast phenotype. The E15 mouse lung mesenchymal cells studied here also share some properties with bone marrow-derived mesenchymal stem cells. Differentiation of cultured fetal lung mesenchymal cells into multiple lineages may represent developmental events in vivo. Among the mesenchymal lineages in the lung, chondrocytes arise from mesenchymal cells and form the cartilaginous rings surrounding the trachea and the cartilage plates adjacent to bronchi [19,20]. Therefore, the formation of Alcian-Blue colonies in fetal lung mesenchymal cell cultures treated with chondrogenic media could be modeling this differentiation process. Interestingly, lung mesenchymal cells did not develop lipid-rich vesicles when cultured in adipogenic media, thus suggesting a more restricted potential than bone marrow-derived mesenchymal stem cells. Although fetal lung mesenchymal cells were clearly capable of osteogenic mineralization, the significance of this osteogenic potential during normal lung development is not clear.
We did not expect the degree of reversibility observed when EGM-2 media was removed and fetal lung mesenchymal cells were placed back in basal media. The changes in gene expression, immunostaining, and morphology demonstrate that fetal lung mesenchymal cells have inherent plasticity. These results also raise the obvious question of potential mesenchymal cell plasticity during normal development or in disease pathogenesis. Abnormal differentiation and proliferation of lung mesenchymal cells likely plays a role in idiopathic pulmonary fibrosis, pulmonary arterial hypertension, and bronchopulmonary dysplasia [21–23]. The potential for mesenchymal cell plasticity in vivo, especially in adult tissues after injury, is not completely clear [24,25]. If epithelial cells are major sources of VEGF, then epithelial injury and reduced VEGF production may cause loss of endothelial cells and instead differentiation and proliferation of myofibroblasts. Better understanding of the cell and molecular events regulating changes in cell phenotype could, therefore, lead to new therapeutic strategies.
Acknowledgments
This work was supported by the March of Dimes (5-FY07-555, L.S.P.) and the NIH (HL097195 and HL086324, L.S.P). Confocal imaging was performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126). The authors thank Brian Halloran, Amanda Im, Liping Du, Chen Xie, and Jin-Hua Liu for their technical assistance as well as their scientific colleagues for their helpful comments and suggestions.
Author Disclosure Statement
The authors do not have any financial interests or commercial associations that pose a conflict of interest in connection with this article.
References
- 1.Morrisey EE. Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburton D. El-Hashash A. Carraro G. Tiozzo C. Sala F. Rogers O. De Langhe S. Kemp PJ. Riccardi D, et al. Lung organogenesis. Curr Topics Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deMello DE. Sawyer D. Galvin N. Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 4.Hall SM. Hislop AA. Pierce CM. Haworth SG. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol. 2000;23:194–203. doi: 10.1165/ajrcmb.23.2.3975. [DOI] [PubMed] [Google Scholar]
- 5.Gebb SA. Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn. 2000;217:159–169. doi: 10.1002/(SICI)1097-0177(200002)217:2<159::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Schachtner SK. Wang Y. Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol. 2000;22:157–165. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark KR. Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Parera MC. van Dooren M. van Kempen M. de Krijger R. Grosveld F. Tibboel D. Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol. 2005;288:L141–L149. doi: 10.1152/ajplung.00148.2004. [DOI] [PubMed] [Google Scholar]
- 9.White AC. Lavine KJ. Ornitz DM. FGF9 and SHH regulate mesenchymal VEGFa expression and development of the pulmonary capillary network. Development (Cambridge, England) 2007;134:3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shifren JL. Doldi N. Ferrara N. Mesiano S. Jaffe RB. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J Clin Endocrinol Metab. 1994;79:316–322. doi: 10.1210/jcem.79.1.8027247. [DOI] [PubMed] [Google Scholar]
- 11.Roth-Kleiner M. Post M. Similarities and dissimilarities of branching and septation during lung development. Pediatr Pulmonol. 2005;40:113–134. doi: 10.1002/ppul.20252. [DOI] [PubMed] [Google Scholar]
- 12.Han RN. Liu J. Tanswell AK. Post M. Expression of basic fibroblast growth factor and receptor: immunolocalization studies in developing rat fetal lung. Pediatr Res. 1992;31:435–440. doi: 10.1203/00006450-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Akeson AL. Wetzel B. Thompson FY. Brooks SK. Paradis H. Gendron RL. Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn. 2000;217:11–23. doi: 10.1002/(SICI)1097-0177(200001)217:1<11::AID-DVDY2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Schniedermann J. Rennecke M. Buttler K. Richter G. Stadtler AM. Norgall S. Badar M. Barleon B. May T. Wilting J. Weich HA. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 2010;11:50. doi: 10.1186/1471-2121-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez DF. Huang L. King JA. ElZarrad MK. Yoder MC. Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol. 2008;294:L419–L430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 16.Jat PS. Noble MD. Ataliotis P. Tanaka Y. Yannoutsos N. Larsen L. Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edlund H. Pancreas: how to get there from the gut? Curr Opin Cell Biol. 1999;11:663–668. doi: 10.1016/s0955-0674(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 18.Wang S. Yan J. Anderson DA. Xu Y. Kanal MC. Cao Z. Wright CV. Gu G. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol. 2010;339:26–37. doi: 10.1016/j.ydbio.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sala FG. Del Moral PM. Tiozzo C. Alam DA. Warburton D. Grikscheit T. Veltmaat JM. Bellusci S. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development (Cambridge, England) 2011;138:273–282. doi: 10.1242/dev.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J. Zhang JJ. Moro A. Kushida M. Wegner M. Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239:514–526. doi: 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 21.Scotton CJ. Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 22.Arciniegas E. Frid MG. Douglas IS. Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 23.Torday JS. Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res. 2007;62:2–7. doi: 10.1203/PDR.0b013e31806772a1. [DOI] [PubMed] [Google Scholar]
- 24.Anglani F. Mezzabotta F. Ceol M. Cristofaro R. Del Prete D. D'Angelo A. The regenerative potential of the kidney: what can we learn from developmental biology? Stem Cell Rev. 2010;6:650–657. doi: 10.1007/s12015-010-9186-6. [DOI] [PubMed] [Google Scholar]
- 25.Psaltis PJ. Zannettino AC. Worthley SG. Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem cells (Dayton, Ohio) 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]