Abstract
Rat embryonic stem cell (ESC) lines are not widely available, and there are only 2 lines available for distribution. Here, ESC lines were derived and characterized from Fischer 344 (F344) rats that express marker transgenes either β-galactosidase or human placental alkaline phosphatase (AP), nontransgenic F344 rats, and from Dark Agouti (DA) rats. The ESC lines were maintained in an undifferentiated state as characterized by colony morphology, expression of Oct4, Nanog, Sox-2, Cdx2, and Stella, staining for AP, and stage-specific embryonic antigen-1. Pluripotency was demonstrated in vitro by differentiation to embryoid bodies, followed by embryonic monsters. The Cdx2 expression by ESCs was unexpected and was confirmed via reverse transcriptase-polymerase chain reaction, immunocytochemistry. Pluripotency of ESCs was demonstrated in vivo by production of teratoma after an injection into F344 nontransgenic rats, and by an injection of male DA ESCs into F344 or Sprague-Dawley rat blastocysts and the generation of chimeric rats and germline contribution. ESCs from both F344 and DA contributed to chimeric rats, and one DA ESC line was proved to be germline competent. ESC sublines were created by transfection with a plasmid expressing enhanced green fluorescent protein (eGFP) under the control of a beta actin promoter and cytomegalovirus enhancer (pCX-eGFP) or by transfection with a plasmid expressing GFP under the control of a 3.1 kb portion of the rat Oct4 promoter (pN1-Oct4-GFP). In pN1-Oct4-GFP sublines, GFP gene expression and fluorescence were shown to be correlated with endogenous Oct4 gene expression. Therefore, these new ESC lines may be useful for tissue engineering and transplantation studies or for optimizing culture conditions required for self-renewal and differentiation of rat ESCs. While they made chimeric rats, further work is needed to confirm whether the transgenic F344 rat ESCs described here are germline-competent ESCs.
Introduction
As highlighted in recent reviews [1,2], the rat is an important laboratory species that has lagged behind the mouse within the field of functional genomics, but continues to out-publish the mouse in other areas of scientific research, such as the cardiovascular system and the nervous system. Embryonic stem cells (ESCs) have been important tools used to manipulate the mouse genome via gene targeting. It was after 2 decades of trying that “genuine” rat ESCs were produced [3,4], and only last year, genuine rat ESCs were demonstrated to be useful for knockout rat production [5]. To date, the efficiency of rat ESCs for gene targeting approaches is lower than in the mouse.
ESCs are derived from the inner cell mass of the blastocyst, can be maintained indefinitely in culture in the undifferentiated state, and can differentiate into a variety of cell types in vitro and in vivo [3,4,6–12]. Early work had suggested that the growth factors and cytokine requirements to maintain rat ESCs so that they continue to self-renew and prevent their differentiation was different from the rat. For example, leukemia inhibitory factor (LIF) supplementation of the medium together with inactivated mouse embryonic fibroblasts feeders (MEFs) was different from mouse ESCs [9]. Later, it was suggested that rat ESCs are similar to mouse ESCs [13,14], as the use of medium containing inhibitors of 2 pathways, glycogen synthase kinase 3 (GSK3) and mitogen-activated protein kinase kinase (MEK) pathways, restrict their differentiation and maintain their self-renewal. Therefore, the similarities/differences between rat and mouse ESCs remain to be fully understood.
Using medium containing the 2 inhibitors and no serum (called 2i medium), rat ESCs have been produced from Brown Norway, Wistar, Dark Agouti (DA), Sprague-Dawley (SD), and Fischer 344 (F344) strains [3,4,15,16]. To date, DA ESCs have shown germline competence and are termed “genuine” ESCs. In contrast, F344 ESCs have not produced chimera or germline-competent ESCs. The reasons for the differences between the 2 strains are unknown. The observed differences might be due to incompatibility between host blastocyst and F344 ESCs, sa decreased efficiency has been seen in the mouse unless the C57BL/6 is used as a host blastocyst (eg, [17,18]). The F344 blastocyst is a compatible host to DA ESCs, but efficiency to produce germline transmission is low compared with the efficiency observed in C57BL/6 mice or Sv129 mice.
We speculate that ESCs derived from F344 rats would have important advantages for gene targeting strategies. Specifically, F344 rats are inbred, have a bacterial artificial chromosome (BAC) library available to facilitate construction of targeting vectors, and are a relatively efficient strain in terms of litter size, compared with other inbred strains. Further, the F344 rat has proved utility for toxicological testing, aging studies, etc. We posited that F344 strains that express a marker transgene would be valuable for producing ESC-derived cells for cell-transplantation therapy, tissue engineering, biotechnology, etc. To that end, here, rat ESC lines and sublines were derived from a transgenic (Tg) F344 rat strain expressing either a β-galactosidase (β-gal) transgene under a cytomegalovirus (CMV) promoter or expressing human placental alkaline phosphatase (AP) under the Rosa26 promoter. These ESCs lines were characterized by gene expression for Oct4, Sox2, Nanog, Cdx2, and Stella, staining for AP and stage-specific embryonic antigen-1 (SSEA1), and expression of their marker transgene and chimera formation. These rat ESCs were differentiated to embryoid bodies (EBs) and to spontaneously contracting tissue (“beating structures”) in vitro and could form teratoma after injection into non-Tg F344 rats. To evaluate the ability to introduce genes into rat ESCs, a rat Oct4 reporter construct called pN1-Oct4-GFP was made, as others have done in the mouse species [19,20]. Rat pN1-Oct4-GFP ESCs lines were selected and clonally derived. A pN1-Oct4-GFP ESC reporter line was validated by correlating Oct4 RNA expression with green fluorescent protein (GFP) gene expression and by correlating GFP fluorescence and Oct4 protein expression. The cell lines and tools developed here will assist with refinement of rat ESC culture conditions and tracking of rat ESC-derived cells after transplantation or chimera formation.
Materials and Methods
Animals
The animal work was approved by the KSU Institutional Animal Care and Use Committee. F344 Tg rats that express the β-gal reporter under a CMV promoter produced by Ozgene for Mahendra S Rao (NIH, NIA), and F344 Tg rats that express the human placental AP gene under the Rosa26 promoter that were previously described [21], were used. The Tg rats supplied by Dr. Rao were bred to homozygousity, and the colony was maintained Specific-Pathogen-Free (SPF). At the time of these experiments, the Tg F344 rats were SPF and homozygous for the transgene. Non-Tg F344 and DA rats were obtained from Harlan and bred in-house.
ESC culture
A number of ESC lines were derived; these lines are listed in Table 1. The ESC culture was performed using standard methods. ESC culture: Embryos were collected on 4.5 days post coitum (dpc) and were placed on mitotically inactivated MEFs in medium described next. Blastocysts outgrowths were lifted with a mouth pipette with an interior diameter of 150 μm and disrupted with Trypsin/EDTA, then the trypsin was inactivated by adding serum containing medium, and the cells were dispersed by pipetting 4–6 times. The cells were centrifuged for 3 min at 250×g, resuspended in medium, and plated in 1-well of a 96-well plate. After 2–7 days, ESC-like colonies were observed and picked based on morphology for plating on a fresh feeder layer. The colonies were grown until the largest colony reached 200 μm, then they were lifted using trypsin/EDTA and plated into a new well of a 96-well plate. At passage, ESCs were usually split 1:3 or 1:5. ESCs were frozen using ESC freezing medium according to the manufacturer's recommendations (GlobalStem). At thaw, ESC viability was checked with trypan blue exclusion, and the ability to re-establish culture was checked.
Table 1.
Polymerase Chain Reaction Primers
| Gene | Sequence | Product Size | |
|---|---|---|---|
| RT-PCR | |||
| Pou5F1 (Oct3/4) | F | CGA GGA GTC CCA GGA TAT GA | 446 |
| Pou5F1 (Oct3/4) | R | GCC GGT TAC AGA ACC ACA CT | |
| Sox2 | F | ACC AGC TCG CAG ACC TAC AT | 388 |
| Sox2 | R | CCC TCC CAA TTC CCT TGT AT | |
| Nanog | F | GCC CTG AGA AGA AAG AAG AG | 356 |
| Nanog | R | CTG ACT GCC CCA TAC TGG AA | |
| Pbgd | F | TAG CAT GCA AGA GAC CAT GC | 354 |
| Pbgd | R | GGC CGA AGT CTC AAC AAC TC | |
| hPAP | F | CTG ATG AAT GGG AGC AGT GGT GGA ATG | 360 |
| hPAP | R | GCA GAC ACT CTA TGC CTG TGT GGA G | |
| β-galactosidase | F | CGT CGT TTT ACA ACG TCG TGA C | 423 |
| β-galactosidase | R | CGC CGA GTT AAC GCC ATC | |
| Stella | F | TCC TAC AAC CAG AAA CAC TAG | 304 |
| Stella | R | GTG CAG AGA CAT CTG AAT GG | |
| Cdx2 | F | CAG GAG GAA AGC TGA GTT GG | 373 |
| R | TTC TCA CAG TGT CCG TGC TC | ||
| qRT-PCR | |||
| Pou5F1 (Oct3/4) | F | AGA ACC GTG TGA GGT GGA AC real-time | 132 |
| Pou5F1 (Oct3/4) | R | GCC GGT TAC AGA ACC ACA CT real-time | |
| EGFP | F | GAA GCA GCA CGA CTT CTT CAA real-time | 155 |
| EGFP | R | AAG TCG ATG CCC TTC AGC TC real-time | |
| Pbgd | F | GCA CGG CAG CTT AAT GAT GT real-time | 163 |
| Pbgd | R | CAA GGC CGA AGT CTC AAC AC real-time | |
| Cdx2 | F | CAG GAG GAA AGC TGA GTT GG | 373 |
| Cdx2 | R | TTC TCA CAG TGT CCG TGC TC | |
| D2Rat250-F1 | F | GTC CCT CTC CTG TCC CTC TC | 180/158/146 |
| D2Rat250-R1 | R | GAA GTC TGA ACG CTC ATG CA | 180/158/146 |
ESC culture media tested
Three different media were tested: (1) A serum-free, 2i medium that was based on a previously described recipe [4] with modifications, (2) A 2i medium with serum, and (3) A basic fibroblast growth factor (bFGF) and LIF supplemented medium.
A serum-free, 2i medium that was based on a previously described recipe [4] with modifications: N2B27+LIF medium was prepared by mixing 1 mL N2 (Invitrogen) supplemented with 5 mg/mL bovine serum albumin fraction V (Sigma) and 3 μg/mL progesterone (Sigma) to 100 mL Neurobasal medium (Invitrogen) and adding 2 mL B27 (Invitrogen), 1 mL Glutamax (Invitrogen), and 100 mL DMEM/F12 medium (Sigma) with a final concentration of 0.1 mM 2-mercaptoethanol (Sigma). The GSK3 inhibitor CHIR99021 (Stemgent) was added at 3 μM, and the MEK inhibitor PD0325901 (Stemgent) was added at 0.8 μM and 1,000 IU/mL rat LIF (Chemicon).
A 2i medium with serum: 83% DMEM (Invitrogen), 15% heat-inactivated fetal bovine serum (Hyclone), 1% nonessential amino acids (Invitrogen), 1% Glutamax, 0.1 mM 2-mercaptoethanol, 3 μM CHIR99021, 0.8 μM PD0325901, and 1,000 IU/mL LIF.
bFGF and LIF supplemented medium: 83% DMEM, 15% heat-inactivated fetal bovine serum (Hyclone), 1% nonessential amino acids, 1% Glutamax, 0.1 mM 2-mercaptoethanol, 2–8 ng/mL bFGF (Invitrogen), and 1,000 IU/mL LIF.
Feeder layer
MEFs were obtained from commercial sources (ATCC or Globalstem). The MEFs produced in our laboratory were mitotically inactivated either by irradiation (eg, 20–30G delivered by KSU's linear accelerator or from KSU's nuclear reactor) or by exposure to mitomycin C (Sigma). In some cases, inactivated MEFs were purchased.
Karyotyping analysis
Chromosome counting was performed in house. G-band chromosome analysis was performed by Cell Line Genetics after reviewing at least 20 chromosome spreads.
AP staining
ESCs were stained with an AP staining kit according to the manufacturer's instructions (Millipore). Positive control cells were undifferentiated mouse ESCs (American Type Culture Collection, D3 line).
Blastocyst injection with ESCs
Embryos were collected at 4.5 dpc from nontrangenic F344 or from SD rats and cultured on inactivated MEFs until the blastocoel had expanded. Blastocysts were injected with 4–15 ESCs. In some cases, the injected blastocysts were moved to individual wells in a 96-well plate onto a feeder layer, and photomicrographs were taken using phase-contrast and epi-fluorescence imaging over the next 8 days. The distribution of ESCs within the outgrowths was noted. In other cases, the injected blastocysts were transferred surgically into the uterine horns of 3.5 dpc pseudopregnant SD rats.
EB formation and differentiation
EBs were generated either using the hanging drop method or by plating on low attachment culture plates. Briefly, ESCs were cultured in 2i+LIF medium in suspension until they formed spheres. The ESCs clumps were then cultured in suspension in EB medium that consisted of 2i+LIF medium without the inhibitors and without LIF. The EB culture was maintained in suspension for 6–8 days, and the EBs were collected and plated onto 0.1% gelatin-coated or matrigel-coated wells. EBs were cultured in EB medium for another 2 weeks to form embryonic monsters.
Immunocytochemistry
ESCs were plated on an MEF layer on top of gelatin-coated coverslips. After 3 or 4 days of culture, ESCs were rinsed with Dulbecco's phosphate buffered saline (DPBS), fixed with 4% buffered paraformaldehyde for 3–4 min at room temperature, and triple rinsed with DPBS. Nonspecific binding was blocked with DPBS containing 5% normal goat serum (0.2% Triton X-100 was added for intracellular staining) for 30 min at room temperature. Primary antibodies included SSEA1, SSEA3, SSEA4 (Developmental Studies Hybridoma Bank), Oct4 (1:500–3,000; Chemicon or BD Biosciences), Sox2 (1:1,000–5,000; Chemicon), Nanog (1:500–3,000; Chemicon), β-gal (1:1,000–5,000; Abcam or Sigma-Aldrich), or Cdx2 (Biogenex, prediluted). Primary antibodies were detected with highly cross-absorbed fluorescent secondary antibodies (eg, Alexafluor 488, 568, or 594; Molecular Probes, 1:100–1,000). Before mounting, the DNA was stained with 6-diamidino-2-phenylindole for 5 min, and the plates were triple rinsed with DPBS. Coverslips were mounted with 70% glycerol containing anti-fade solution (2% n-propyl gallate in 0.1M Tris Buffer, pH 9.0) and sealed with rubber cement or nail polish. Slides were viewed using a Nikon Eclipse microscope, and images were collected using a Roper CoolSnap ES camera controlled by Metamorph v.7 software. The images were imported into Canvas 12 (ACD systems), cropped, scaled, colored, and assembled into plates.
Teratoma formation
Up to 2×106 Tg F344 ESCs were injected subcutaneously along the backs of F344 recipients, for example, β-gal-Tg ESCs were injected into non-Tg F344 rats. Injections were made 1 cm lateral to the spine and cranial to the hind limbs. The recipients were observed for 2–3 months for teratoma formation. At euthanasia, the teratomas were photographed in situ, then excised, and sent to the histopathology lab for preparation and analysis by a board-certified veterinary pathologist who was blind to the experimental conditions.
Reverse transcriptase-polymerase chain reaction
Total RNA was isolated using an RNeasy kit (Qiagen) or TRIZOL using the manufacturer's protocol. RNA was treated with DNase before storage. Complementary DNA was synthesized from total RNA using Superscript III First-Strand Synthesis Supermix kit (Invitrogen) primed with oligo-dT 12–18 per the manufacturer's protocol. polymerase chain reaction (PCR) was performed using a BioRad iCycler or iQ5: the initial denaturation at 95°C for 3 min, 30 cycles of [94°C for 1 min, 53°C–55°C for 30 s, and 72°C for 30 s], and the final extension at 72°C for 10 min. After PCR, the products were resolved on a 1%–2% agarose gel with 100 bp DNA ladder and imaged. Primers sequences are listed in Table 1.
Semi-quantitative reverse transcriptase-polymerase chain reaction
Semi-quantitative RT-PCR was performed using a BioRad iQ5 System and Sybr Green/Fluoroscein PCR Master Mix (SABiosciences). Pbgd was used for quantitative (q)RT-PCR normalization via the ΔCt method [22]. Reactions for qRT-PCR were run in duplicates and averaged and were also run using 2 or 20 ng per tube cDNA loading concentrations. Control RNA was collected from dpc 4.5, 9.5, 10.5, or 12.5 rat embryos, MEFs, undifferentiated mouse ESCs (D3 line; ATCC), undifferentiated human teratocarcinoma cells (NT2 line; ATCC), and mouse teratocarcinoma cells (F9; ATCC). Additional controls were supplied by Drs. Mohamed Rumi and Michael Soares at University of Kansas Medical Center who supplied cell pellets from trophoblast stem cells (TS cells, these cells were isolated, expanded, and derived as described in [23]) and extraembryonic endoderm cells (XEN) and Dr. Qilong Ying at University of Southern California who supplied “genuine” rat ESC pellet (Dac8, passage 35, these cells were isolated, expanded, and derived as described in [4]).
ESC transfection
ESCs were transfected using an Amaxa Nucelofector (A23 or B16 programs) under the following conditions: 106 ESCs, 90 μL of nucleofection solution, and 2 μg of plasmid DNA. The pCX-eGFP plasmid (pCX-eGPF) was supplied by Dr. A Nagy's lab. The pN1-GFP plasmid was made in-house. Plasmid N1-Oct4-GFP contains a 3.1 kb portion of the rat Oct4 promoter including both the proximal enhancer and a portion of the distal enhancer. The Oct4 promoter and the rat Oct4 ATG site was inserted 5’ to GFP, polyA sequence [22]. pN1-Oct4-GFP also contains a neomycin/kanamycin selection cassette under a separate SV40 promoter. ESCs transfected with pN1-Oct4-GFP were plated in one well of a 6-well plate on a feeder layer in rat ESC medium 2 for 2 days. Transfected cells were selected using G418 (Invitrogen) for 6 days. Colonies showing ESC morphology and GFP fluorescence were individually picked up and plated in one well of a 96-well plate on a feeder layer.
Results
Derivation of ESC cells
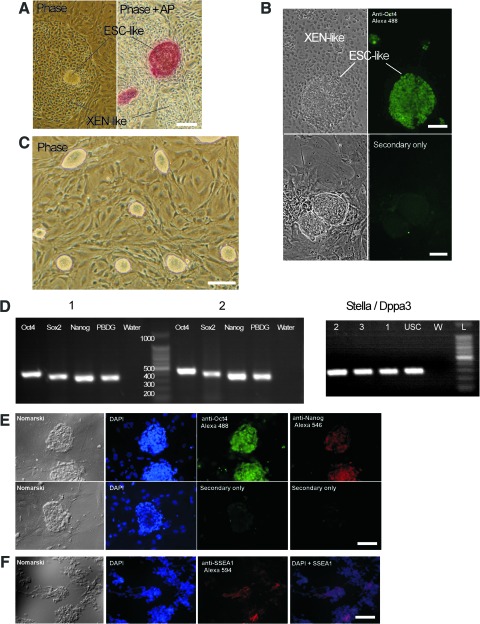
Rat ESC lines were derived from homozygous Tg F344, or non-Tg F344 and DA blastocysts (see Table 2). Outgrowths of the inner cell mass became distinct within a week and could be lifted manually and disrupted using trypsin-EDTA. ESC colonies could be selected based on morphology and could be manually moved to a new well on feeders. Sometimes, ESC colonies were observed growing on or adjacent to an unknown, flattened cell type, possibly extraembryonic endoderm cells (XEN) (Fig. 1A, B). Both the ESCs and the second cell type could be passaged and expanded in identical growth conditions and grew side by side or with the ESCs on top of the flat cells. Both cell types could be cryopreserved, reanimated, and expanded at approximately the same efficiency. To enrich ESCs, ESCs colonies were manually picked and moved to fresh wells (Fig. 1C). As shown in Fig. 1A and B, the XEN-like cell type was negative for Oct4 and AP. The XEN-like cells were not characterized further.
Table 2.
List of Rat Embryonic Stem Cell Lines That Were Derived and How They Have Been Characterized
| Cell line strain | Derivation conditions | Morphology | Sex | Tg? | OCT4 | NANOG | SOX2 | Karyotype | Tetraploid percentage | |
|---|---|---|---|---|---|---|---|---|---|---|
| Embryo-derived Stem Cell LINES | ||||||||||
| 1 | F344 β-galactosidase transgenic >10 other lines derived in bFGF/LIF/15% FBS | LIF/bFGF-S | Flat, individual | NO | Y | Y | No | Y | ||
| Embryonic Stem Cell LINES | ||||||||||
| 9 | F344 wild-type | 2i/LIF-SF | Round colonies | ND | Y | Y | Y | |||
| 11 | F344 wild-type | 2i/LIF-SF | Round colonies | M | Y | Y | Y | |||
| 12 | F344 wild-type | 2i/LIF-SF | Round colonies | ND | Y | Y | Y | |||
| 13 | F344 wild-type | 2i/LIF-SF | Round colonies | ND | Y | Y | Y | |||
| 38 | F344 wild-type | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | |||
| 39 | F344 wild-type | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | |||
| 40 | F344 wild-type | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | |||
| 2 | F344 β-galactosidase transgenic | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | Y | Euploid | 60% |
| 3 | F344 β-galactosidase transgenic | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | Y | Aneuploid | 16% |
| 4 | F344 β-galactosidase transgenic | 2i/LIF-SF | Round colonies | M | Y | Y | Y | Y | ||
| 32 | F344 hPAP transgenic | 2i/LIF-SF | Round colonies | M | Y | Y | Y | Y | ||
| 33 | F344 hPAP transgenic | 2i/LIF-SF | Round colonies | M | Y | Y | Y | Y | ||
| 34 | F344 hPAP transgenic | 2i/LIF-SF | Round colonies | F | Y | Y | Y | Y | ||
| 48 | DA | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | Euploid | 14% | |
| 49 | DA | 2i/LIF-FBS | Round colonies | F | ND | ND | ND | Euploid | 18% | |
| 50 | DA | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | Euploid | 32% | |
| 51 | DA | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | |||
| 52 | DA | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | |||
| 53 | DA | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | |||
| 54 | DA | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | Euploid | 32% | |
| 55 | DA | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | |||
| 56 | DA | 2i/LIF-FBS | Round colonies | F | Y | Y | Y | |||
| 57 | DA | 2i/LIF-FBS | Round colonies | M | Y | Y | Y | |||
| 58 | SD | 2i/LIF-SF | Round colonies | ND | ND | ND | ND | |||
| 60 | SD | 2i/LIF-SF | Round colonies | ND | ND | ND | ND | |||
| Sublines | Strain | Notes |
|---|---|---|
| 1.1 | F344 β-galactosidase transgenic | pCX |
| 2.1 | F344 β-galactosidase transgenic | pCX |
| 2.2 | F344 β-galactosidase transgenic | Neural |
| 3.1 | F344 β-galactosidase transgenic | pN1 |
| 3.2 | F344 β-galactosidase transgenic | pCX |
| 4.1 | F344 β-galactosidase transgenic | pN1 |
| 30 | F344 β-galactosidase transgenic | PE-like |
| 48.1 | DA | pCX |
| 53.1 | DA | pCX |
| 54.1 | DA | pCX |
LIF, leukemia inhibitory factor; F344, Fischer 344; Tg, transgenic; bFGF, basic fibroblast growth factor; DA, Dark Agouti; SD, Sprague-Dawley; 2i, 2 inhibitor.
FIG. 1.
Generation and characterization of rat embryonic stem cells (ESCs) from transgenic F344 and nontransgenic Dark Agouti (DA) rats. (A, B) Rat ESCs and primitive endoderm-like (XEN-like) cells in a mixed culture. After collapse of the blastocyst, the inner cell mass starts expanding. Phase-bright colonies with ESC morphology are found on or adjacent to XEN-like cells (A). The ESCs are positive for alkaline phosphatase (AP, shown in A) and Oct4 staining (B), and the XEN-like cells are negative for both Oct4 and AP. (C) Both XEN and ESCs can be expanded, frozen/thawed, and maintained in the 2i medium. To enrich ESCs, the colony is manually selected, dissociated, and expanded (see C). (D) RT-PCR characterization of pluripotency transcription factors expression by ESCs. Lanes from left to right: Oct4, Sox2, Nanog, PBGD, and water only (control). Block 1 (left): Positive-control ESCs (supplied by Dr. Qilong Ying, University of Southern California). Block 2: ESCs derived from a β-galactosidase (β-gal) transgenic rat (line 3). At left: Dppa3/Stella expression by ESCs. Lanes labeled by rat ESC lines (lines are listed in Table 1). Lines 2 and 3 were derived using 2i medium with 15% heat-inactivated fetal bovine serum (CHIR99021, GSK-3β inhibitor and PD0325901, MEK inhibitor) and leukemia inhibitory factor (LIF). Line 1 was derived using human basic fibroblast growth factor (bFGF) and LIF and 15% heat-inactivated fetal bovine serum. Lane labeled USC represents positive control rat ESCs from Qilong Ying (University of Southern California). Lane labeled W, water only (control). Lane labeled L is a 100 bp ladder. (E) Oct4 and Nanog immunocytochemical staining. Top: ESCs derived from a F344 β-gal transgenic rat (line 3) at passage 4 stained for Oct4 (green), or Nanog (red). Bottom: Secondary antibody only controls. Panels from left to right: Normarski illumination shows colony size and morphology, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, blue) staining for DNA, immunofluorescence micrograph for Oct4 (green), and at the far right, immunofluorescent staining for Nanog (red). Lower panel shows ESCs stained with secondary antibody only. (F) SSEA1 immunocytochemical staining. ESC derived from a F344 β-gal transgenic rat (line 2) at passage 12 stained for SSEA1 (red). From LEFT to RIGHT: Normarski illumination, DAPI nuclear stain (blue), SSEA1 (red), and at the far right, merged DAPI and SSEA1 staining. In all images, scale bar is 100 μm. PCR, polymerase chain reaction; SSEA1, stage-specific embryonic antigen-1; GSK, glycogen synthase kinase; MEK, mitogen-activated protein kinase; F344, Fischer 344; USC, University of Southern California; 2i, 2 inhibitor. Color images available online at www.liebertonline.com/scd
Comparison of media formulations
DMEM medium supplemented with LIF and bFGF supported initial derivation of rat ESCs. ESCs could not be maintained in this medium. At initial derivation and at early passage, ESC colonies were comprised of loosely packed, round cells that formed colonies with ragged borders (data not shown). When stained for AP, these colonies were variably stained with light homogenous AP staining or were composed of negative cells with a few positive AP-stained cells buried in the colony (data not shown). The ESCs lost AP staining between passage 4–8. Therefore, we concluded that this medium could not maintain the undifferentiated state as assessed by immunocytochemistry, AP staining, or RT-PCR for the markers of the pluripotent state: Oct4, Sox2, and Nanog. The ESCs lost staining Nanog staining first and later lost Oct4 staining. Thus, ESCs completely differentiated by passage 8 in this medium. In summary, medium supplemented with bFGF and LIF might be used to derive ESCs, and did not maintain rat ESCs in the undifferentiated state.
In contrast, media formulations that contained GSK3 and MEK inhibitors with or without rat LIF (2i medium+LIF) with or without serum could derive rat ESCs and maintain them in the undifferentiated state (the undifferentiated state was indicated by morphology, AP staining, Oct4, Sox2, Nanog immunocytochemistical staining, and by Oct4, Nanog, Rex1 gene expression, etc, data presented next). When comparing the 2 media containing the 2i with or without serum, minor differences were observed. For example, medium that contained serum promoted better adherence to the feeder layer, and 2 ESC like populations were observed (data not shown). One population had a more-firm adherence to the feeder layer with colonies that were less refractile in phase contrast (less highly domed), the other population grew in suspension (balls of phase bright ESCs) or was loosely adherent to feeders. Both serum-free and serum-containing 2i+LIF media maintain ESCs when they were grown in the absence of feeders for at least 2 passages. However, ESCs expanded in suspension culture were more difficult to work with, as cells were lost during feeding and other manipulations.
Rat ESCs express Cdx2
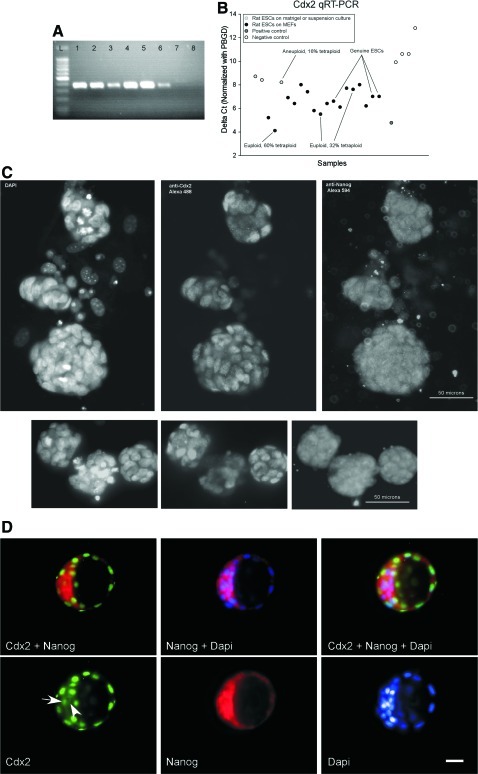
The rat ESC colonies were rounded and phase-bright; stained for AP and Oct4; and had a similar appearance to mouse ESCs (Fig. 1A–C). RNA extracted from rat ESCs was collected, and RT-PCR revealed that rat ESCs express transcription factors: Oct4, Sox2, and Nanog at levels comparable to genuine rat ESCs supplied by Dr. Qilong Ying at University of Southern California (Fig. 1D). In addition, rat ESCs express Oct4 and Nanog protein in the nucleus as seen by immunocytochemistry (Fig. 1E). Finally, some but not all rat ESCs expressed SSEA1 (Fig. 1F). Rat ESCs did not express SSEA3 or SSEA4 (data not shown). As shown in Fig. 2A, rat ESCs express the trophoectoderm marker, Cdx2. As shown in Fig. 2A–C, this finding was confirmed by immunocytochemistry, RT-PCR, and semi-quantitative RT-PCR. In Fig. 2A by using RT-PCR, Cdx2 expression levels were compared between genuine rat ESCs (supplied by Dr. Qilong Ying at University of Southern California) and the rat ESCs derived here (lines 1, 2, and 3). As a positive control, rat TS cells were used. As a negative control, undifferentiated mouse ESCs (D3 line) were used. As expected, undifferentiated mouse ESCs did not express Cdx2 (Fig. 2A, Lane 1: ESC line 2 at passage 6, Lane 2: ESC line 3 at passage 6, Lane 3: ESC Control from Qilong Ying, Lane 4: Positive Control TS #1, Lane 5: Positive Control TS #2 supplied by Dr. Michael Soares and Mohammed Rumi at University of Kansas Medical Center, Lane 6: ESC line 1 at passage 10, Lane 7: Negative Control mouse D3 ESCs obtained from ATCC). In Fig. 2B, to determine whether Cdx2 expression was correlated with aneuploidy or high tetraploidy, Cdx2 expression was evaluated in aneuploid and euploid ESC lines with different levels of tetraploidy, and uncharacterized ESC lines along with TS cell positive control cells and adult heart and liver, rat embryonic fibroblast (REF), and rat umbilical cord mesenchymal stromal cells (RUC) negative control cells. Genuine rat ESCs were included (samples from Dr. Qilong Ying and our line 53) as were samples from line 53 ESCs that were differentiated to EBs for 5 or 10 days. The delta Ct values are provided in Table 3, and a subset of the results are graphically shown in Fig. 2B. Except for one euploid ESC line with 60% tetraploidy, these data show that rat TS cells express Cdx2 at a higher level than rat ESCs. In summary, no strong correlation of Cdx2 expression levels and the number of complete chromosome sets was observed. In contrast, rat adult heart and liver, REFs, and RUCs express Cdx2 at lower levels than rat ESCs. Note that the rat ESC lines derived here and the genuine rat ESCs (DAc8 line supplied by Dr. Ying and line 53 derived here) express Cdx2 at about the same level. Of potential importance, growing rat ESCs on Matrigel or growing them in suspension culture may decrease Cdx2 expression (see Fig. 2B, Table 3).
FIG. 2.
Cdx2 staining of rat embryonic stem cells and rat blastocysts. (A) RT-PCR of Cdx2. From left to right: (L) 100 bp Ladder, Lane 1: ESC line 2 at passage 6, Lane 2: ESC line 3 at passage 6, Lane 3: ESC Control from Qilong Ying, Lane 4: Positive Control TS #1, Lane 5: Positive Control TS #2 supplied by Dr. Michael Soares and Mohammed Rumi at University of Kansas Medical Center, Lane 6: ESC line 1 at passage 10, Lane 7: Negative Control mouse D3 ESCs obtained from ATCC, Lane 8: Negative Control (water). (B) Semi-quantitative RT-PCR for Cdx2. 18 rat ESC lines derived here and two rat ESC control supplied by Dr. Qilong Ying express Cdx2 gene at levels higher than negative control cells lines: rat adult tissues and rat embryonic fibroblasts (REFs) and rat umbilical cord mesenchymal stromal cells (RUCs). The positive control cells, rat trophoblast stem cells (TS) express Cdx2 at a higher level than all rat ESCs lines except one euploid rat ESC line that was 60% tetraploid. The delta threshold count (delta Ct) was averaged from two technical replicates and normalized using the Ct value of PBGD. The data from all lines is shown in Table 3. (C) Immunocytochemistry for Cdx2 and Nanog. Left panels are DAPI staining of nuclei. Middle panels are immunofluorescence for Cdx2. Right panels are immunofluorescence for Nanog. As seen in the middle panels, while the staining intensity of Cdx2 varies throughout the colonies, it is more intense at the edges of the colony. While Cdx2 staining varies all Nanog staining cells also had some degree of Cdx2 staining. There was consistent overlap of Cdx2 and Nanog staining. (D) Immunocytochemistry for Cdx2 and Nanog in 4.5 days post coitus rat blastocysts. As seen in the bottom left panel, the inner cell mass contains Cdx2 positively stained nuclei (arrow), albeit at lower staining intensity than in trophectoderm cells. Additionally, Cdx2 staining was observed in the cytoplasm of inner cell mass cells (arrowhead). XEN and TS cell pellets were supplied by Drs. Michael Soares and Mohammed Rumi, University of Kansas Medical Center; rat ESC control cell pellet was supplied by Dr. Qilong Ying, University of Southern California (USC). In C the calibration bar is 50 μm; in D the calibration bar is 25 μm. Color images available online at www.liebertonline.com/scd
Table 3.
Semi-Quantitative RT-Polymerase Chain Reaction Data Showing Cdx2 Expression by Rat Embryonic Stem Cell Lines and by Control Cell Lines (ΔCt Values Referenced to PBGD)
| Cell line strain | Karyotype | Tetraploid percentage | Cdx2? | Cdx2 ΔCt | Special culture conditions | |
|---|---|---|---|---|---|---|
| Embryo-derived Stem Cell LINES | ||||||
| 1 | F344 β-galactosidase transgenic | Y | 7.0 | |||
| 11 | F344 wild-type | Y | 8.7 | Suspension | ||
| 12 | F344 wild-type | Y | 8.4 | Suspension | ||
| 38 | F344 wild-type | Y | 5.2 | |||
| 2 | F344 β-galactosidase transgenic | Euploid | 60% | Y | 4.1 | |
| 3 | F344 β-galactosidase transgenic | Aneuploid | 16% | Y | 8.2 | Matrigel |
| 4 | F344 β-galactosidase transgenic | Y | 6.9 | |||
| 32 | F344 hPAP transgenic | Y | 6.4 | |||
| 33 | F344 hPAP transgenic | Y | 8.0 | |||
| 34 | F344 hPAP transgenic | Y | 7.4 | |||
| 48 | DA | Euploid | 14% | Y | 5.8 | |
| 50 | DA | Euploid | 32% | Y | 5.5 | |
| 51 | DA | Y | 6.4 | |||
| 52 | DA | Y | 6.6 | |||
| 53 | DA | Y | 6.1 | |||
| 54 | DA | Euploid | 32% | Y | 7.7 | |
| 55 | DA | Y | 7.6 | |||
| 56 | DA | Y | 8.0 | |||
| 57 | DA | Y | 6.2 | |||
| Cdx2 controls | ||||||
| DAC8 P35 | Y | 7 | ||||
| DAC8 P35 | Y | 7 | ||||
| Rat adult liver | Y | 9.9 | ||||
| REF | Y | 10.3 | ||||
| RUC | Y | 10.6 | ||||
| Rat adult heart | Y | 12.8 | ||||
| TS cells | Y | 4.9 | ||||
| 54 differentiated to EBs 5 days | Y | 8 | ||||
| 54 differentiated to EBs 10 days | Y | 7.6 | ||||
EBs, embryoid bodies; TS, trophoblast stem; REF, rat embryonic fibroblast; RUC, rat umbilical cord mesenchymal stromal cells.
Next, the expression of Cdx2 and Nanog was analyzed in rat ESCs using immunocytochemistry (Fig. 2C). As seen in Fig. 2C, Cdx2 expression was found in the nucleus of virtually all ESCs. Variability in Cdx2 staining intensity was observed (compare Top and Bottom panels) with higher expression found on the outer surface of the colony. There was a high degree of correspondence between Nanog and Cdx2 staining, suggesting that Cdx2 was found in rat ESCs and not in a contaminating population of TS or other embryonic cells. In contrast to what we observed in rat ESCs, as shown in Fig. 2D, in rat blastocysts, Cdx2 expression was found at high levels in trophectoderm cells and at lower levels in the ICM. We found Cdx2 expression in both the cytoplasm (arrowhead in Fig. 2D) and the nucleus of ICM cells (arrow).
In an adjacent study under review elsewhere, we developed and tested a focused gene array for characterizing rat ESCs and compared undifferentiated ESCs with rat TS cells, rat extraembryonic endoderm cells (XEN), MEFs, and differentiated ESCs. Our array is similar to commercially available arrays for characterizing human and mouse ESCs (eg, arrays from SABioscience). When undifferentiated and differentiated rat ESC lines were compared, Cdx2 expression in undifferentiated ESCs was confirmed. In contrast, Hand1, Eomes, and Fgfr2 were not highly expressed in undifferentiated rat ESCs. After differentiation by removal of MEK and GSK inhibitors and EB formation for 5 or 10 days, rat ESCs increased expression of Eomes, Fgfr2, and Hand1 and decreased expression of Nanog, Oct4, Sox2, Stella, Rex1, Esrrb, and Cdx2 (report in preparation).
ESC lines were derived from male and female blastocysts
The isolation and expansion of rat ESCs produce about an equal distribution of male and female ESC lines as determined by PCR for SrY (see Table 2). In some cases, the sex of the ESC lines was validated by karyotyping by G-banding (performed by Cell Line Genetics). Karyotyping revealed that most lines were euploid, and one line (line 3) was aneuploid (+3 and −16). It was noted that some rat ESC lines had a significant amount of tetraploidy (see Table 2), and so care was taken to select diploid, male ESCs for blastocyst injection.
Rat ESCs derived from Tg F344 rats form teratoma after injection into wild-type F344 rats
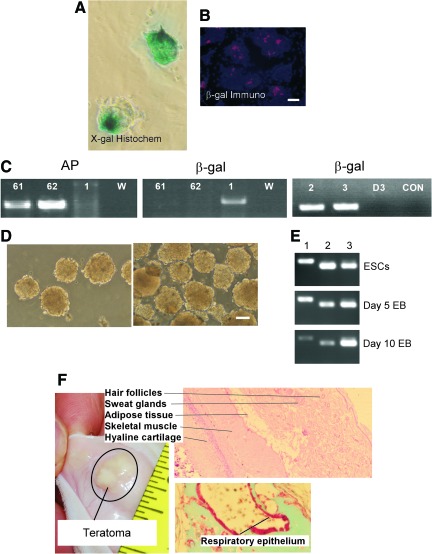
Rat ESC lines derived from Tg β-gal or human placental AP F344 rats continued to express the marker transgenes. As shown in Fig. 3A–C, this was demonstrated by X-gal histochemistry (Fig. 3A), immunocytochemical staining for β-gal (Fig. 3B), by RT-PCR (not shown), and by PCR (Fig. 3C). As shown in Fig. 3D, when ESCs were exposed to medium without GSK and MEK inhibitors and without LIF in a hanging drop (Fig. 3D left) or by plating ESCs on low adherence plates (Fig. 3D right), rat ESCs could be induced to differentiate to EBs. After 8 days of culture, the EBs were moved to a cell culture plate treated with gelatin, fibronectin, or Matrigel, and the EBs attached to the substrate and proceeded to differentiate. In 2 to 4 weeks, adherent cells were confluent and formed complex 3 dimensional structures that consist of a variety of tissue types. For example, differentiated ESC lines produced spontaneously contracting (beating) structures (data not shown). Further characterization of rat ESCs differentiated in vitro is beyond the scope of this report. Rat ESCs from Tg F344 strains were injected subcutaneously into wild-type (non-Tg) F344 rats without immune suppression. One of the 4 rats injected developed a palpable tumor approximately 2 months after injection at the injection site (Fig. 3F, left). The tumor was photographed in situ and histologically prepared, sections were stained with hematoxylin and eosin. Histological evaluation by a veterinary histologist and a board-certified veterinary clinical pathologist revealed that the tumor was a teratoma containing organized tissues derived from all 3 germlayers (Fig. 3F, right). The other 3 rats were asymptomatic for 4 months and were sacrificed. On gross examination at autopsy, there was no evidence of tumors or ESCs.
FIG. 3.
Characterization of rat ESCs derived from transgenic rats. (A–C) Detection of transgenes genes in ESCs derived from transgenic F344 rats. (A) X-gal histochemistry (X-gal Histochem) to detect β-gal in ESCs (line 2, passage 11) derived from β-gal transgenic rat. (B) Immunocytochemical staining for β-gal protein (β-gal Immuno, red) in rat ESC line 2 counterstained with DAPI for DNA (blue). Scale bar is 100 μm. (C) PCR on genomic DNA extracted from rat ESC lines 61, 62, 1, 2, and 3, to detect human placental AP (left) or β-gal transgene expression (β-gal, middle and right). ESC lines 61 and 61 were derived from the human placental AP transgenic rats and lines 1, 2, and 3 were derived from the β-gal transgenic rats. Lane W, water (negative control). (D) Embryoid bodies (EBs) derived from ESCs. Left: EBs formed using the hanging-drop method for 8 days. Right: EBs formed using suspension culture method for 8 days. EBs were derived from line 2 (β-gal F344 ESCs) at passage 12. Scale bar 100 μm. (E) RT-PCR characterization of pluripotency transcription factor expression in EBs. EBs cultured in the presence of bone morphogenetic protein 4 (BMP4) for 5 or 10 days show progressive down-regulation of Oct4 (lane1), and Nanog (lane 2) and not the control gene (PBGD, lane 3). Top: ESCs maintained in 2i medium to prevent differentiation (undifferentiated control). Middle and bottom: EBs maintained in medium with BMP4 for 5 days (middle) or 10 days (bottom). (F) ESCs derived from transgenic F344 rats form teratoma after injection into parental strain F344 rats. ESCs from Line 1 were injected subcutaneously into parental strain F344 rats. Ten weeks later, a tumor was palpated at the injection site (tumor in situ, left). The tumor was isolated, collected, and histologically processed by the KSU veterinary medicine diagnostic laboratory. Hematoxylin and eosin staining (right) of paraffin sections showed hair follicles, sweat glands, adipose tissue, skeletal muscle and hyaline cartilage (right at top), and respiratory epithelium (right, bottom). Thus, ESC-derived tumors formed tissues from all 3 germ layers. Color images available online at www.liebertonline.com/scd
Transfection with pCX-GFP
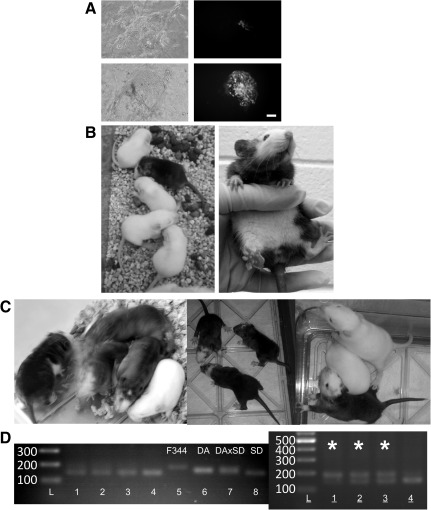
Rat ESCs were transfected with pCX-GFP using the nucleofector, and an estimated 5%–10% of the cells that survived were fluorescent. Since the pCX-GFP vector did not contain antibiotic resistance gene, at passage, cells were plated at a low density to produce clonally derived sublines. In some cases, single cells were moved to wells in a 96-well tray onto inactivated MEFs. In either way, brightly fluorescent clones were derived and expanded as sublines. GFP ESC sublines were cryopreserved/thawed, re-expanded, and used for blastocyst injection (Fig. 4). Rat GFP-F344 ESCs were injected into F344 or SD blastocysts (see Table 4). In some cases, the injected blastocysts were cultured up to 8 days after injection. When blastocysts were cultured after GFP ESC injection, ESC distribution within the blastocyst was tracked by monitoring the GFP cells overtime. Between 4 and 8 days of culture, the blastocysts collapsed. GFP cells were observed within the ICM (100% of the blastocysts, n=14) and the trophoendoderm (100% of the blastocysts, n=14) after the injection. However, as shown in Fig. 4A, by 8 days of culture, GFP ESC cells were observed within the inner cell mass outgrowths (Fig. 4A). These observations suggest that Tg F344 GFP-ESCs might contribute to the ICM and extraembryonic tissues after blastocyst injection and that long-term culture in 2i conditions encouraged expansion of ESCs and not extraembryonic cells (Fig. 4A). In other experiments, Tg F344 ESCs or DA ESCs were injected into F344 or SD blastocysts and transferred into pseudopregnant SD recipients (see Table 3). Chimeric animals were found after injection of DA ESCs into F344 (Fig. 4B) or SD blastocysts (Fig. 4C), and one ESC line was proved to be germline competent. Using the SD line as blastocyst donors and recipients after embryo transfer was more efficient for producing chimeric rats than F344 (see Table 3). Using SD blastocysts, we were successful at producing SD-F344 chimeric rats (Fig. 4D). Since SD and F344 are albino rats, chimerism was shown by microsatellite genotyping (Fig. 4D).
FIG. 4.
Chimera formation after injection of rat 4.5 dpc blastocysts. (A) ESC line 3 cells were nucleofected with the pCX-eGFP plasmid and expanded to passage 11. These cells were injected into non-eGFP, parental strain F344 4.5 dpc blastocysts. Injected blastocysts were cultured for 10 days on inactivated mouse embryonic fibroblast feeder (MEF) cells after injection (top) or after 14 days (bottom) of culture on inactivated MEFs. The same field is shown in each panel using phase-contrast illumination (left), GFP fluorescence (middle), and a merged image is shown on the right. Scale Bar 100 μm. (B) A single female chimera from a litter of 9 pups after injection of the male line 54.1 into F344 blastocysts. (C) Left: 4 chimera from a litter of 5 pups after injection of male line 53.1 into SD blastocysts. Note that this ESC line demonstrated germline transmission. Note that there is a variation in the degree of chimerism for each ESC line. Middle and right: 4 chimera from 2 litters of 3 pups after injection of male line 52 into SD blastocysts. (D) PCR microsatellite genotyping data after injection of male F344 line into SD blastocysts (lanes 1–4 on left and lines 1–4 on right). Lanes 5–8 are control samples for F344, DA, DA× SD, and SD, respectively. Note that 3 F344× SD chimera are indicated by astrisks in gel on the right. eGFP, enhanced green fluorescent protein; dpc, days post coitum; SD, Sprague-Dawley.
Table 4.
Results from Blastocyst Injection with Rat Embryonic Stem Cells
| ESC Line | Passage | ESC Strain | Sex | # Cells injected | Host Blastocyst | # Blastocyst Transferred | Pups Born | Coat Chimerism | Germline? | Embryo Culture Prior to Injection | Culture of Injected Blastocysts |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 54.1 | 11 | DA | M | 4-10 | F344 | 8^^^ | 1 | 0 | 2 hours | 2 hours | |
| 54.1 | 11 | DA | M | 4-10 | F344 | 11 | 9 | 1 (F) | 2 hours | 2 hours | |
| 54.1 | 11 | DA | M | 4-10 | F344 | 8 | 0 | 0 | 2 hours | 2 hours | |
| 54.1 | 11 | DA | M | 4-10 | F344 | 6 | 0 | 0 | 2 hours | 2 hours | |
| 48 | 11 | DA | M | 10-12 | F344 | 13 | 0! | N/A | 1-2 hours | 0 hours | |
| 53.1 | 10 | DA | M | 10-12 | F344 | 12 | 3* | N/A | 1-2 hours | 3-5 hours^^ | |
| 53.1 | 10 | DA | M | 10-12 | SD | 12 | 0 | 0 | 1-2 hours | 3-5 hours^^ | |
| 53.1 | 10 | DA | M | 10-12 | SD | 12 | 1* | N/A | 1-2 hours | 3-5 hours^^ | |
| 53.1 | 10 | DA | M | 10-12 | SD | 12 | 1* | N/A | 1-2 hours | 3-5 hours^^ | |
| 53.1 | 10 | DA | M | 10-12 | SD | 18 | 6* | 4 (2M, 2F) | Y | 1-2 hours | 3-5 hours^^ |
| 52 | 9 | DA | M | 8-10 | SD | 16^^^ | 3 | 3 | Y | >1 hour 30 mins | 3-4 hours^^ |
| 52 | 9 | DA | M | 8-10 | SD | 14^^^ | 4** | 1 | >1 hour 30 mins | 3-4 hours^^ | |
| 38.1 | 12 | F344 | M | 10-12 | SD | 15 | 3 | 1 | Y | >1 hour 30 mins | 3-4 hours^^ |
| 38.1 | 12 | F345 | M | 10-13 | SD | 15 | 9 | 5 | >1 hour 30 mins | 3-4 hours^^ |
duration in culture affected by time consumed for injection of blastocysts with embryonic stem cells.
one uninjected embryo per side to sustain pregnancy.
pups died neonatally.
1 pup died neonatally.
!showed signs of birth yet there were no pups found.
Transfection with pN1-GFP
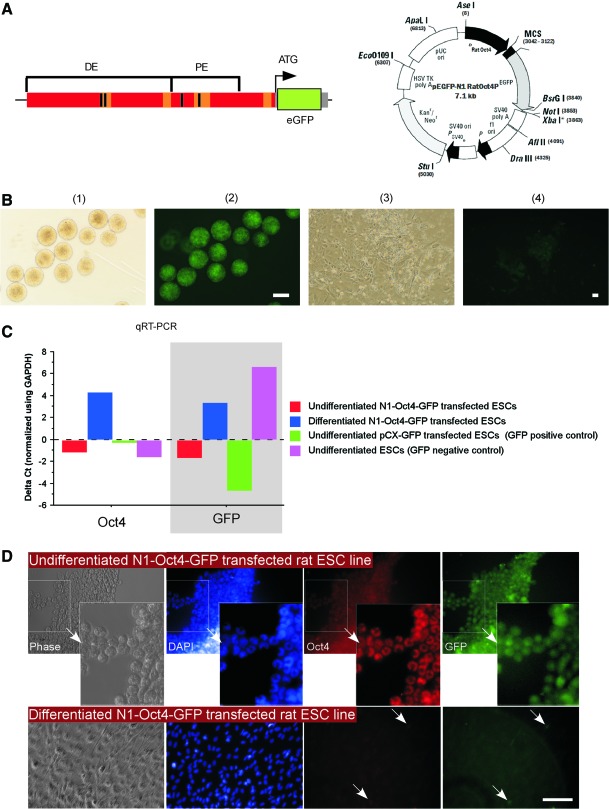
ESCs were transfected with pN1-Oct4-GFP plasmid shown in Fig. 5A. The data presented are from stable, clonally derived pN1-Oct4-GFP-ESC sublines that were derived from nucleofected ESCs. The transfected cells were expanded for 1 week after transfection before being placed under 6 days of G418 selection (200–800 μg/mL). Cells that survived G418 selection and were fluorescent were evaluated for Oct4 and GFP expression by RT-PCR and by immunofluorescence in undifferentiated ESCs and in ESCs that were induced to differentiate for up to 8 days via EB formation as just described. Undifferentiated pN1-Oct4-ESCs were maintained in 2i+LIF medium, and they express GFP fluorescence (Fig. 5B, panels 1 and 2). When these cells were differentiated for 1 week by removing LIF and the 2i, the percentage of ESCs that display GFP fluorescence decreases, and this decrease correlates with the change in cellular morphology and with increasing survival time (Fig. 5B, panels 3 and 4). These data suggested that the pN1-Oct4-GFP reporter line report Oct4 expression with fidelity. To confirm these observations, semi-quantitative RT-PCR analysis of Oct4 and GFP expression was performed. Note that undifferentiated pN1-Oct4-GFP ESCs had higher expression of Oct4 and GFP (see Fig. 5C, red bars) than pN1-Oct4-GFP ESCs that were differentiated for 6 days (blue bars in Fig. 5C). Note that the GFP gene expression in the pN1-Oct4-GFP ESCs after 6 days of differentiation was not as low as the negative-control cells (undifferentiated rat ESCs that were not transfected, Fig. 5C purple bars). Undifferentiated rat ESCs that were transfected with the pCX-GFP vector had similar Oct4 expression compared with the other undifferentiated rat ESC lines and higher expression of GFP owing to the strong promoter enhancer in the pCX vector (Fig. 5C, green bars). The fidelity of the pN1-Oct4-GFP reporter was evaluated by analyzing the protein expression using immunofluorescence staining for Oct4 and GFP fluorescence (Fig. 5D). Note that many undifferentiated pN1-Oct4-GFP ESCs had strong positive staining for Oct4 that was confined to the nucleus. In contrast, the GFP fluorescence was found in the cytoplasm (compare Oct4 and GFP staining in Top right panels of Fig. 5D). When pN1-Oct4-GFP ESCs were differentiated for 6 to 8 days, few GFP fluorescent cells were found in the differentiated cultures (Fig. 5D lower panel), and these GFP-positive cells appeared to stain for Oct4 too (see arrows in Fig. 5D lower panel). This observation suggests that a few ESCs continue to have the Oct4 promoter active following the differentiation protocol, and this observation fits with the Oct4 immunocytochemistry staining.
FIG. 5.
Construction and Characterization of Oct4 GFP reporter ESCs. (A, left) Schema of the Oct4 promoter reporter construct. (A, right) Plasmid map for the N1 Oct4 GFP reporter. (B) (1 and 2) ESCs in suspension culture after transfection with the N1 Oct4 GFP reporter vector and G418 selection. Phase contrast (1) and fluorescence images (2) of the same field showing the high degree of GFP fluorescence in undifferentiated ESCs. (3 and 4) In contrast, when the same ESCs are differentiated for 4-6 days by removal of the 2i inhibitors and LIF, the cell morphology indicates that the ESCs differentiated and the GFP fluorescence decreases. Scale bar 100 μm. (C) qRT-PCR analysis of the Oct4 and EGFP transgene expression. Oct4 expression in undifferentiated cells (red bars) and differentiated cells (blue bars). Undifferentiated pCX-EGFP transgenic ESCs (green bars) were used as a positive control for both Oct4 and EGFP expression. Undifferentiated nontransgenic ESCs (pink bars) were used as a positive control for Pou5F1 expression and as a negative control for EGFP expression. The control, housekeeping gene was PBGD. (D) Immunohistochemistry of N1-Oct4-EGFP reporter ESC line in culture. Top, undifferentiated N1-Oct4-GFP reporter ESCs were stained with an antibody to Oct4 (red) to observe the colocalization of eGFP (green) and Oct4 expression. Bottom panel shows the same ESC line after differentiation induced by withdrawal of PD0325901 (MEK inhibitor), CHIR99021 (GSK3β inhibitor) and leukemia inhibitory factor (LIF) for 6 days. 4',6-diamidino-2-phenylindole dihydrochloride (DAPI) was used to counterstain the DNA. The field indicated in the inset is shown at higher magnification on the right. Note that the Oct4 staining is localized to the nucleus, and in contrast the GFP signal is found throughout the cytoplasm (arrows indicates one cell that shows nuclear localization of Oct4 and cytoplasmic staining of GFP). Bottom row: Once the N1-Oct4-EGFP reporter line is differentiated by removal of 2i inhibitors and LIF, the cells change morphology (indicating their differentiation). In addition, the DNA changes to a more heterochromatin state (DAPI staining) and the Oct4 staining is lost. Note that when Oct4 immunofluorescence is decreased, so is GFP fluorescence. Note that some cells continue to express Oct4 and they continue to express GFP (examples indicated by arrows). These data suggest that N1-Oct4-EGFP reporter cells faithfully report Oct4 promoter activity and Oct4 protein expression. Scale bar 100 μm. Color images available online at www.liebertonline.com/scd
Discussion
Here, we show for the first time that rat ESC lines and sublines can be derived from F344 blastocysts, and that these cells can continue to self-renew in an undifferenatiated state for more than 35 passages and form chimera after an injection into SD blastocysts. These ESCs lines have the features of undifferentiated ESCs, as (1) they can be maintained following numerous passages without losing the undifferentiated morphology and phenotype, (2) they express ESC-specific transcription factor genes: Nanog, Sox2, and Oct4 at levels comparable to “genuine rat ESCs,” (3) they express the early embryonic stage markers, SSEA1 and AP, (4) they form a variety of tissues on in vitro differentiation including spontaneous beating structures, and they form teratoma after the injection of Tg F344 ESCs into non-Tg F344 rats with an intact immune system without immune suppression, (5) in vitro culturing for up to 8 days after the injection of Tg F344 ESCs into non-Tg F344 blastocysts showed that Tg rat ESCs contribute to the ICM and extraembryonic tissues, (6) similar to genuine rat ESCs from DA rats, the Tg F344 ESCs express Cdx2, and (7) F344 ESCs can contribute to the ICM to form chimera after an injection into SD blastocysts and embryo transfer. Finally, using DA ESCs, we found that using SD blastocysts as the embryo donor was more efficient for producing chimeric rats than F344 blastocysts. These results strongly suggest that rat ESC lines were derived from Tg F344 rats and that SD blastocysts can serve as efficient hosts to F344 and DA ESCs.
Here, rat ESCs were manipulated by gene transfection with a ubiquitous marker gene, GFP, under a strong promoter (pCX-GFP) and sublines of ESCs were derived. GFP ESC sublines were clonally expanded and manually selected. The sublines had similar properties to the parental ESC line in terms of morphology, Oct4 expression, proliferation rate, and staining. GFP-ESCs were injected into non-Tg F344 blastocysts and tracked 2–8 days in culture. These results showed that F344 blastocysts are compatible host blastocyst for Tg F344 ESCs in vitro and that the Tg ESCs were found in the ICM and in the trophoectoderm during days 2–6 of culture. In contrast, GFP ESC cells were found in the ICM outgrowths exclusively after 8 days of culture.
In other laboratories, F344 ESCs have not produced chimeric rats after an injection into DA or SD blastocysts [3,4]. Here, for the first time, F344 ESCs that were injected intor SD blastocysts produced chimeric rats. In other laboratories, F344 blastocysts were more efficient host blastocysts to produce germline transmission of ESCs when injected with DA ESCs [3,4], and F344 blastocysts produce the first ESC gene targeted knockout rats after an injection with DA ESCs [5]. In contrast, here we found that SD blastocysts were more efficient to produce germline transmission of ESCs. We speculate that the DA and F344 blastocyst is not a compatible blastocyst for Tg or non-Tg F344 ESCs. The production of gene targeting might be more efficient using F344 ESCs, as a BAC library is available for F344 to enable a recombineering approach.
Comparison of rat ESCs to other cells derived from rat embryos
From the rat embryo, extraembryonic endoderm cells (XEN) epiblast stem cells (EpiSC), and TS cells have been described [24–31]. Based on these previous reports, the present cells are ESCs and not XEN, EpiSC, or TS. First, ESCs have a different morphology from these other cell types. Here, rat ESCs grew as rounded or oblate, regular, phase-bright colonies that had a morphology similar to “genuine” rat ESCs derived by others [3,4] and mouse D3 ESCs. Second, at passage, rat ESCs could be grown from a single-cell suspension (clonogenic expansion properties), and the clonogenic nature of rat ESCs was also demonstrated by clonogenic expansion after transfection with either the pCX-GFP or the pN1-Oct4-GFP plasmid. Third, rat ESC lines express Oct4, Sox2, Nanog, Cdx2, SSEA1, and AP. These ESC lines express Rex1, Stella, and Cdx2. The expression of Cdx2 was found to colocalize with Nanog (Fig. 2). In contrast, Cdx2 expression was not found in rat XEN, undifferentiated mouse ESCs, REFs, RUCs, adult tissues, or MEFs, suggesting that this is the true expression of Cdx2. It is known that undifferentiated mouse ESCs do not usually express Cdx2 and can be induced to express Cdx2 [32]. Further, we examined Cdx2 protein expression in rat blastocysts and rat ESCs. We found intense nuclear Cdx2 expression in trophectoderm and less nuclear Cdx2 expression in inner cell mass cells.
We found Cdx2 gene expression in more than 14 rat ESC lines including 2 genuine rat ESCs (one provided by Dr. Qilong Ying and the other derived here). We noted down regulation of Cdx2 gene expression in lines that had been expanded on Matrigel or in suspension culture, which might be shown in the future to improve germline-competency of rat ESCs. Cdx2 gene expression was confirmed by immunocytochemistry that showed Cdx2 protein in the nucleus of undifferentiated rat ESCs as indicated by colocalization with Nanog. Heterogeneity was noted in the Cdx2 staining intensity within ESC colonies. Cdx2 gene expression was found in rat ESCs that had differentiated to EBs for 5 or 10 days after the removal of GSK and MEF inhibitors. In contrast, TS cells rapidly down-regulate Cdx2 during differentiation [23]. Fourth, after differentiation on removal of GSK and MEK inhibitors and LIF from the medium, rat ESCs differentiated into EBs that could further differentiate into a variety of different tissues including spontaneously contracting (beating) structures. In preliminary experiments, ESCs differentiated to neurosphere-like structures (data not shown). Since Tg F344 ESC produced teratoma consisting of all 3 germ layers after an injection into non-Tg F344, we have in vivo and in vitro demonstration of multilineage differentiation capacity. Fifth, after GFP, Tg F344 ESCs were injected into rat blastocysts and cultured up to 8 days. During days 2–6 of culture, ESCs were observed to be distributed in the ICM and into trophoectoderm. In contrast, after culture of >8 days, GFP ESCs were confined to the expanded ICM. Sixth, after injection of DA ESCs into rat blastocysts and embryo transfer into recipient females, chimeric rats were born showing a varying degree of coat color chimerism, and germline competency was found in one DA ESC line. Additionally, F344 ESCs produced chimeric rats after an injection into SD blastocysts. Till date, the F344 chimeric animals have not produced offspring that confirm germline transmission.
Rat ESCs differ from rat epiblast cells (EpiSC) in terms of growth factor requirements, morphology, gene expression, and differentiation potential [24,29]. Specifically, EpiSC have a flattened morphology, are not clonogenic, require bFGF and TGFβ/activin signaling to be maintained, and they do not express Stella or Rex1. Epiblast cells also do not contribute efficiently to chimera (or enter the germline). Here, rat ESCs were maintained in stable, undifferentiated culture under LIF/Stat3 stimulation and GSK3 and MEK inhibition, and had Rex1 and Stella expression, and rat ESCs efficiently colonized the blastocyst after the injection, and produced chimera and germline transmission. These results suggest that ESC lines have been derived and not EpiSCs.
The rat ESCs differ from rat XEN cells in terms of culture conditions, morphology, gene expression, and differentiation potential [25,31]. Specifically, XEN cells grow in high serum medium containing LIF (15% serum and 2,500 IU/mL LIF) and appear as loosely packed colonies of flattened cells. Randomly distributed within XEN colonies, individual round cells express Oct4 or AP. XEN cells do not express Sox2, Nanog, Cdx2, or Fgf4, but do express Oct4, Rex1, Gata4, and Gata6. After blastocyst injection, XEN cells integrate into the ICM at a lower frequency than trophectoderm or endoderm. The ESC lines described here that were grown in 2i plus LIF medium clearly differ from XEN cells in terms of morphology, growth factor requirements, gene and protein expression, and their distribution after blastocyst injection. In contrast, rat ESC lines that were derived and maintained in medium supplemented with LIF and bFGF resembled XEN cells in terms of gene expression, AP staining, colony morphology, etc. In summary, our results suggest that LIF and bFGF containing medium might support production of XEN cells, and not ESCs.
ESCs differ from TS cells in terms of isolation procedure, expansion conditions, morphology, gene and protein expression, etc [27,33–35]. Here, we demonstrated that TS cells express Cdx2 at higher levels than all ESCs lines tested except for one ESC line that was euploid with >60% tetraploidy. The literature reports that TS cells do not stain for AP, SSEA1, Nanog, and Sox2, in contrast to what was shown here. Therefore, our results suggest that the cells isolated here are ESCs and not TS cells.
Our findings indicate that the ESCs isolated here meet many requirements to be called pluripotent stem cells. We have not, however, directly demonstrated whether Tg F344 ESCs can contribute to the germline after blastocyst injection and embryo transfer. In contrast, our work shows that DA ESCs can contribute to generate chimera at good efficiency, especially when SD blastocysts act as the recipient blastocyst. Note that others, using similar isolation and expansion techniques, have already queried whether F344 ESCs are germline competent, and till now, they have not shown chimera formation [3,4]. We contend that insufficient data are available to conclude that there are deficiencies in F344 ESCs' pluripotency, as it is presently unknown whether the failure is due to the blastocyst or the ESCs. Despite the missing germline transmission data, our findings suggest that Tg F344 ESCs may have utility in transplantation studies into parental F344 rats and may yet be shown to be germline competent.
Medium
Here, rat ESCs could not be maintained when grown on inactivated MEFs in medium containing serum, LIF, and bFGF. ESCs grown in this medium, lost AP staining, did not have ESC characteristic dense, smooth, phase-bright colony morphology, lost Nanog, then Oct4 staining, and apparently differentiated by passage 6. In contrast, ESCs were maintained in the undifferentiated state and continued to self-renew in 2i medium supplemented with LIF with or without serum. Further, rat ESCs were maintained in this same medium formulation for 2 passages without an inactivated MEF layer without apparent detrimental effect. We found that the MEF layer and serum were important for ESC adherence. We observed that ESC adherence to the substrate was decreased when ESCs were grown serum free. Here, we report that the addition of serum to the 2i medium with LIF enhanced ESC attachment to feeders. This is not an optimal solution, as serum contains undefined factors that may trigger differentiation. In summary, ESCs could be maintained using MEFs with 2i medium plus LIF in an apparently undifferentiated state for prolonged periods. We found that the presence of serum facilitated expansion and characterization by providing better adherence of the ESC colonies.
Efficiency of derivation of ESCs from Tg F344 rats
We established ESC lines with high efficiency from Tg F344 lines (see Table 2). Previous work had reported similar efficiency at producing ESC lines from the parental strain F344 using 2i (28 lines from 47 ICM; [3]). We also produced ESC lines that have yet to be fully characterized for chimera formation and germline transmission from SD, wild-type (parental strain) F344, and DA rats. We did not find significant problems establishing ESCs in any of these strains.
Oct4 GFP marker
Here, we transfected rat ESCs with a plasmid made by fusing a 3.1 kb fragment of the rat Oct4 promoter that contains the proximal enhancer and some of the distal enhancer [22] immediately upstream of a eGFP and poly A and neomycin resistance cassette (Fig. 5A). We showed that the GFP signal in the pN1-Oct4-GFP ESCs correlated with gene expression and protein expression of Oct4 such that under conditions that maintained self-renewal and the undifferentiated state, Oct4 protein via nuclear immunocytochemical staining was found, and this was accompanied by GFP fluorescence in the cytoplasm. In contrast, Oct4 staining and Oct4 gene expression and GFP fluorescence and gene expression decreased when pN1-Oct4-GFP ESCs were differentiated to EBs for 6–8 days. Taken together, these data support the notion that pN1-Oct4-GFP plasmid is a useful tool for marking Oct4 expression in rat ESCs. This ESC line and the pN1-GFP plasmid will be useful tools for optimizing rat ESC culture conditions and for marking the fate of Oct4-expressing ESCs during rat embryonic development.
Acknowledgments
The authors thank J. Cox, M. McHaney, and Y. Lopez-Rodriguez and other members of the Weiss laboratory, Drs. B. Petroff, S. Paul, D. Albertini, M. Rumi, and M. Soares at University of Kansas Medical Center, and W. Rall and M.S. Rao at National Institutes of Health for their encouragement and assistance with this work. Drs. J. Auerbach, A. Toumadje, S. Medicetty, and J. Peters are thanked for helpful conversations. The KSU Comparative Medicine group (Dr. B. Carter, Dr. T. Miesner, S. Barrett, and R. Page) is thanked for assistance with the rat colony. Drs. M. Larson and R. Wesselschmidt provided helpful suggestions about rat blastocyst injection. The authors thank people who shared their resources: Dr. Qilong Ying and the Ying's lab provided genuine rat ESC cell pellets and answered questions about rat ESC culture. Drs. M. Soares and M. Rumi provided RNA from rat TS cells and extraembryonic endoderm cells and independently verified RT-PCR findings. Dr. H. Scholer's lab provided the mouse Oct4 reporter plasmid (GOF18 plasmid, data not shown). Dr. T. Ochiya provided REFs 3Y-1B cells (data not shown). Dr. A. Nagy provided the pCX-GFP plasmid. Dr. D. Marcus and the NIH/NCRR P20-RR017686 provided access to the Nanodrop. This work was supported by NIH-NIA funds to MLW during a sabbatical project in M.S. Rao's laboratory (2003–4), and by grants from the Terry C. Johnson Center for Basic Cancer Research, the National Institute of Health (NS34160), the Kansas Biosciences Authority, the University of Kansas Cancer Center pilot project program 2010, the KSU College of Veterinary Medicine Dean's office, and by the State of Kansas Legislature to the Midwest Institute for Comparative Stem Cell Biology. A portion of this work was supported by an anonymous donation to The Spinal Cord Injury program at the University of Kansas Medical School. A portion of this work (rat Oct4-GFP plasmid (pN1-Oct4-GFP) and the methylation of the rat ESC's Oct4 promoter) was presented at ISSCR June, 2008 in Philadelphia, PA. MLW acknowledges BGW for her constant support and encouragement. Mr. and Mrs. Howard Walker are thanked for their hospitality during the fall of 2010.
Author Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Hamra FK. Gene targeting: enter the rat. Nature. 2010;467:161–163. doi: 10.1038/467161a. [DOI] [PubMed] [Google Scholar]
- 2.Iannaccone PM. Jacob HJ. Rats! Dis Model Mech. 2009;2:206–210. doi: 10.1242/dmm.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buehr M. Meek S. Blair K. Yang J. Ure J. Silva J. McLay R. Hall J. Ying QL. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Li P. Tong C. Mehrian-Shai R. Jia L. Wu N. Yan Y. Maxson RE. Schulze EN. Song H, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong C. Li P. Wu NL. Yan Y. Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannaccone PM. Taborn GU. Garton RL. Caplice MD. Brenin DR. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev Biol. 1994;163:288–292. doi: 10.1006/dbio.1994.1146. [DOI] [PubMed] [Google Scholar]
- 7.Ruhnke M. Ungefroren H. Zehle G. Bader M. Kremer B. Fandrich F. Long-term culture and differentiation of rat embryonic stem cell-like cells into neuronal, glial, endothelial, and hepatic lineages. Stem Cells. 2003;21:428–436. doi: 10.1634/stemcells.21-4-428. [DOI] [PubMed] [Google Scholar]
- 8.Vassilieva S. Guan K. Pich U. Wobus AM. Establishment of SSEA-1- and Oct-4-expressing rat embryonic stem-like cell lines and effects of cytokines of the IL-6 family on clonal growth. Exp Cell Res. 2000;258:361–373. doi: 10.1006/excr.2000.4940. [DOI] [PubMed] [Google Scholar]
- 9.Buehr M. Nichols J. Stenhouse F. Mountford P. Greenhalgh CJ. Kantachuvesiri S. Brooker G. Mullins J. Smith AG. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol Reprod. 2003;68:222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- 10.Demers SP. Yoo JG. Lian L. Therrien J. Smith LC. Rat embryonic stem-like (ES-like) cells can contribute to extraembryonic tissues in vivo. Cloning Stem Cells. 2007;9:512–522. doi: 10.1089/clo.2007.0029. [DOI] [PubMed] [Google Scholar]
- 11.Schulze M. Ungefroren H. Bader M. Fandrich F. Derivation, maintenance, and characterization of rat embryonic stem cells in vitro. Methods Mol Biol. 2006;329:45–58. doi: 10.1385/1-59745-037-5:45. [DOI] [PubMed] [Google Scholar]
- 12.Ueda S. Kawamata M. Teratani T. Shimizu T. Tamai Y. Ogawa H. Hayashi K. Tsuda H. Ochiya T. Establishment of rat embryonic stem cells and making of chimera rats. PLoS One. 2008;3:e2800. doi: 10.1371/journal.pone.0002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying QL. Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 14.Ying QL. Wray J. Nichols J. Batlle-Morera L. Doble B. Woodgett J. Cohen P. Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirabayashi M. Kato M. Kobayashi T. Sanbo M. Yagi T. Hochi S. Nakauchi H. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol Reprod Dev. 2010;77:94. doi: 10.1002/mrd.21123. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X. Lv Z. Liu L. Wang L. Tong M. Zhou Q. Derivation of embryonic stem cells from Brown Norway rats blastocysts. J Genet Genomics. 2010;37:467–473. doi: 10.1016/S1673-8527(09)60066-7. [DOI] [PubMed] [Google Scholar]
- 17.Schwartzberg PL. Goff SP. Robertson EJ. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989;246:799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- 18.Seong E. Saunders TL. Stewart CL. Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K. Lopes SM. Tang F. Surani MA. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payer B. Chuva de Sousa Lopes SM. Barton SC. Lee C. Saitou M. Surani MA. Generation of stella-GFP transgenic mice: a novel tool to study germ cell development. Genesis. 2006;44:75–83. doi: 10.1002/gene.20187. [DOI] [PubMed] [Google Scholar]
- 21.Kisseberth WC. Brettingen NT. Lohse JK. Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 22.He H. McHaney M. Hong J. Weiss ML. Cloning and characterization of 3.1kb promoter region of the Oct4 gene from the Fischer 344 Rat. Open Stem Cell J. 2009;1:30–39. doi: 10.2174/1876893800901010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asanoma K. Rumi MA. Kent LN. Chakraborty D. Renaud SJ. Wake N. Lee DS. Kubota K. Soares MJ. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brons IG. Smithers LE. Trotter MW. Rugg-Gunn P. Sun B. Chuva de Sousa Lopes SM. Howlett SK. Clarkson A. Ahrlund-Richter L. Pedersen RA. Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 25.Debeb BG. Galat V. Epple-Farmer J. Iannaccone S. Woodward WA. Bader M. Iannaccone P. Binas B. Isolation of Oct4-expressing extraembryonic endoderm precursor cell lines. PLoS One. 2009;4:e7216. doi: 10.1371/journal.pone.0007216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galat V. Binas B. Iannaccone S. Postovit LM. Debeb BG. Iannaccone P. Developmental potential of rat extraembryonic stem cells. Stem Cells Dev. 2009;18:1309–1318. doi: 10.1089/scd.2009.0115. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS. Rumi MA. Konno T. Soares MJ. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis. 2009;47:433–439. doi: 10.1002/dvg.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma GT. Soloveva V. Tzeng SJ. Lowe LA. Pfendler KC. Iannaccone PM. Kuehn MR. Linzer DI. Nodal regulates trophoblast differentiation and placental development. Dev Biol. 2001;236:124–135. doi: 10.1006/dbio.2001.0334. [DOI] [PubMed] [Google Scholar]
- 29.Tesar PJ. Chenoweth JG. Brook FA. Davies TJ. Evans EP. Mack DL. Gardner RL. McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 30.Nichols J. Smith A. Buehr M. Rat and mouse epiblasts differ in their capacity to generate extraembryonic endoderm. Reprod Fertil Dev. 1998;10:517–525. doi: 10.1071/rd98075. [DOI] [PubMed] [Google Scholar]
- 31.Chuykin I. Lapidus I. Popova E. Vilianovich L. Mosienko V. Alenina N. Binas B. Chai G. Bader M. Krivokharchenko A. Characterization of trophoblast and extraembryonic endoderm cell lineages derived from rat preimplantation embryos. PLoS One. 2010;5:e9794. doi: 10.1371/journal.pone.0009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K. Sengupta S. Magnani L. Wilson CA. Henry RW. Knott JG. Brg1 is required for Cdx2-mediated repression of Oct4 expression in mouse blastocysts. PLoS One. 2010;5:e10622. doi: 10.1371/journal.pone.0010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faria TN. Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- 34.Hunt JS. Atherton RA. Pace JL. Differential responses of rat trophoblast cells and embryonic fibroblasts to cytokines that regulate proliferation and class I MHC antigen expression. J Immunol. 1990;145:184–189. [PubMed] [Google Scholar]
- 35.Wang KI. Ho HN. Misra DN. Kunz HW. Gill TJ., III Isolation and characterization of rat trophoblast cells. Am J Reprod Immunol Microbiol. 1988;16:8–14. doi: 10.1111/j.1600-0897.1988.tb00170.x. [DOI] [PubMed] [Google Scholar]