Abstract
Royal jelly (RJ) is a honeybee product that contains proteins, carbohydrates, fats, free amino acids, vitamins, and minerals. RJ has been reported to have antitumor, antibacterial, and wound-healing activities. We previously reported that RJ enhanced the migration of human dermal fibroblasts and altered the levels of cholesterol and sphinganine in an in vitro wound-healing model in addition to regulating skin photoaging following exposure to ultraviolet-B radiation. We established an animal model of skin aging in the context of estrogen deficiency and assessed the antiaging effects of RJ on skin. To establish an in vivo model of skin aging, bilateral ovariectomies were performed in 12-week-old virgin female Sprague-Dawley rats. Induction of osteoporosis was confirmed through two-dimensional images of the trabecular bone in the left femoral necks using microcomputed tomography. The protective effects of RJ ovariectomy-induced skin aging were examined by determining the protein expression of type I procollagen and matrix metalloproteinase (MMP)-1. The collagen content and epidermal thickness of skin tissue were measured by staining techniques. There was a significant difference in weight between sham-operated and ovariectomized groups. Food efficiency ratio did not differ significantly among the groups. The level of procollagen type I protein was increased in the dorsal skin of ovariectomized rats fed with a dietary supplement containing 1% RJ extract, but the level of MMP-1 was not altered. In particular, the amount of collagen recovered was close to the normal level. RJ may protect against skin aging by enhancing collagen production in rats with ovariectomy-induced estrogen deficiency.
Key Words: collagen, estrogen deficiency, royal jelly, skin aging
Introduction
Skin aging is a complex biological process that is a consequence of intrinsic aging that occurs over time and extrinsic aging caused by environmental factors such as ultraviolet (UV) radiation. Intrinsic aging of the skin is largely genetically determined and is clinically associated with increased fragility and loss of elasticity. These effects are enhanced by extrinsic aging and hormonal aging.1–4 The importance of intrinsic skin aging is also increasing. Hormones, which include the growth hormone/insulin-like growth factor-I (IGF-I) axis and sex hormones, namely, estradiol, testosterone, and dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S), contribute to the skin aging process.5,6 Skin is one of the most important nonreproductive target organs of estrogen, which is known to play an essential role in regulating skin maintenance and turnover.7,8 In postmenopausal women, estrogen deficiency leads to decreased skin thickness and collagen content, together with increased dryness, elasticity, and wrinkling in skin.9,10 Several studies have shown that these symptoms can be reversed by estrogen replacement therapy (ERT).11–13 ERT also has a similar effect on wound contraction in ovariectomized rats.14 ERT has been associated with increased collagen synthesis and reduced collagen degradation, and estrogen has been implicated as a modulator of matrix metalloproteinase (MMP) production and function.15,16 Although ERT has been used for many years, recent trials have reported significantly increased risks of breast cancer and other pathologies with this treatment.17 Many studies are being performed to discover effective and nontoxic materials with estrogen-like activity to replace estrogen in therapies for postmenopausal women. Many natural products with estrogen-like effect include the following: Piper sarmentosum,18 Cirsium japonicum,19 olive oil,20 and soy isoflavone.21 We also previously reported the protective effects of apigenin, as a new material associated with ERT, on osteoporosis in ovariectomized rats as a postmenopausal bone loss model.22
Royal jelly (RJ), a yellowish material secreted from the hypopharyngeal and mandibular glands of worker-caste (nurse) honeybees, is a widely consumed health tonic with various perceived benefits. RJ consists of proteins, carbohydrates, fats, free amino acids, vitamins and minerals, and significant amounts of bioactive substances such as unsaturated fatty acids of 10-hydroxy-2-decenoic (10H2DA), 3,10-dihydroxydecanoic, and sebacic acids.23,24 Pharmacologically, RJ displays vasodilative, hypotensive, antitumor, antihypercholesterolemic, anti-inflammatory, and antioxidative activities. The major fatty acid component of RJ, 10H2DA, has antitumor, collagen synthetic, and MMP-inhibitory activities.25–27 In a previous study, we demonstrated that RJ enhanced the migration of human fibroblasts and increased the level of sphingolipids in an in vitro wound-healing model.28 Furthermore, we revealed that RJ protected human skin fibroblasts against ultraviolet-B (UVB)-induced photoaging by enhancing collagen production.29 RJ and RJ-derived fatty acids have also been shown to exert estrogen-like effects in vitro and in vivo, similar to those evoked by 17b-estradiol (E2).30,31 Estrogen deficiency following ovariectomy results in significant decreases in the level of vascular endothelial growth factor (VEGF) gene expression in the uterus. RJ treatment resulted in an increase in VEGF gene expression above the level of a sham-operated group. These findings suggest that RJ has antiphotoaging and estrogenic activities in skin. However, the effects of RJ on skin aging due to estrogen deficiency have not been reported. In this study, we established an animal model of skin aging due to estrogen deficiency induced by ovariectomy in rats. The protective effects of RJ on the elasticity and epidermal thickness of skin in a model of menopause-induced aging were assessed.
Materials and Methods
Reagents
Distilled water and acetonitrile were purchased from Burdick & Jackson, SK Chemicals; 10H2DA, from Nacalai USA, Inc.; and trifluoroacetic acid, from Alfa Aesar. All chemicals and solvents were of high-performance liquid chromatography (HPLC) grade and are commercially available. Fresh RJ was obtained from the RDA and a voucher specimen (KHUKSY-HPOP001 or KHUKSY-HOOC001) was deposited in the herbarium at the Department of Medical Science, Graduate School of East-West Medical Science, Kyung Hee University (Yongin-si, Korea).
Quantitative analysis of 10H2DA from RJ by HPLC
The standardized major bioactive component of RJ, 10H2DA, was determined by HPLC (Agilent 1100 HPLC System; Agilent Technologies, Inc.) with a Symmetry C18 column (250 mm×4.6 mm i.d., S-4 μm, 80 Å; YMC Co., Ltd.). The standards and samples were separated using a linear gradient consisting of acetonitrile (A) and water (B) under the following conditions: 0–20 minutes, 30–100% A; 20–25 minutes, 100% A with a run time of 25 minutes. The detection wavelength was set at 210 nm. The flow rate was 1.0 mL/min and the column was maintained at 25°C. The injection volume for all test samples and standards was 10 μL.
Sample collection
Fresh RJ was obtained from the RDA and a voucher specimen (KHUKSY-HPOP001 or KHUKSY-HOOC001) was deposited in the herbarium at the Department of Medical Science, Graduate School of East-West Medical Science, Kyung Hee University (Youngin, Korea).
Animal treatment and biological sampling
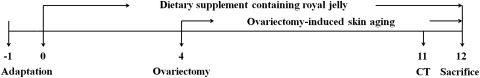
Twelve-week-old female Sprague-Dawley rats (155±4 g each) were purchased from Central Lab Animal, Inc. (Seoul, Korea). The animals were kept in an air-conditioned room at 22°C±1°C and 60%±5% humidity under a 12-hour-light/12-hour-dark cycle with free access to food pellets and drinking water throughout the experiment. After 1 week of acclimation, the rats were divided into four groups using a randomized block design in accordance with body weight: SHAM (sham-operated rats that were fed a control diet, n=12), OVX (ovariectomized rats that were fed a control diet, n=11), OC (ovariectomized rats that were fed a diet containing 1% RJ from Cheorwon, n=12), and OP (ovariectomized rats that were fed a diet containing 1% RJ from Pocheon, n=12). The experimental schedule is described in Figure 1. The animals in each group were maintained on experimental diets for 12 weeks. The compositions of the experimental diets are shown in Table 1. Dietary fat was supplied as corn oil and fixed at 10% of the dietary weight. To investigate food efficiency ratio (FER) from each group, body weight and food intake values were measured once per week during the experimental period. In vivo microcomputed tomography (micro-CT) was used to quantify alterations in bone volume and bone mineral density in the same animals at 7 weeks after OVX surgery. Rats were ovariectomy induced or sham operated after 4 weeks from the beginning of the dietary supplementation. After 12 weeks of dietary supplementation, the rats were anesthetized with chloral hydrate solution (0.3 mL/kg; Sigma-Aldrich). Exsanguination was performed by cardiac puncture to collect blood, followed by cervical dislocation. Blood serum samples were harvested for assays after centrifugation. The backs of the rats were shaved using clippers, and dorsal skin samples were collected. The serum and skin samples were promptly frozen at −80°C until use.
FIG. 1.
Experimental design.
Table 1.
Composition of Diet (g/kg Dry Diet)
| |
Experimental groups |
|||
|---|---|---|---|---|
| SHAM (n=12) | OVX (n=11) | OC (n=12) | OP (n=12) | |
| Casein | 230 | 230 | 230 | 230 |
| L-cystine | 3 | 3 | 3 | 3 |
| Corn oil | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 |
| Vitamin mix | 10 | 10 | 10 | 10 |
| Mineral mix | 35 | 35 | 35 | 35 |
| Sucrose | 200 | 200 | 200 | 200 |
| Corn starch | 372 | 372 | 362 | 362 |
| Royal jelly (Cheorwon) | — | — | 10 | — |
| Royal jelly (Pocheon) | — | — | — | 10 |
Group SHAM, SD rats control diet only; group OVX, ovariectomized rats fed control diet; groups OC and OP, ovariectomized rats fed diets containing 1% royal jelly originated from Cheorwon and Pocheon, respectively.
The experimental protocol [KHUASP-08-008] was approved by the Institutional Animal Care and Use Committee of Kyung Hee University.
Zoom-in micro-CT analysis
Induction of estrogen-deficiency-induced osteoporosis was confirmed through two-dimensional images of the trabecular bone in the left femoral necks by micro-CT, 7 weeks after ovariectomy. Three-dimensional tomographic images were taken by micro-CT according to the previously published method.32 Rats were anesthetized (1.5% isofluorane, 70% N2O, and 30% O2 gas) and maintained on anesthetic gases for the duration of each measurement. The 3D images (512×512×512) were reconstructed from 450 projection data and the isotropic pixel resolution was 25 μm.
Analysis of alkaline phosphatase activity and osteocalcin level in rat blood serum
Alkaline phosphatase (ALP) activity was determined by using a commercial alkaline phosphatase kit (LabAssay™ ALP; Wako Pure Chemical Industries, Ltd.). Quantitative analysis of osteocalcin was performed at a professional facility (Seoul Medicinal Science Institute).
Histological analysis
Skin tissue samples were fixed in 4% para-formaldehyde and embedded in paraffin. Sections that were 6 μm thick were subjected to hematoxylin and eosin (H&E) and Masson's trichrome staining. Dermal thickness was assessed in the H&E sections and collagen content was evaluated in the Masson's trichrome sections, which allowed areas of mature collagen deposition to be detected.
Western blot analysis
Frozen skin tissues were homogenized in cell lysis buffer and the proteins were subjected to western blot analysis. To investigate the expression of procollagen type I and MMP-1 proteins in skin tissue, 40 μg of total protein extracts was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech). The membrane was blocked with 5% skim milk and incubated with primary antibodies (procollagen type I, Santa Cruz Biotechnology, Inc.; MMP-1, Millipore). After washing with Tris-buffered saline Tween-20, horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) were applied. The blots were developed using ECL western blotting detection reagents (Amersham Pharmacia Biotech).
Statistical analysis
All data were analyzed using Statistical Analysis System software (PASW Statistics 18.0; SPSS, Inc.), and are presented as the mean±SE or ±SEM. Statistically significant differences were evaluated using Duncan's multiple-range test. Statistical significance was assigned at P<.05.
Results
Quantitative analysis of 10H2DA from RJ
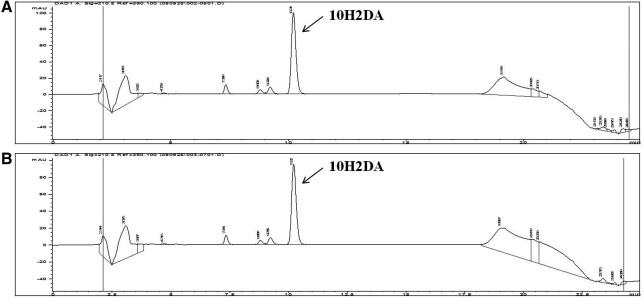
The content of 10H2DA, a main fatty acid from RJ, was measured by HPLC. The retention time of the 10H2DA standard compound was 10.229 minutes (Fig. 2). RJ from Cheorwon and Pocheon contained 2.114±0.0065 μg/g (0.211%) and 2.019±0.0080 μg/g (0.202%) of 10H2DA, respectively. The contents were not significantly different between samples.
FIG. 2.
High-performance liquid chromatography chromatograms of (E)-10-hydroxy-2-decenoic acid (10H2DA) of royal jelly from Cheorwon (A) and from Pocheon (B).
Body weight gain and the FER
To examine the antiaging effects of RJ on estrogen-deficiency-induced skin in vivo, ovariectomy-induced rats were orally administrated 1% of RJ in the base diet for 12 weeks. The body weight and food consumption in each group were measured every week. There were no significant differences in body weight between groups at the beginning of the experimental period. After 12 weeks, the body weights in SHAM and OVX groups were significantly different, 169.50±11.03 and 221.17±14.40 g, respectively. In contrast, the body weights in the ovariectomized groups (groups OVX, OC, and OP) did not differ significantly. Food intakes (g/week) in each group were 1349.57±300.39, 1632.67±311.17, 1369.96±289.35, and 1344.29±274.95 g, respectively. The FER (body weight [g]/food intake [g]) was higher in groups OP and OC than in the SHAM and OVX groups, but the results were not statistically significant (Table 2).
Table 2.
Body Weight Gain, Food Intake, and Food Efficiency Ratio of Experimental Rats
| Group | SHAM | OVX | OC | OP |
|---|---|---|---|---|
| Initial weight (g) | 155.8±2.39a | 153.3±1.94a | 155.7±3.07a | 155.5±2.51a |
| Final weight (g) | 325.3±10.44b | 374.4±15.85a | 395.7±10.13a | 385.8±8.75a |
| Weight gain (g) | 169.5±11.03b | 221.2±14.40a | 240.0±7.03a | 230.3±8.48a |
| Food intake (g/week) | 112.5±25.03 | 148.4±28.29 | 114.2±24.11 | 112.0±22.91 |
| FER | 0.149±0.3333a | 0.148±0.0282a | 0.209±0.0440a | 0.204±0.0417a |
All values are mean±SEM (groups SHAM, OC, and OP: n=12; group OVX: n=11). FER=[gain of body weight (g)/week]/[amount of food intake (g)/week].
Means with different letters differ at P<.05 level by ANOVA and Duncan's multiple range test.
Group SHAM, SD rats control diet only; group OVX, ovariectomized rats fed control diet; groups OC and OP, ovariectomized rats fed diets containing 1% royal jelly originated from Cheorwon and Pocheon, respectively, for 12 weeks (4 weeks before OVX and 8 weeks after OVX); FER, food efficiency ratio.
Confirmation of skin aging in ovariectomized rats
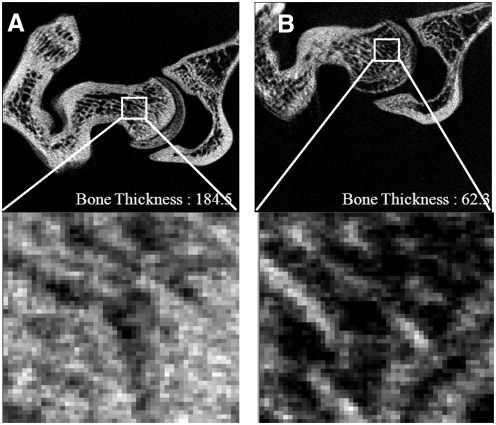
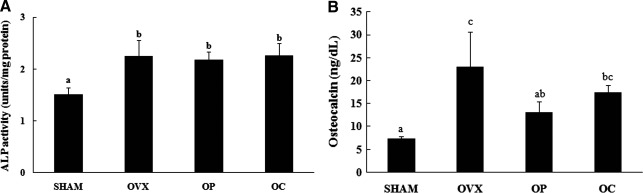
Estrogen-deficiency-induced osteoporosis was induced through ovariectomy in rats. Seven weeks after ovariectomy, two-dimensional images of the femoral neck showed significant differences in trabecular architecture between the SHAM and OVX groups (Fig. 3). While serum ALP activity (Fig. 4A) and osteocalcin level (Fig. 4B) were both higher in the OVX group than in the SHAM group, osteocalcin concentration was significantly decreased in the OC group compared with the OVX group at the end of the 12-week study. There were no significant differences between the OVX group and the RJ-treated groups in ALP activity. Based on the result of micro-CT analysis and changes of bone formation markers, osteocalcin and bone-specific ALP, we confirmed the induction of osteoporosis in a rat of ovariectomy.
FIG. 3.
Two-dimensional images of trabecular bone in the femoral necks of the SHAM (A) and OVX (B) groups using microcomputed tomography parameters.
FIG. 4.
Alkaline phosphatase (ALP) activities (A) and osteocalcin levels (B) in sham-operated rats that were fed a control diet only (SHAM) and ovariectomized rats that were fed a control diet (OVX), a diet including royal jelly from Pocheon (OP), and a diet including royal jelly from Cheorwon (OC) for 12 weeks. All values are mean±SEM (n=12 for SHAM, OP, and OC; n=11 for OVX). The data were evaluated for statistical significance with one-way ANOVA followed by Duncan's multiple range test. abcMeans with different letters are statistically different.
Changes in epidermal thickness and collagen
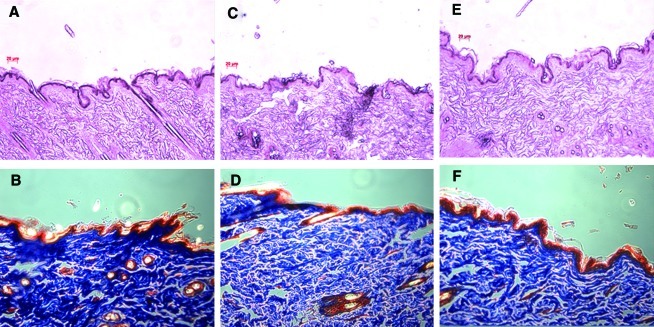
Masson's trichrome staining and H&E staining were applied to evaluate collagen content and epidermal thickness according to dietary supplementation with 1% RJ in ovariectomized estrogen-deficient rats. Figure 5A, C, E, G showed representative histological sections before and after dietary supplementation. The histological structures of rat skin were studied in the SHAM, OVX, OC, and OP groups. The variation of epidermal thickness in each group was a little different after dietary supplementation for 12 weeks.
FIG. 5.
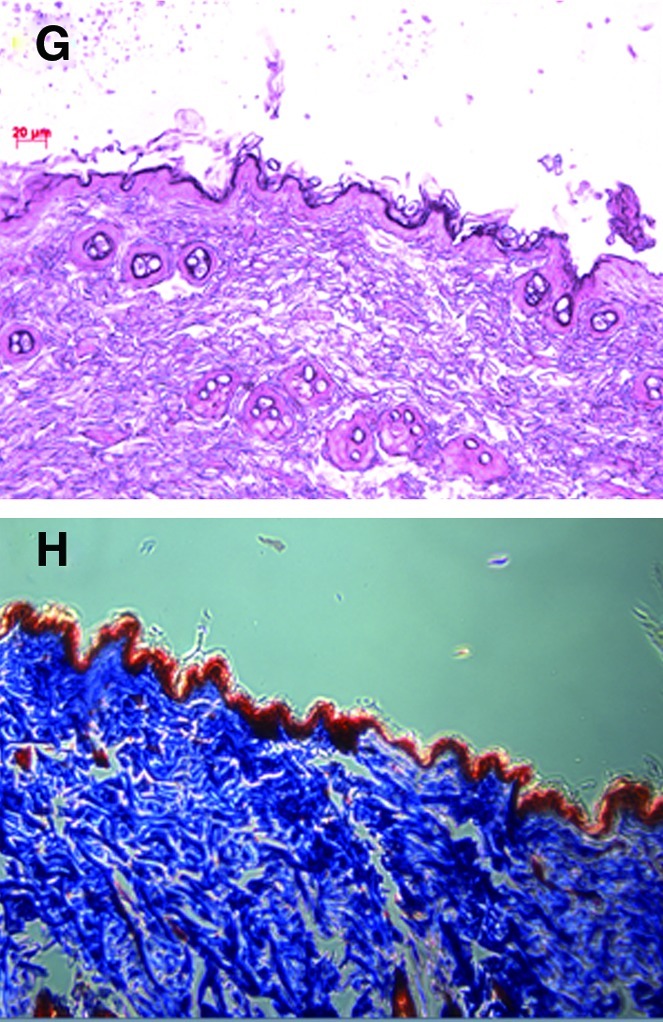
(A, C, E, G) The histological appearance and (B, D, F, H) Masson's trichrome staining of (A, B) sham-operated rats that were fed a control diet only (SHAM), and (C, D) ovariectomized rats that were fed a control diet (OVX) and diets including royal jelly from (E, F) Pocheon (OP) and (G, H) Cheorwon (OC) for 12 weeks. Color images available online at www.liebertonline.com/jmf
Based on the results of Masson's trichrome staining, the amounts of collagen were significantly decreased in the OVX group compared with the SHAM group (Fig. 5B, D, F, H). Collagen levels in the OC group almost recovered to the value of the SHAM group after being fed RJ from Cheorwon.
Changes in procollagen type I and MMP-1 protein levels
To investigate the mechanism underlying the effects of RJ in ovariectomized rat skin, the protein expression of procollagen type I and MMP-1 were analyzed by western blot analysis (Fig. 6). A significant difference in the level of procollagen type I protein was detected in skin samples from the SHAM and OVX groups. The OVX group showed lower levels of procollagen type I protein than the SHAM group, whereas rats that received RJ, particularly the OP group, showed a greater increase than the OVX group. MMP-1 protein levels did not change among the groups. Therefore, dietary supplementation with 1% RJ suppresses skin aging through the regulation of collagen synthesis in ovariectomized rats.
FIG. 6.
Expression of procollagen type I and matrix metalloproteinase (MMP)-1 proteins in rats fed a control diet only (SHAM) and ovariectomized rats fed a control diet (OVX), and diets including royal jelly from Pocheon (OP) and Cheorwon (OC) for 12 weeks.
Discussion
We created an animal model of skin aging due to estrogen deficiency following ovariectomy and verified osteoporosis by observing 2D images of the trabecular bone in the neck of the femur using micro-CT. Biochemical markers such as serum ALP activity and osteocalcin levels were measured. The trabecular thickness, which was measured by zoom-in tomography, was significantly different between the SHAM and OVX groups (Fig. 2). In ovariectomized rats, serum ALP activity and osteocalcin level were increased in comparison with the SHAM group (Fig. 3).
Ovariectomized experimental animals are widely used as an experimental model of female menopause. Ovariectomy increases the concentrations of serum ALP and osteocalcin, as well as biochemical markers of bone formation due to increases in the exchange ratio of bone.33,34 Ovariectomy is sufficient for accelerating spontaneous skin aging and stimulating UV-irradiation-induced photoaging of murine skin.35 Moreover, in ovariectomized rats, the induction of osteoporosis leads to severe structural alterations in skin and bone collagen in parallel with hypoestrogenism.36 The results of the present study agree with those of previous studies. Collagen content and protein levels of procollagen type I significantly decreased in the dorsal skin of rats after ovariectomy (Figs. 5E–H and 6). Furthermore, we examined the effects of RJ on collagen content, and MMP-1 and protein levels of procollagen type I in established models of skin aging. RJ did not affect FER among the experimental groups. Therefore, the effects of RJ did not depend upon amounts of food or energy intake. A dietary supplement of 1% RJ from Cheorwon or Pocheon in ovariectomized rats resulted in a significant decrease in serum osteocalcin levels compared with the OVX group, but not ALP activities. In addition, RJ increased the levels of collagen and procollagen type I protein in the OVX group, but did not alter MMP-1 production. These results are consistent with those of our previous study on the effects of RJ on UVB-induced photoaging of the skin in vitro.29 We hypothesized that increases in collagen content and type I procollagen by RJ represent the synergistic interactions of RJ components, especially unsaturated fatty acids. Evidence also suggests that RJ and some of the RJ-derived fatty acids, in particular 10H2DA, possess the ability to promote collagen production by inducing transforming growth factor-β1 (TGF-β1) production,26,37 suppressing MMP activity through the inhibition of p38 and JNK-AP-1 signaling pathways, and providing estrogenic effects.26,27,30 Estrogen has several pharmacological functions, such as the maintenance of bone mass and protection from cardiovascular and neurodegenerative diseases. Estrogen also promotes collagen production by stimulating the secretion of TGF-β1, increasing the elasticity and firmness of the skin, while decreasing wrinkle depth and pore size.38 Surazynski et al.39 studied how estradiol increases estrogen receptor (ER) mRNA levels, prolidase activity, and IGF-I receptor expression, all of which influence collagen biosynthesis in human skin fibroblasts. All things considered, the estrogenic effect of RJ maybe mediated by the mechanism of TGF-β and ER signaling. The estrogen deprivation that accompanies menopause exacerbates the deleterious effects of both intrinsic and environmental aging.40 ERT has an obvious, visible effect on the skin and is efficient for combating skin aging.13,41 However, long-term ERT is associated with increased risk of cancer in estrogen target tissues.42 RJ has potential as a regulator of skin aging due to estrogen deficiency after menopause and does not have remarkable side effects. Unfortunately, we were unable to explore mechanisms of action in this study. Therefore, further studies are required to thoroughly investigate the mechanisms of the estrogen-like effects of RJ against skin aging.
Acknowledgement
This work was supported by a grant from the BioGreen 21 Program (No. 20090801-060-001-001-02-00: PJ007178), Rural Development Administration, Republic of Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 2.Rabe JH. Mamelak AJ. McElgunn PJ. Morison WL. Sauder DN. Photoaging: Mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.McCullough JL. Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323–331. doi: 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- 4.Karol MH. How Environmental agents influence the aging process. Biomol Ther. 2009;17:113–124. [Google Scholar]
- 5.Kassira N. Glassberg MK. Jones C, et al. Estrogen deficiency and tobacco smoke exposure promote matrix metalloproteinase-13 activation in skin of aging B6 mice. Ann Plast Surg. 2009;63:318–322. doi: 10.1097/SAP.0b013e318184ac15. [DOI] [PubMed] [Google Scholar]
- 6.Makrantonaki E. Schönknecht P. Hossini AM, et al. Skin and brain age together: The role of hormones in the ageing process. Exp Gerontol. 2010;45:801–813. doi: 10.1016/j.exger.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Phillips TJ. Demircay Z. Sahu M. Hormonal effects on skin aging. Clin Geriatr Med. 2001;17:661–672. doi: 10.1016/s0749-0690(05)70092-6. [DOI] [PubMed] [Google Scholar]
- 8.Emmerson E. Campbell L. Ashcroft GS. Hardman MJ. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol Cell Endocrinol. 2010;321:184–193. doi: 10.1016/j.mce.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Sumino H. Ichikawa S. Abe M, et al. Effects of aging and postmenopausal hypoestrogenism on skin elasticity and bone mineral density in Japanese women. Endocr J. 2004;51:159–164. doi: 10.1507/endocrj.51.159. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson S. Nelson LD. Sharpe DT. Thornton MJ. 17β-estradiol regulates the secretion of TGF-β by cultured human dermal fibroblasts. J Biomater Sci Polym Ed. 2008;19:1097–1109. doi: 10.1163/156856208784909354. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs KO. Solis O. Tapawan R. Paranjpe J. The effects of an estrogen and glycolic acid cream on the facial skin of postmenopausal women: a randomized histologic study. Cutis. 2003;71:481–488. [PubMed] [Google Scholar]
- 12.Sator PG. Sator MO. Schmidt JB, et al. A prospective, randomized, double-blind, placebo-controlled study on the influence of a hormone replacement therapy on skin aging in postmenopausal women. Climacteric. 2007;10:320–334. doi: 10.1080/13697130701444073. [DOI] [PubMed] [Google Scholar]
- 13.Sator PG. Schmidt JB. Sator MO. Huber JC. Hönigsmann H. The influence of hormone replacement therapy on skin ageing: A pilot study. Maturitas. 2001;39:43–55. doi: 10.1016/s0378-5122(00)00225-5. [DOI] [PubMed] [Google Scholar]
- 14.Calvin M. Dyson M. Rymer J. Young SR. The effects of ovarian hormone deficiency on wound contraction in a rat model. Br J Obstet Gynaecol. 1998;105:223–227. doi: 10.1111/j.1471-0528.1998.tb10057.x. [DOI] [PubMed] [Google Scholar]
- 15.Claassen H. Steffen R. Hassenpflug J, et al. 17β-estradiol reduces expression of MMP-1, -3, and -13 in human primary articular chondrocytes from female patients cultured in a three dimensional alginate system. Cell Tissue Res. 2010;342:283–293. doi: 10.1007/s00441-010-1062-9. [DOI] [PubMed] [Google Scholar]
- 16.Voloshenyuk TG. Gardner JD. Estrogen improves TIMP–MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am J Physiol Regul Integr Comp Physiol. 2010;299:R683–R693. doi: 10.1152/ajpregu.00162.2010. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE. Anderson GL. Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Estai MA. Suhaimi FH. Das S, et al. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: A radiological study. Clinics (Sao Paulo) 2011;66:865–872. doi: 10.1590/S1807-59322011000500025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon HY. Rhyu MR. Lee YJ. The effects of Cirsium japonicum on lipid profile in ovariectomized rats. Biomol Ther. 2008;16:293–298. [Google Scholar]
- 20.Saleh NK. Saleh HA. Olive oil effectively mitigates ovariectomy-induced osteoporosis in rats. BMC Complement Altern Med. 2011;11:10. doi: 10.1186/1472-6882-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shedd-Wise KM. Alekel DL. Hofmann H, et al. The soy isoflavones for reducing bone loss study: 3-yr effects on pQCT bone mineral density and strength measures in postmenopausal women. J Clin Densitom. 2011;14:47–57. doi: 10.1016/j.jocd.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JA. Ha SK. Kang TH, et al. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci. 2008;82:1217–1223. doi: 10.1016/j.lfs.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Takaki-Doi S. Hashimoto K. Yamamura M. Kamei C. Antihypertensive activities of royal jelly protein hydrolysate and its fractions in spontaneously hypertensive rats. Acta Med Okayama. 2009;63:57–64. doi: 10.18926/AMO/31859. [DOI] [PubMed] [Google Scholar]
- 24.Izuta H. Chikaraishi Y. Shimazawa M. Mishima S. Hara H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid Based Complement Alternat Med. 2009;6:489–494. doi: 10.1093/ecam/nem152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii A. Kobayashi S. Kuboyama N, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn J Pharmacol. 1990;53:331–337. doi: 10.1254/jjp.53.331. [DOI] [PubMed] [Google Scholar]
- 26.Koya-Miyata S. Okamoto I. Ushio S. Iwaki K. Ikeda M. Kurimoto M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci Biotechnol Biochem. 2004;68:767–773. doi: 10.1271/bbb.68.767. [DOI] [PubMed] [Google Scholar]
- 27.Yang XY. Yang DS. Wei-Zhang , et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J Ethnopharmacol. 2010;128:314–321. doi: 10.1016/j.jep.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Kim J. Kim Y. Yun H, et al. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr Res Pract. 2010;4:362–368. doi: 10.4162/nrp.2010.4.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HM. Hwang E. Lee KG. Han SM. Cho Y. Kim SY. Royal jelly protects against UVB-induced photoaging in human skin fibroblasts via enhancing collagen production. J Med Food. 2011;14:899–906. doi: 10.1089/jmf.2010.1363. [DOI] [PubMed] [Google Scholar]
- 30.Mishima S. Suzuki KM. Isohama Y, et al. Royal jelly has estrogenic effects in vitro and in vivo. J Ethnopharmacol. 2005;101:215–220. doi: 10.1016/j.jep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Moutsatsou P. Papoutsi Z. Kassi E, et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS One. 2010;5:e15594. doi: 10.1371/journal.pone.0015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho MH. Chun IK. Lee SC. Cho MH. Lee SY. Trabecular thickness measurement in cancellous bones: Postmortem rat studies with the zoom-in micro-tomography technique. Physiol Meas. 2005;26:667–676. doi: 10.1088/0967-3334/26/5/008. [DOI] [PubMed] [Google Scholar]
- 33.Kim SK. Lee MH. Rhee MH. Studies on the effects of biomedicinal agents on serum concentration of Ca2+, P and ALP activity in osteoporosis-induced rats. J Vet Sci. 2003;4:151–154. [PubMed] [Google Scholar]
- 34.Li XX. Hara I. Matsumiya T. Effects of osthole on postmenopausal osteoporosis using ovariectomized rats; comparison to the effects of estradiol. Biol Pharm Bull. 2002;25:738–742. doi: 10.1248/bpb.25.738. [DOI] [PubMed] [Google Scholar]
- 35.Tsukahara K. Nakagawa H. Moriwaki S, et al. Ovariectomy is sufficient to accelerate spontaneous skin ageing and to stimulate ultraviolet irradiation-induced photoageing of murine skin. Br J Dermatol. 2004;151:984–994. doi: 10.1111/j.1365-2133.2004.06203.x. [DOI] [PubMed] [Google Scholar]
- 36.Kafantari H. Kounadi E. Fatouros M. Milonakis M. Tzaphlidou M. Structural alterations in rat skin and bone collagen fibrils induced by ovariectomy. Bone. 2000;26:349–353. doi: 10.1016/S8756-3282(99)00279-3. [DOI] [PubMed] [Google Scholar]
- 37.Koya-Miyata S. Takei Y. Ushio S. Iwaki K. Ikeda M. Kurimoto M. Royal jelly and ascorbic acid 2-O-alpha-glucoside (AA-2G) increase collagen production in normal hamster skin fibroblast cultures. Nat Med. 2002;56:191–194. (In Japanese.) [Google Scholar]
- 38.Schmidt JB. Binder M. Demschik G. Bieglmayer C. Reiner A. Treatment of skin aging with topical estrogens. Int J Dermatol. 1996;35:669–674. doi: 10.1111/j.1365-4362.1996.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 39.Surazynski A. Jarzabek K. Haczynski J. Laudanski P. Palka J. Wolczynski S. Differential effects of estradiol and raloxifene on collagen biosynthesis in cultured human skin fibroblasts. Int J Mol Med. 2003;12:803–809. [PubMed] [Google Scholar]
- 40.Verdier-Sévrain S. Effect of estrogens on skin aging and the potential role of selective estrogen receptor modulators. Climacteric. 2007;10:289–297. doi: 10.1080/13697130701467157. [DOI] [PubMed] [Google Scholar]
- 41.Nouveau S. Bastien P. Baldo F. de Lacharriere O. Effects of topical DHEA on aging skin: A pilot study. Maturitas. 2008;59:174–181. doi: 10.1016/j.maturitas.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol. 2005;17:493–499. doi: 10.1097/01.cco.0000174034.62032.08. [DOI] [PubMed] [Google Scholar]