Abstract
Alders exhibit several uses in different areas and also offer some nutritional and medicinal values. The bark and leaves from black alder [Alnus glutinosa (L.) Gaertn] are used in folk medicine for the treatment of inflammatory processes and other health disorders. This study assessed if an extract of A. glutinosa stem bark exhibits some biological properties linked to improving the inflammatory state, which could partly justify its ethnopharmacological use. Therefore, various aspects of antioxidant activity as well as the effect on tumor necrosis factor-α (TNF-α) production were evaluated. The phytochemical study revealed the presence of terpenes, saponins, tannins, flavonoids, and anthraquinones (by high-performance thin-layer chromatography). The betulinic acid content in the extract, determined by reversed-phase high-performance liquid chromatography (validated method), was 0.72±0.027%. In addition, high amounts for total phenols as well as flavonoids were determined. The extract exhibited a 2,2′-diphenylpicrylhydrazyl radical scavenging capacity similar to that of ascorbic acid and had a significant effect on superoxide anion scavenging, superior to that of ascorbic acid. It was also able to protect HeLa cells from induced oxidative stress. In the TNF-α assay, levels of this citokine were depressed by the extract in HL-60 cells. To test the effect of the extract on cell proliferation, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed. According to the results, the antioxidant properties displayed by the extract of A. glutinosa stem bark, together with the effect on TNF-α levels, suggest that these activities, linked to a successful reduction in inflammatory processes, may support, in part, its ethnopharmacological use.
Key Words: black alder, free radical scavenging activities, reactive oxygen species, tumor necrosis factor-α
Introduction
Alnus glutinosa (L.) Gaertn. (Family Betulaceae), commonly known as “European alder,” native to several countries in northern Africa, temperate Asia, and Europe, is one of the approximately 30 species of trees and shrubs of the genus Alnus.1 Various types of secondary plant metabolites, including anthraquinones, phenolic glycosides, flavonol glycoside, terpenoids, xanthones, etc., have previously been reported in the barks, buds, leaves, and pollens of A. glutinosa.2
Alder bark is commonly used for cooking, being traditionally used for smoking fish and meat, and also offers some nutritional and medicinal value. Alder catkins are edible and high in protein, with a bitter and unpleasant taste.
The A. glutinosa stem bark (AGSB) is traditionally used as an astringent,3,4 cathartic, febrifuge,5 emetic (fresh),6 hemostatic, and tonic.7 In addition, a decoction of AGSB is used to treat swelling, inflammation,8 and rheumatism.9,10
As is well known, inflammation is associated with the progression of numerous diseases and is accompanied by the chronic release of cytokines and reactive oxygen species (ROS), which may be involved in increased tissue injury.11 Several studies have shown that the production of ROS, mainly superoxide anion radical (O2•−), occurs at the inflammation site and contributes to tissue damage.12 Moreover, in inflammatory cells, ROS contribute to the expression of a variety of different inflammatory cytokines such as tumor necrosis factor-α (TNF-α), which is considered to be a primary mediator of the inflammatory response.13
The above traditional usages suggest that AGSB may contain active metabolites related to the inflammation process. In this sense, the present study was designed to investigate whether AGSB extract shows some biological properties that can partly justify its use against the inflammatory state and to improve it. With this aim, we evaluated the capacity of free radical scavengers by two in vitro assays: the inhibition of ROS generation in H2O2-induced oxidative stress in cultured HeLa cells and the effect of the extract on production of the cytokine TNF-α from the HL-60 cell line.
Materials And Methods
Plant material
The stem bark of A. glutinosa (L.) Gaertn (Family Betulaceae) was harvested in San Agustín de Guadalix (Madrid, Spain) (40°41′ N, 3°36′ W) and identified in the Department of Botany (School of Pharmacy, CEU San Pablo University, Madrid). A voucher specimen (number 2642/09) has been deposited at the Herbarium of the School of Pharmacy, San Pablo University.
Methanolic extract from dried and powered AGSB was obtained under reflux in an extraction system (B-811, Buchi, Flawil, Switzerland) for 2 h and concentrated to dryness under vacuum using a rotary evaporator (Rotavapor R-200, Buchi).
Phytochemical screening
A preliminary phytochemical screening of the extract was carried out in order to investigate the presence of terpenes, antraquinones, tannins, alkaloids, saponins, and flavonoids. This determination was carried out as follows: tannins were identified with 1% gelatin solution, saponins by the froth test, and anthraquinones with 10% potassium hydroxide solution in methanol. Alkaloids were detected with Dragendorff's reagent in the alkaloid fraction obtained by a classical acid/base extraction procedure. Terpenes were analyzed by high-performance liquid chromatography (HPTLC) (Silicagel 60 plates, Merck, Darmstadt, Germany) with hexane:ethyl acetate:acetic acid (7:3:0.03 by volume) as the solvent system and detected with anisaldehyde spray reagent (heating for 10 min at 100°C). For flavonoids, HPLTC plates were developed in an ethyl acetate:acetic acid:formic acid:water (100:11:11:26 by volume) system, detected with natural products/polyethylene glycol spray reagent and visualizing in the ultraviolet (UV) (365 nm).
High-performance liquid chromatography profile and quantification of betulinic acid
Quantification of betulinic acid for standardization of the extract was done using reversed phase (RP) high-performance liquid chromatography (HPLC).14 A standard HPLC system (Kontron Instruments, Montigny-le-Bretonneux, France; 325 pump system) equipped with a UV detector (absorbance at 433 nm) and a model 465 autosampler was used. Data were acquired and processed by Kromasystem 2000 software (Kontron). Chromatographic analysis was performed by an isocratic elution with acetonitrile (analytical grade):water (86:14, vol/vol) in a Kromasil-C18 RP column (250 mm×4.6 mm i.d.; pore size, 5 μm). Betulinic acid was quantified with a UV detector at a wavelength of 210 nm. The flow rate and injection volume were 1.0 mL/min and 20 μL, respectively. The method used was fully validated according to the Internation Conference on Harmonization guidelines in terms of linearity, precision, and accuracy.15 A linear relationship for the response of the betulinic acid standard was proven. These validation results demonstrated the suitability of the method for the precise and accurate determination of betulinic acid in AGSB extract. Dried extract was dissolved with methanol for sample solution preparation. After filtration through a membrane filter (pore size, 0.45 μm), clean solution was injected into the HPLC system. All chromatographic operations were performed at ambient temperature. The identity of the peak of betulinic acid in the extract chromatogram was established by comparing retention time and UV spectra with the betulinic analytical standard (Sigma-Aldrich Quimica SA, Madrid) and confirmed by spiking with its standard. Quantification was done by the integration of the peak using the Kromasystem 2000 program.
Total phenolic and total flavonoid content
The concentration of total phenols in the extract was determined with Folin–Ciocalteau reagent, following the colorimetric method adapted by Lee et al.16 The extract (200 μL) was mixed with 1 mL of 10% Folin–Ciocalteau reagent and 800 μL of 7% Na2CO3 solution. Milli-Q (Millipore Corp., Bedford, MA, USA) water was used as the negative control. The mixture was kept for 2 h at room temperature before the absorbance was measured at 765 nm with a Shimadzu (Kyoto, Japan) model UV-1601 spectrophotometer. Measurements were performed in triplicate, and calculations were based on a calibration curve obtained with gallic acid. The levels of total phenols were expressed as milligrams of gallic acid equivalents per gram of dry weight.
Levels of flavones and flavonols in the extract were estimated as quercetin equivalents. The standard solutions or extract (0.2 mL) were mixed with 0.8 mL of water and 0.06 mL of 5% NaNO2. Five minutes later, 0.06 mL of 10% AlCl3 was added, and after 6 min, 0.4 mL of 1 M NaOH was added. After incubation of the reaction mixture at room temperature for 30 min, the absorbance was measured at 510 nm with a Shimadzu model UV-1601 spectrophotometer. Quercetin was used to obtain the calibration curve. The mean of three readings was used, and the total flavonoid content was expressed in milligrams of quercetin per gram of dry weight.17
Cell culture and assay for measuring cell proliferation
The human cervical carcinoma HeLa (ATCC CCL-2) and human promyelocytic leukemia HL-60 (ATCC CCL-240) cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The HeLa cell line was maintained grown in Eagle's minimal essential medium (Sigma) with 1% nonessential amino acids (×100) (Sigma). For the HL-60 cell line, RPMI 1640 medium (Gibco, Grand Island, NY, USA) was used. Both cultures were supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine, and cells were grown at 37°C in an atmosphere of 5% CO2 in humidified air.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay for measuring cell proliferation
The cytotoxic effect of the extract was evaluated by conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.18 In brief, in 96-well microplates (Costar, Corning® Life Sciences Inc., Lowell, MA, USA), the cells were treated with different concentrations of the AGSB extract, diluted in phosphate-buffered saline (PBS) solution, and incubated for 72 h. At 4 h prior to the end of the exposure period, 50 μL per well of MTT solution (1 mg/mL in PBS, pH 7.4) (Sigma) was added, and the cells were continuously cultured until termination. Then, dimethyl sulfoxide was added to each well for solubilization of formazan, a product generated from the viable cells, and the absorbance was measured at 570 nm with an Opsys MR™ automatic plate reader (Dynex Technologies, Chantilly, VA, USA). The dose concentration that inhibited 50% growth after the incubation period (IC50) was calculated. All the experiments were carried out in triplicate, and three independent experiments were performed for each sample.
Antioxidant capacity
Two in vitro assays were performed to assess the antioxidant activity of the AGSB extract and its ability to protect HL-60 cells against oxidative stress.
2,2′-Diphenylpicrylhydrazyl radical scavenging activity
The 2,2′-diphenylpicrylhydrazyl (DPPH) scavenging activity of the AGSB extract was measured from the bleaching of a purple-colored methanol solution of DPPH, which was used as a reagent in a spectrophotometric assay.19 For the assay, 100 μL of methanolic extract at different concentrations (10.0–50.0 μg/mL) was mixed with 100 μL of DPPH (1 mM) in microplate wells. The microplate was kept in the dark at room temperature for 20 min. Absorbance was read against a blank at 517 nm using a microplate reader (VersaMax™, Molecular Devices, Sunnyvale, CA, USA). All experiments were carried out in triplicate. Ascorbic acid was used as a positive control.20 Inhibition of free radical DPPH in percentage was calculated as scavenging activity (%): ([A0 – A1]/A1)×100, where A0 is the absorbance of the blank at 517 nm. The IC50 value (extract concentration required to scavenge 50% of DPPH radicals) was obtained through interpolation from linear analysis.
Superoxide anion scavenging assay
The xanthine–xanthine oxidase reaction is a suitable system for the generation of superoxide anion (O2•−).21 Using this reaction, we evaluated the ability of the AGSB extract to scavenge O2•− using the nitro blue tetrazolium (NBT) reduction assay.20 The reaction mixture contained 65 μL of buffer (50 nM KH2PO4/KOH, pH 7.4), 10 μL of Na2EDTA (15 mM), 15 μL of hypoxanthine (3 mM), 25 μL of NBT (0.6 mM), and 12.5 μL of various extract concentrations, all in phosphate buffer, pH 7.4. The reaction was started by adding 25 μL of xanthine oxidase (1 U/10 mL), and its rate was continuously monitored spectrophotometrically (Shimadzu model UV-1601) at 560 nm every 5 min for 40 min. A negative control was prepared without AGSB extract, and ascorbic acid was used as a positive control. The ability of the extract to scavenge O2•− was calculated as a percentage of the inhibition of NBT reduction in samples, with the extract compared with the control. Results are expressed as IC50 (concentration required to inhibit 50% of NBT reduction) and were obtained through interpolation from linear analysis. All experiments were carried out in triplicate. To ensure there was no NBT reduction due to extract compounds, a control was made by mixing the NBT solution with the extract in a phosphate buffer.
Measurement of ROS production
Measurement of intracellular ROS was based on ROS-mediated conversion of the nonfluorescent 2′,7′-dichlorofluorescein diacetate (DCFH-DA) into 2′,7′-dichlorofluorescin (DCFH).22 The intensity of fluorescence reflects enhanced oxidative stress. After a 24-h incubation, HeLa cells that had been seeded in black 96-well plates were washed with PBS (pH 7.4) and then incubated with DCFH-DA (20 μM) in PBS at 37°C for 30 min. At the end of incubation, PBS (control) and different concentrations of the AGSB extract were added, followed by 0.5 mM H2O2 (as the inducer for ROS production). Then, DCFH fluorescence of the cells from each well was measured every 30 min for 120 min, at an emission wavelength of 530 nm and an excitation wavelength of 485 nm,23 using a Fluostar Optima fluorescence plate reader (BMG Labtech GmbH, Ortenberg, Germany). The background was from cell-free conditions. Results were expressed as percentage of fluorescence intensity over control (nonstimulated HeLa cells).
TNF-α production
The effect of the extract at 50 μg/mL, 100 μg/mL, and 200 μg/mL on production of the cytokine TNF-α from HL-60 cells was determined after stimulation and intervention lasting 16 h with an enzyme-linked immunosorbent assay kit (Amersham Co., Little Chalfont, United Kingdom) according to the manufacturer's instructions. The following chemical inductors of TNFα synthesis were used: okadaic acid (OA) (50 μM) or 12-O-tetradecanoylphorbol 13-acetate (TPA) (20 μM), in the absence or presence of extracts. PBS was used as a negative control. Thalidomide (Sigma) (30 μM) was used as a positive control. Then cell number and cell morphology were evaluated microscopically. Cells were collected by centrifugation (0.3 g for 10 min), and the TNF-α supernatant concentration was quantified following the supplier's protocol. Five independent experiments were performed.
Results And Discussion
The traditional uses of AGSB prompted us to study an extract to analyze if it presents biological properties linked to inflammation that could partly justify its effects. To this aim, the ability of the AGSB extract to scavenge free radicals, its possible inhibition of ROS generation, and its effect on production of the cytokine TNF-α were evaluated.
Once the AGSB extract was obtained (the yield was 20.3% [wt/wt] of dried material), an initial phytochemical study using HPTLC was performed. This revealed the presence of terpenes, saponins, tannins, flavonoids, and anthraquinones. However, the screening was negative for alkaloids.
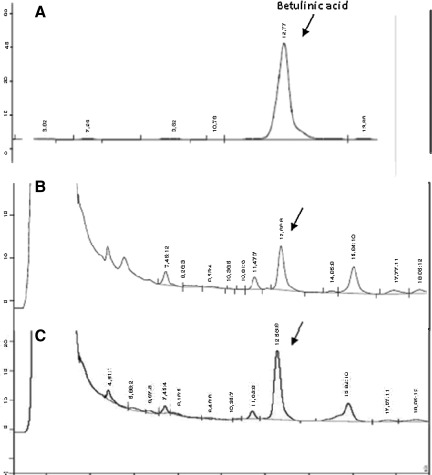
Furthermore, the HPLC profile and the quantification of betulinic acid, for standardization, were performed using RP-HPLC, following a validated method. Table 1 shows the main parameters obtained in the validation procedure. The resultant chromatograms are present in Figure 1A for betulinic acid, Figure 1B for the extract, and Figure 1C for the extract with betulinic acid added, in order to verify the presence of this compound. Betulinic acid was shown at the retention time of 12.66 min. The average content of betulinic acid found in AGSB extract was 0.72±0.027% (wt/wt) (n=3).
Table 1.
Main Validation Parameters of the Reversed-Phase High-Performance Liquid Chromatography Method Used for the Quantification of Betulinic Acid
| Parameter | Results |
|---|---|
| Linear range (μg/mL) | 10–80 |
| Correlation coefficient | 0.998 |
| Intercept | −0.0541±0.0277 |
| Slope | 0.0396±5.6×10−4 |
| LOQ (μg/mL) | 4.3 |
| LOD (μg/mL) | 1.3 |
| Intraday precision | |
| RSD (n=5, Day 1) | 1.584 |
| RSD (n=5, Day 2) | 1.553 |
| Interday precision RSD (n=10) | 2.883 |
| Recovery (% average) (19–70 μg/mL) | 102±10 |
LOD, limit of detection; LOQ, limit of quantification; RSD, relative SD.
FIG. 1.
HPLC profiles of (A) betulinic acid, (B) A. glutinosa stem bark extract, and (C) the extract with betulinic acid added. The retention time of the betulinic acid peak is indicated.
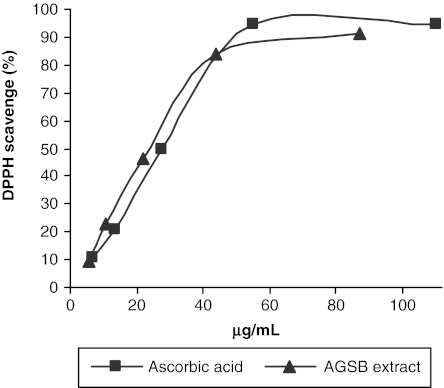
The determination of antioxidant activity of diverse biological samples is generally based on the inhibition of a particular reaction in the presence of antioxidants. The most commonly used methods are those involving chromogenic compounds of a radical nature: the presence of antioxidant leads to the disappearance of these radical chromogens. In order to assess the antioxidant capacity, the AGSB extract was first screened for its total phenolic content and flavonoid content. A high amount for both total phenols (508.86±12.97 mg of gallic acid/g of extract) and flavonoids (34.55±0.19 mg of quercetin/g of extract) was obtained. Then, the ability of the extract to scavenge free radicals was evaluated using two distinct in vitro assays. The stable DPPH radical scavenging model is a widely used method to estimate the antioxidant activity of complex mixtures such as plant extracts. The effect of the AGSB extract and that of the reference substance on the DPPH radical were found to be concentration dependent (Fig. 2). The assay results, expressed as IC50, indicate a free radical scavenging ability for the AGSB extract comparable to that of ascorbic acid. The effect reported by Middleton et al.24 for a methanolic extract of seeds of A. glutinosa (IC50=127 μg/mL) was lower than the AGSB extract activity.
FIG. 2.
2,2′-Diphenylpicrylhydrazyl (DPPH) free radical scavenging effects of A. glutinosa stem bark (AGSB) extract (▴) and ascorbic acid (▪, positive control). The 50% inhibition concentration values obtained were 26.50±0.35 μg/mL (extract) and 29.23±0.28 μg/mL (ascorbic acid).
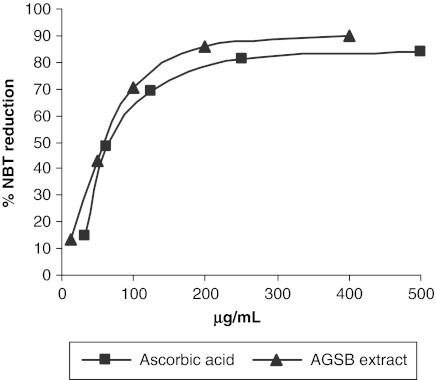
Additionally, we evaluated the ability of the AGSB extract to scavenge superoxide anion radical (O2•−) using the NBT reduction assay. The effect of AGSB extract and ascorbic acid on superoxide anion scavenging is shown in Figure 3. The IC50 (in μg/mL) values obtained suggest that the AGSB extract displays a significant capacity for scavenging O2•− radical, even better than ascorbic acid. Therefore, the results of both antioxidant assays may establish the radical scavenging ability of the AGSB extract. This activity is probably due to the flavonoid compounds, whose effectiveness as a free scavenging agent is well documented.25
FIG. 3.
Inhibition of nitro blue tetrazolium (NBT) reduction obtained for AGSB extract (▴) and ascorbic acid (▪, positive control). The 50% inhibition concentration values obtained were 66.11±0.17 μg/mL and 84.08±0.20 μg/mL, respectively.
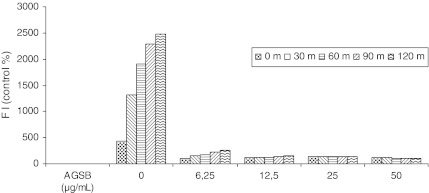
A link exists between antioxidants with respect to scavenging ROS and anti-inflammatory effects, and therefore they play an important role in the treatment of inflammatory diseases.11 Thus, we examined the inhibitory effect of the AGSB extract on ROS generation in H2O2-induced oxidative stress in cultured HeLa cells. Previously, the proliferation assay on the cell line showed that the extract did not significantly affect cell viability (data not shown). The intracellular ROS concentration was determined by measuring the intensity of DCFH fluorescence. When DCFH-DA-labeled cells were incubated in medium, a sudden increment in fluorescence intensity indicated the oxidation of DCFH-DA by intracellular radicals. The results were expressed as a percentage of control (nonstimulated HeLa cells) fluorescence intensity (Fig. 4). The production of DCFH fluorescence in HeLa cells with H2O2 (control group) increased significantly in a time-dependent way, which means a high ROS production, whereas preincubation with AGSB extract (6.25–50 μg/mL) significantly reduced the increased fluorescence induced by H2O2. This effect had already been clearly with the lower concentration of the extract. This suggests that the AGSB extract presents intracellular radical scavenging ability and therefore demonstrated a high protection level against oxidative stress induced by H2O2 in the HeLa cell line. However, we were unable to conclude as to whether the AGSB extract presented a direct quenching effect on H2O2-induced oxidant species or by other different mechanisms. Hence, further experiments should be performed to clarify this position.
FIG. 4.
Inhibitory effects of the AGSB extract, at different concentrations (in μg/mL), on the production of intracellular reactive oxygen species in cultured HeLa cells. The fluorescence intensity (FI) was measured every 30 min for 120 min.
Conversely, in inflammatory cells, ROS contribute to the expression of a variety of different inflammatory cytokines such as TNF-α, which is considered to be a primary mediator of the inflammatory response.13 Several compounds isolated from other plants but present in A. glutinosa (bark and leaves), such as emodin,26 lupeol,27 or glutinone,28 have demonstrated anti-inflammatory effects during in vivo or in vitro experiments. Some of these compounds had a marked effect on TNF-α levels.29
The effect of the AGSB extract on production of the cytokine TNF-α from HL-60 cells was determined. First, a proliferation assay on the cell line was conducted that showed that the extract did not significantly affect cell viability (data not shown). HL-60 cells do not produce detectable amounts of TNF-α under normal cell culture conditions but do begin to produce it in response to OA or TPA.30 Therefore, these chemical inductors of TNF-α synthesis were used. Thalidomide was used as a positive control, and cells treated with PBS (negative control) produced no detectable amount of TNF-α. The results, summarized in Table 2, were dose-dependent and indicate that the production of TNF-α induced by chemical promoters (OA, TPA) was depressed by the AGSB extract in HL-60 cells. The ability to inhibit the synthesis of TNF-α by natural31–33 or synthetic34,35 compounds has been proposed as a successful strategy in the reduction of the inflammatory processes and in different diseases directly related to the action of this cytokine. Some are related to its role as a mediator of the production of ROS.31,34 Numerous plant extracts have demonstrated the inhibitory action of this synthesis factor.
Table 2.
Tumor Necrosis Factor-α Concentration in the Culture Medium of HL-60 Cells Treated with A. glutinosa Stem Bark Extract in the Presence of Okadaic Acid or 12-O-Tetradecanoylphorbol 13-Acetate
| TNF-α (pg/mL) | |
|---|---|
| TPA (20 μM) | |
| Positive control + AGSB extract | 123.4±17.3 |
| 200 μg/mL | 53.8±7.9* |
| 100 μg/mL | 78.2±8.7* |
| 50 μg/mL | 102.5±11.5 |
| OA (50 μM) | |
| Positive control + AGSB extract | 582.5±49.6 |
| 200 μg/mL | 135.5±14.7* |
| 100 μg/mL | 284.2±37.3* |
| 50 μg/mL | 540.08±70.2 |
Data are mean±SD values of five replicates. Tumor necrosis factor-α (TNF-α) with phosphate-buffered saline (negative control) is under the kit's detection limit.
P<.05 versus 12-O-tetradecanoylphorbol 13-acetate (TPA) or okadaic acid (OA) (positive controls) by Student's t test.
In conclusion, the AGSB extract possesses a radical scavenging capacity, and it is able to protect cells from induced oxidative stress and may be able to decrease TNF-α levels. These biological properties are linked to a successful reduction in inflammatory processes and may support, in part, its ethnopharmacological use. The results obtained encourage us to pursue this line of investigation, and further research is required to evaluate other factors related to inflammation through the use of targets useful for developing new anti-inflammatory drugs and exploring their molecular mechanisms. Elucidating the implication of betulinic acid and other secondary metabolites of AGSB extract is also an objective.
Acknowledgments
We are grateful to the Universidad CEU San Pablo for financial support (grant USP-08/05) and to Brian Crilly for his language assistance in the preparation of this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mitchell A. Wilkinson J. Pareys Buch der Bäume. Nadel- und Laubbäume in Europa nördlich des Mittelmeeres. Blackwell Wissenschafts-Verlag; Berlin: 1997. pp. 150–288. [Google Scholar]
- 2.Dictionary of Natural Products. Chapman & Hall/CRC; Boca Raton, FL: 2001. [Google Scholar]
- 3.Chiej R. Encyclopaedia of Medicinal Plants. MacDonald; London: 1984. [Google Scholar]
- 4.Grieve MA. A Modern Herbal. Penguin; London: 1984. [Google Scholar]
- 5.Holtom JA. Hylton WH. Complete Guide to Herbs. Rodale Press; New York: 1979. [Google Scholar]
- 6.Lust J. The Herb Book. Bantam Book; New York: 1974. [Google Scholar]
- 7.Uphof JCT. Dictionary of Economic Plants. Verlag von J. Cramer; Lehre, Germany: 1968. [Google Scholar]
- 8.Grieve M. A Modern Herbal Alder. 2004. www.botanical.com/botanical/mgmh/a/alder019.html www.botanical.com/botanical/mgmh/a/alder019.html
- 9.Launert E. Guide to Edible and Medicinal Plants of Britain and Northern Europe. Hamlyn; London: 1981. [Google Scholar]
- 10.Chevalier A. The Encyclopedia of Medicinal Plants. Dorling Kindersley; London: 1996. [Google Scholar]
- 11.Conner EM. Grisham MB. Inflammation, free radicals and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 12.Salvemini D. Masferrer JL. Interactions of nitric oxide with cyclooxygenase: in vitro, ex vivo, and in vivo studies. Method Enzymol. 1996;269:12–25. doi: 10.1016/s0076-6879(96)69005-3. [DOI] [PubMed] [Google Scholar]
- 13.Almeida MV. Teixeira FM. de Souza MVN. Amarante GW. Alves CCS. Cardoso SH. Mattos AM. Ferreira AP. Teixeira HC. Thalidomide analogs from diamines: synthesis and evaluation as inhibitors of TNF-α production. Chem Pharm Bull. 2007;55:223–226. doi: 10.1248/cpb.55.223. [DOI] [PubMed] [Google Scholar]
- 14.Zhao G. Yan W. Cao D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J Pharmaceut Biomed. 2007;43:959–962. doi: 10.1016/j.jpba.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 15.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Methods: Definitions and Terminology. ICH Secretariat; Geneva: 2010. [Google Scholar]
- 16.Lee KW. Kim YJ. Lee HJ. Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 17.Zhishen J. Mengcheng T. Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects of superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 18.Borenfreund E. Babich H. Martin-Alguacil N. Comparison of two in vitro cytotoxicity assays: the neutral red (NR) and tetrazolium (MTT) tests. Toxicol in Vitro. 1988;2:1–6. doi: 10.1016/0887-2333(88)90030-6. [DOI] [PubMed] [Google Scholar]
- 19.Koleva J. Beek TA. Linssen J. Groot A. Evstatieva L. Screening of plant extracts for antioxidant activity: a comparative study of three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 20.McCune LM. Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus by the indigenous peoples of the North American boreal forest. J Ethnopharmacol. 2002;82:197–205. doi: 10.1016/s0378-8741(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM. Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 22.Chang MC. Ho YS. Lee PH. Chan CP. Lee JJ. Hahn LJ. Wang YJ. Jeng JH. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis. 2001;22:1527–1535. doi: 10.1093/carcin/22.9.1527. [DOI] [PubMed] [Google Scholar]
- 23.Carmody RJ. Cotter TG. Oxidative stress induces caspase independent retinal apoptosis in vitro. Cell Death Differ. 2000;7:282–291. doi: 10.1038/sj.cdd.4400646. [DOI] [PubMed] [Google Scholar]
- 24.Middleton P. Stewart F. Al-Qahtani S. Egan P. O'Rourke C. Abdulrahman A. Byres M. Middleton M. Kumarasamy Y. Shoe M. Naharb L. Delazar A. Dey Sarker S. Antioxidant, antibacterial activities and general toxicity of Alnus glutinosa, Fraxinus excelsior and Papaver rhoeas. Iran J Pharm Res. 2005;2:81–86. [Google Scholar]
- 25.Dudonné S. Vitrac X. Coutière P. Woillez M. Mérillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas G. Babykutty S. Sathiadevan PP. Srinivas P. Molecular mechanism of emodin action: transition from laxative to an antitumor agent. Med Res Rev. 2007;25:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]
- 27.Hodges ID. Kweifio-Okai G. Macrides TA. Antiprotease effect of anti-inflammatory lupeol esters. Mol Cell Biochem. 2003;252:91–96. doi: 10.1023/a:1025569805468. [DOI] [PubMed] [Google Scholar]
- 28.Bermejo BP. Abad MJ. Diaz AM. Villaescusa L. Gonzalez MA. Silvan AM. Sesquiterpenes from Jasonia glutinosa: in vitro anti-inflammatory activity. Biol Pharm Bull. 2002;25:1–4. doi: 10.1248/bpb.25.1. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y. Zhao L. Mei H. Zhang SL. Huang ZH. Duan YY. Ye P. Exploration of emodin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur J Pharmacol. 2008;590:377–386. doi: 10.1016/j.ejphar.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Braña MF. Acero N. Añorbe L. Muñoz Mingarro D. Llinares F. Domínguez G. Discovering a new analogue of thalidomide which may be used as a potent modulator of TNF-α production. Eur J Med Chem. 2009;44:3533–3542. doi: 10.1016/j.ejmech.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Hu C. Kitts DD. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine. 2002;12:588–597. doi: 10.1016/j.phymed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Kim N. Ussin L. Cheng X. Murali R. Sullivan KE. TNF-alpha inhibition in MRL/lpr mice ameliorates pulmonary but not renal disease. J Autoimmun. 2002;19:215–222. doi: 10.1006/jaut.2002.0617. [DOI] [PubMed] [Google Scholar]
- 33.Pae HO. Oh GS. Choi BM. Shin S. Chai KY. Oh H. Kim JM. Kim JM. Jang SI. Chung HT. Inhibitory effects of the stem bark of Catalpa ovata G. Don. (Bignoniaceae) on the production of tumor necrosis factor-alpha and nitric oxide by the lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2003;88:287–291. doi: 10.1016/s0378-8741(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim BH. Cheng EY. Ryu JC. Jung SH. Min KR. Kim Y. Anti-inflammatory mode of isoflavone glycoside sophoricoside by inhibition of interleukin-6 and cyclooxygenase-2 in inflammatory response. Arch Pharm Res. 2003;26:306–311. doi: 10.1007/BF02976960. [DOI] [PubMed] [Google Scholar]
- 35.Gavrin LK. Green N. Hu Y. Janz K. Kaila N. Li HQ. Tam SY. Thomason JR. Gopalsamy A. Ciszewski G. Cuozzo JW. Hall JP. Hsu S. Telliez JB. Lin LL. Inhibition of Tpl2 kinase and TNF-alpha production with 1,7-naphthyridine-3-carbonitriles: synthesis and structure-activity relationships. Bioorg Med Chem Lett. 2005;15:5288–5292. doi: 10.1016/j.bmcl.2005.08.029. [DOI] [PubMed] [Google Scholar]