Abstract
Several polymorphisms in the Disrupted-in-Schizophrenia-1 (DISC1) gene are reported to be associated with schizophrenia. However, to date, there has been little effort to evaluate the evidence for association systematically. We carried out an imputation-driven meta-analysis, the most comprehensive to date, using data collected from 10 candidate gene studies and three genome-wide association studies containing a total of 11 626 cases and 15 237 controls. We tested 1241 single-nucleotide polymorphisms in total, and estimated that our power to detect an effect from a variant with minor allele frequency >5% was 99% for an odds ratio of 1.5 and 51% for an odds ratio of 1.1. We find no evidence that common variants at the DISC1 locus are associated with schizophrenia.
Keywords: association, case–control, DISC1, GWAS, meta-analysis, schizophrenia
Introduction
Schizophrenia is a debilitating psychiatric illness with a lifetime prevalence of around 0.4%1 and a heritability estimated to be between 80 and 85%.2, 3 One schizophrenia susceptibility gene that has attracted much attention is Disrupted-in-Schizophrenia-1 (DISC1). DISC1 is one of the three genes (with DISC2 and TSNAX) lying at the site of a balanced t(1;11)(q42.1;q14.3) translocation, which has a strong association (logarithm of the odds ratio=7.1) with schizophrenia and other mental illness in a large Scottish pedigree.4, 5
Further evidence that DISC1 genetic variants predispose to schizophrenia in particular comes from four sources. First, the expression of the protein product DISC1 is reduced in the Scottish pedigree in which the translocation segregates. Second, DISC1 interacts physically with PDE4B. Another Scottish family with schizophrenia has a translocation that reduces the expression of PDE4B.6 Third, association between schizophrenia and variants at the DISC1 locus has been reported.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Fourth, sequencing part of the DISC1 gene in 288 cases identified six rare coding variants not found in 10 000 controls.21
These findings, along with evidence of DISC1's involvement in other mental illnesses including bipolar disorder,19 depression11 and autism,22 have spurred research into understanding what DISC1 does in the brain. Studies of DISC1's interactome have revealed that it is involved in synaptic development pathways23 and interacts with proteins important for neural development such as NDEL1.24, 25, 26 These results seem promising for DISC1's role in schizophrenia in light of the common hypothesis that the origin of the condition is neurodevelopmental.
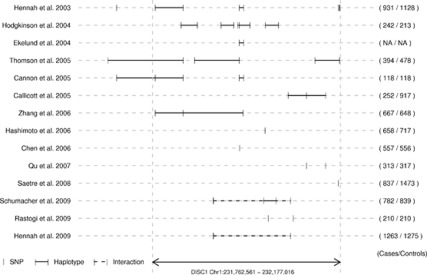
However, there has been no comprehensive meta-analysis of schizophrenia association study findings for DISC1, despite the fact that the association evidence is not totally consistent. Association has been reported over almost the entire gene (Figure 1; refs.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20), but the largest study to date failed to detect any association,27 and genome-wide association studies (GWAS) have not found a clear signal at DISC1.
Figure 1.
Reported Disrupted-in-Schizophrenia-1 (DISC1) associations including both family and association studies from populations of all ethnicities and indicating regions reported as being significantly associated with schizophrenia.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 These includes family-based linkage studies and studies in non-European populations, which were not included in this study. The numbers to the right of the graph indicate numbers of cases/controls in the study.
Meta-analysis of DISC1 findings is difficult to carry out because the gene is large (414 kb) and includes many variants (2603 single-nucleotide polymorphisms (SNPs) in single-nucleotide polymorphism database (dbSNP) 131). There is no consensus about which markers have the strongest association, no markers are tested in all studies and most are tested in only a small number of studies. Therefore, we decided to try to combine data from studies that use different markers by imputation. This method estimates results from a marker that has not been genotyped by exploiting linkage disequilibrium with markers that have been genotyped.
Until recently, there was insufficient sequence information at the DISC1 locus to make imputation feasible; however, release of data from phase 3 of the International HapMap project28 and from the first 1000 Genomes pilot29 provide the most comprehensive catalog of common human genetic variation available today, including rare variants. We set out to use these publicly available data to impute genotypes in DISC1 association studies and to combine results from all available sources, including both GWAS and candidate gene studies, thus overcoming the problems described above.
Methods
Selection of studies for inclusion
We identified candidate gene studies and GWAS for schizophrenia or schizoaffective disorder in unrelated case–control samples drawn from populations of predominantly European ancestry with at least 100 cases and controls. Studies reporting data on both male and female cases and controls were included. We did not include studies where data from multiple individuals was pooled and analyzed together. We excluded studies where the samples overlapped significantly with another identified study.
Systematic literature search
We considered all studies listed for DISC1 on the SZGene database.30 We also searched PubMed with the search terms ‘DISC1' AND ‘Schizophrenia' AND ‘Association' and also for the terms ‘Schizophrenia' AND ‘GWAS'. Once these had been collected, their bibliographies were then searched for additional references. The abstracts of studies thus identified were examined with respect to the inclusion criteria above. Studies failing to meet the criteria were discarded, and the full text of remaining studies was examined to determine whether or not they should be included.
Data extraction
For each study, the following data were extracted: (1) author(s) and year of publication; (2) sample size; and (3) data (individual genotypes, genotype counts or minor allele frequencies (MAFs) in order of preference). Where the required data were not available in the published study or from web-based supplementary information, we contacted the authors of the paper by email to request access to the data. For GWAS, we requested data for the region extending 100 kb upstream of DISC1 and downstream of TSNAX (Chr1: 231 572 000–232 277 000 in the hg19 genome assembly), which covers all reported associations.
Quality control
In addition to any quality control performed by the authors of the studies, we excluded any SNPs that failed the following tests: (1) MAF in cases or controls <0.01. (2) Where alleles were given in the study data, we checked them against dbSNP alleles31 and excluded the SNP if the reported alleles differed from the dbSNP alleles and could not be explained by a strand flip. We also excluded the SNP if no alleles were specified in the study data and the MAF was >0.45; otherwise, we coded the SNP by the minor allele. (3) For studies where we had genotype counts available, we excluded SNPs that had a Hardy–Weinberg equilibrium test P-value of <0.001.
Imputation
One of the problems encountered in previous attempts at meta-analysis is the lack of overlap between studies in terms of tested SNPs. We addressed this problem by imputing genotypes for studies where we had full genotype data. Imputation was carried out using IMPUTE v.2,32, 33 with reference panels of CEU haplotypes from HapMap 3 and the 1000 Genomes pilot 1. The CEU haplotypes are taken from samples from the Centre d'Etude du Polymorphisme Humain collected from individuals living in Utah, USA, with ancestry from northern and western Europe. The haplotype data were obtained from the IMPUTE website (http://mathgen.stats.ox.ac.uk/impute/impute.html). For studies where we did not have full genotype data (we had genotype counts or MAFs), we imputed MAFs using the linear predictor implemented in the BLIMP package34 and the CEU haplotypes from 1000 Genomes pilot 1. We attempted to impute for all studies that genotyped at more than 10 SNPs.
Size and power calculations
To calculate the size and power of the meta-analysis, we simulated case–control data from haplotypes drawn from the 1000 Genomes pilot 1. To calculate the P-value required to obtain a given significance level, we sampled data from the 1000 Genomes haplotypes, with the same characteristics as the studies in the sample (numbers of cases, controls and testing the same SNPs), calculated effect sizes and P-values as described below and obtained the empirical significance levels for each SNP. We repeated the simulation and determined the required nominal point-wise significance level required to achieve a gene-wide significance level of 0.05.
To calculate the power of the meta-analysis, we again sampled from the 1000 Genomes data with the same characteristics as the meta-analysis samples, but in each simulation, we chose a SNP at random and weighted the sampling probabilities in cases to ensure that the chosen SNP had a specified odds ratio (OR) in cases relative to controls. We performed the analysis as described below and recorded whether the significance level determined above was reached by any SNP. The proportion of tests with any SNP reaching significance gave us an estimate of the power of the meta-analysis for a given effect size. For size calculations, we performed 10 000 simulations, and for power calculations, we performed 1000 per data point.
Statistical analysis
Log-ORs (equivalent to logistic regression coefficients) and their standard deviations were calculated for each SNP in each study and then combined across studies using fixed and random effects models.35 We tested whether any of the pooled P-values were more significant than the thresholds calculated above. We also tested whether the distribution of P-values differed significantly from that expected under the null hypothesis. For each SNP, we investigated whether there was any evidence for heterogeneity using the I2 statistic.36
For the imputed genotypes, we calculated the test statistics using SNPTEST,32 which takes into account the genotype uncertainty generated by the imputation. For imputed allele frequencies, we calculated the proportion of the genotypic variance explained by the imputation, and filtered out any SNP where this was below 80%. We then calculated test statistics as for the original data. These were then combined with the test statistics from genotyped SNPs as above. We considered separately: (1) the genotyped data; (2) the genotyped data plus the imputed data, but considered only loci that were genotyped in at least one study; and (3) the genotyped data plus all the imputed data. We removed each study from the meta-analysis in turn to determine whether any single study had a disproportionate effect on the analysis. We also calculated Wakefield approximate Bayes factors37 from estimates of the standardized effect sizes and their variances, assuming a prior for the effect size that was normally distributed with mean 0 and variance 0.015. This prior is equivalent to the belief that for each SNP, there is a 95% probability that its OR lies between 0.79 and 1.28.
Results
We obtained data for 11 candidate gene studies and four GWAS containing a total of 11 626 cases and 15 237 controls (Table 1). We could not obtain data from one case–control study and one GWAS matching our criteria because there was no response from the authors. Three studies were excluded because they overlapped substantially with other studies (Table 2). The total number of separate SNPs genotyped in any study was 401.
Table 1. Details of studies analyzed.
| SNPs tested |
|||||
|---|---|---|---|---|---|
| Study | Year | Gen | Imp | Cases | Controls |
| Devon et al.CM53 | 2001 | 3 | 0 | 656 | 344 |
| Hodgkinson et al.CF14 | 2004 | 38 | 62 | 242 | 213 |
| Wood et al.CF54 | 2007 | 74 | 328 | 451 | 291 |
| Song et al.CS21 | 2008 | 3 | 0 | 288 | 288 |
| Saetre et al.CS17 | 2008 | 15 | 52 | 837 | 1473 |
| Sanders et al.CF27 | 2008 | 114 | 521 | 1870 | 2002 |
| O'Donovan et al.GM55 | 2008 | 115 | 180 | 478 | 2937 |
| Betcheva et al.CM56 | 2009 | 14 | 46 | 255 | 556 |
| Hennah et al.CS12 | 2009 | 62 | 136 | 1263 | 1275 |
| Rastogi et al.CS16 | 2009 | 9 | 0 | 210 | 210 |
| Schumacher et al.CM18 | 2009 | 108 | 210 | 782 | 839 |
| Need et al.GS57 | 2009 | 72 | 138 | 673 | 871 |
| ISC et al.GF38 | 2009 | 224 | 832 | 3322 | 3587 |
| Athanasiu et al.GF58 | 2010 | 250 | 955 | 298 | 351 |
Abbreviations: C, candidate gene(s); F, full genotypes; G, genome-wide association studies; Gen, genotyped; Imp, imputed; M, minor allele frequencies; S, summary genotypes; SNP, single-nucleotide polymorphism.
Table 2. Studies that met our inclusion criteria, but which were not included.
| Study | Year | Reason for exclusion |
|---|---|---|
| Thomson et al.19 | 2005 | Samples reported in Hennah et al.12 |
| Zhang et al.20 | 2006 | Samples partially reported in Hennah et al.12 |
| Lencz et al.59 | 2007 | Samples reported in Hodgkinson et al.14 |
| Nicodemus et al.60 | 2007 | No response from authora |
| Stefansson et al.61 | 2009 | No response from authorb |
‘No response from author' refers to email response to the corresponding author, specifically.
We received an initial response, but no response to two further emails.
No response after three initial emails.
Imputation dramatically increased the number of studies testing each SNP. Before imputation, 100 SNPs were tested in a single study only. After imputation, there were only 13 such SNPs. The mean number of studies testing each SNP increased from 2.6 to 4.3, if only sites genotyped in a single study were considered, and to 3.4 if all imputed sites were used. Ultimately, we obtained data on a total of 1241 separate SNPs. Many of the SNPs in the full imputed data set were tested in only two studies and were heavily dependent on the International Schizophrenia Consortium data.38
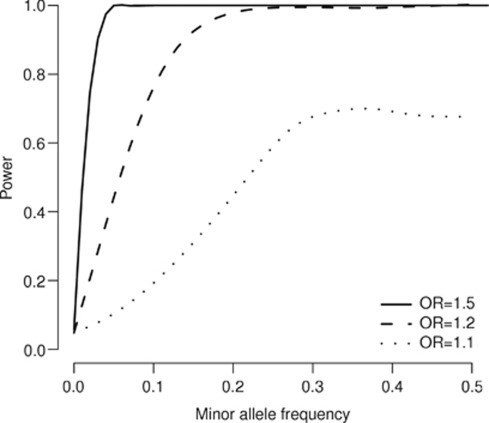
For testing the 401 genotyped loci, we used a point-wise significance level of 1.92 × 10−4 corresponding to a gene-wide significance level of 0.05. We generated this threshold by simulation, using the exact SNPs and sample sizes available in the included studies. It is equivalent to a multiple testing correction for 260 (95% confidence interval 250–269) independent tests. For the 1241 SNPs in the imputed data, the correction is equivalent to 444 independent tests (95% confidence interval 424–467). With these cutoffs, we estimated that for common variants (MAF >5%), the power to detect a true association at OR=1.5, with the imputed data restricted to genotyped loci was 99%. For OR=1.1 the power was 51%.
For rare variants, power is much lower. For variants with MAF between 1 and 5%, the corresponding figures are 92 and 6%. For variants with MAF below 1%, which we did not try to test, the power is very low, even for high ORs. These figures are the power to detect an association at any locus given the OR at the causal locus, which is not necessarily the one detected. Figure 2 shows power as a function of MAF for different ORs at the causal allele.
Figure 2.
Estimated power as a function of minor allele frequency at the causal allele, showing results for different odds ratios. The power was simulated using variants and haplotypes from the 1000 Genomes data and the curves have been fitted as smooth splines of degree 6.
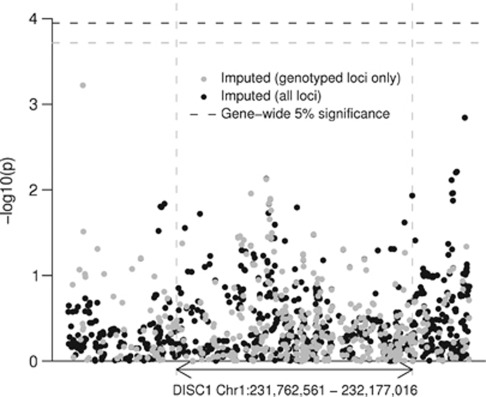
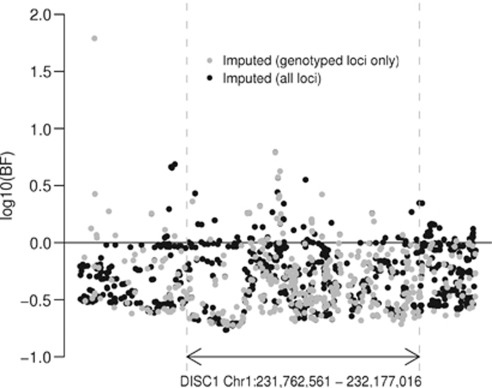
Using these significance thresholds, we detected no significant association with any tested SNP (Figures 3 and 4). The most associated SNP was rs1765805, 160 kb upstream of DISC1 (P=6 × 10−4, genotyped in 1860 cases and 2389 controls), but this was heavily dependent on a single study38 and not supported by nearby SNPs. The next highest associations were found in the regions of rs1331046 (P=1.4 × 10−3, imputed in 3610 cases and 3938 controls), downstream of the gene, and rs17817356 (P=7 × 10−3, genotyped in 4405 cases and 7427 controls, imputed in 2291 cases and 2293 controls), between exons 6 and 7, and about 30 kb upstream from DISC2. Like rs1765805, the association at rs1331046 was driven by the ISC data, but the association at rs17817356 was supported by the ISC and other data sets as well. In the random effects analysis, the associations at rs1765805 and rs17817356 remained at the same significance level, but the association at rs1331046 was substantially reduced (P>0.03).
Figure 3.
Pooled P-values for association. Each point represents a single-nucleotide polymorphism (SNP). The horizontal axis indicates chromosomal position, and the vertical axis indicates negative log10 of the fixed-effect pooled P-value for that SNP. The black points show the imputed SNPs and the gray points show the subset of the imputed SNPs, which were genotyped in at least one study. The horizontal dashed lines show the corrected significance level, which is equivalent to a 5% gene-wide significance level for each data set.
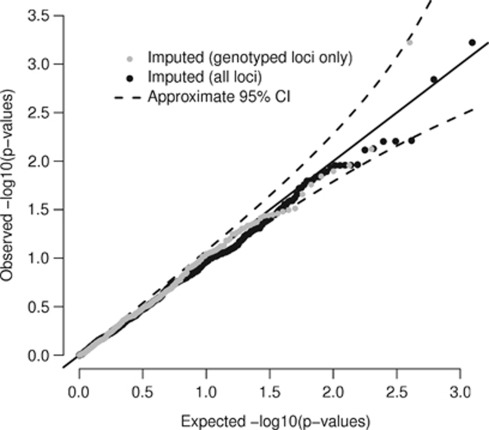
Figure 4.
Quantile–quantile (QQ) plot of P-values. QQ plot of the P-values shown in Figure 2. The solid line shows the expected values under the null hypothesis of no association and the dashed lines show the upper and lower 95% quantiles.
Excluding the ISC study significantly altered our results, and was the only study whose omission did so. As noted above, the associations at rs1765085 and rs1331046 were supported only by the ISC. However, this may be because few other studies tested these SNPs. Both SNPs are outside the gene, in a region where we had lower coverage, as most candidate gene studies have concentrated on SNPs inside the gene.
To assess heterogeneity, we calculated the I2 statistic for all SNPs. This was zero for the majority (70%) of loci and distributed between 0 and 80% for the rest. Tests of heterogeneity are generally underpowered when, as here, there are few studies,39, 40 so we cannot make conclusive statements about the presence or absence of heterogeneity. However, given that most genetic associations display between-study heterogeneity41 and that fixed and random effects models give the same results when there is no heterogeneity, it seems prudent to use the more conservative random effects model to estimate effect size or to prioritize markers for further analysis.
Using the effects estimated by the random effects model, the approximate Bayes factors reveal a similar pattern to the P-values (Figure 5), and indicate that the region around rs17817356 has the most evidence in favor of association and that there is no consistent evidence for association elsewhere in the gene.
Figure 5.
Wakefield approximate Bayes factors for each single-nucleotide polymorphism (SNP). Calculated from the random effects pooled P-values and Z-scores assuming a normal prior on the log-OR, with mean 0 and variance of 0.015. A Bayes factor >0 is evidence for association, whereas a Bayes factor <0 is evidence against.
There are three commonly tested non-synonymous SNPs in the gene, rs3738401, rs821616 and rs6675281. The variants rs821616 and rs6675281 have been reported to have functional effects, affecting centrosomal PCM1 localization,42, 43 expression of splice variants,44 hippocampal function45 and interaction with NDEL1, which is important for neurite outgrowth.46 However, we detected no significant associations at any of these SNPs (P=0.11, 0.51 and 0.93 in the raw data and 0.49, 0.60 and 0.28 in the imputed).
Given the potential contribution of rare variants in DISC1 to schizophrenia risk, we searched additional sources of data to identify further functional variants not represented in dbSNP. In the 1000 Genomes pilot data, we found one additional non-synonymous coding SNP. We also looked in the results from a recent exome sequencing study of 200 Danish individuals47 and identified five novel non-synonymous SNPs. Of these six variants, two had been discovered in cases and controls by Song et al.21 and four were novel (Table 3).
Table 3. Non-synonymous SNPs not found in dbSNP identified in 260 individuals.
| Position (hg18) | NT substitution | AA substitution | Source | Song et al.? |
|---|---|---|---|---|
| 229 896 375 | C/T | A83V | 47 | Yes |
| 229 896 606 | G/T | W160L | 47 | No |
| 229 896 987 | C/T | P287L | 47 | No |
| 229 897 063 | T/G | C312W | 47 | No |
| 229 969 594 | T/C | I452F | 47 | No |
| 230 969 594 | A/T | E751Q | 29 | Yes |
Abbreviation: AA, amino acid; dbSNP, single-nucleotide polymorphism database; NT, nucleotide. Two have been found in a previous study and four are novel.
Discussion
We have carried out a comprehensive analysis of candidate schizophrenia susceptibility variants in the DISC1 gene. We found no significant association with any variant. We calculated approximate Bayes factors for each SNP to identify the regions where there was most evidence of association and found that the best evidence of association was in a region between exons 6 and 7. Our meta-analysis had to deal with the problem that of 401 variants reported in the literature, 120 markers were tested in a single study and a further 110 in just two studies. The lack of overlap in the markers tested in different studies poses a challenge to meta-analysis that we overcame by imputing genotypes. By combining results from genome-wide association and candidate gene studies together with LD data from the HapMap and 1000 Genomes projects, we were able to test the involvement of 1241 SNPs in a combined sample of 11 626 cases and 15 237 controls.
Our results raise four questions. First, what range of effects have we tested? Second, to what extent have we excluded the involvement of common variants throughout the gene? Third, can we draw any conclusions about the role of rare variants at the DISC1 locus? Finally, why have we been unable to replicate the many previously reported associations?
Power calculations help to answer the first question (Figure 2). Power depends critically on the sample size, which is not straightforward to estimate. Although the total number of cases and controls in our sample was 11 626 and 15 237, not every SNP was tested in every individual. In the raw data, the mean numbers of cases and controls testing any single SNP were 2781 and 3782. We cannot directly calculate the sample sizes for the imputed data as we used imputed genotype probabilities in our tests, but comparison of the variance of test statistics suggests that the mean improvement was equivalent to increasing the sample size by 49% to a mean of 4144 cases and 5653 controls. These figures are heavily skewed in the sense that some variants have much larger sample sizes. Imputation also allowed us to test variants that had not been genotyped in any study.
Our power calculations are only approximate as we sampled from a fairly limited set of haplotypes. However, they can give us an indication of what effects we have tested. For variants with MAF >5%, we had 99% power to detect an effect with OR=1.5 and 51% power to detect an effect with OR=1.1. However, it is possible that effects will be too small to detect, even for high-frequency variants. Robust association for common variants with OR of <1.1 have been reported in a study of height48 and it is not inconceivable that similarly small effects operate in schizophrenia.49 It has been argued that such variants can explain a significant proportion of the heritability for common diseases and traits.38, 49, 50
Results from the HapMap and 1000 Genomes projects provide an answer to the second question. Genotyped SNPs included 32% of all SNPs with MAF >5% in the 1000 Genomes, and imputed SNPs included 89%. For SNPs with MAF between 1 and 5%, the corresponding figures are 10 and 41%. For genotyped loci, 95% of the gene is within 5.0 kb of a tested SNP, 50% is within 1.4 kb and the maximum distance without a tested SNP is 13.7 kb. For the imputed data, the figures are 2.6, 0.6 and 7.2 kb. These small regions make it unlikely that any common SNP could exist that we have not, at least indirectly, tested.
Third, the effects may arise from alleles too rare to be tested here. It is not surprising that common variants do not have a large effect on susceptibility to such a debilitating disease, but we might expect rare variants to have larger effects. Although we tested variants down to an MAF of 1%, we had very low power at this level, and we could not even test all the known variants of this frequency. Further stages of the 1000 Genomes project will allow most variants at this frequency to be tested. On the other hand, association between DISC1 and the disease might derive from extremely rare, essentially private, mutations of large effect.51, 52 One study reported the identification of coding mutations in schizophrenia cases that were not detected in 10 000 control subjects.21 However, this study did not search in healthy controls for rare variants that were not found in cases. In two studies sampling 260 individuals in total, we found four novel non-synonymous mutations, and rediscovered two that had been identified in the previous study. This suggests that coding mutations in DISC1 may be more common in healthy individuals than previously assumed. However, it should also be noted that control subjects were not actively screened for the absence of schizophrenia, a family history of schizophrenia or related mental illnesses.
Finally, many previous studies have reported association. There are a number of reasons why we might have failed to replicate these results; publication bias, winner's curse, allelic heterogeneity and population, sex or subgroup-specific heterogeneity. We have not attempted to investigate these here, but it is worth noting that the presence of heterogeneity of effects would particularly tend to confound large GWAS and meta-analyses such as this one.
Our meta-analysis does not address the question of DISC1's involvement in other mental illness, such as bipolar disorder, major depressive disorder or autism spectrum disorder, nor does it answer all the questions with regard to the association of DISC1 with schizophrenia. Although we found no significant association with common variants, many studies have reported sex-specific and interaction effects,9, 11, 12, 18, 19 which we did not test. Consideration of the power required to detect such effects suggests that if they do exist, significantly larger studies will be required to detect them convincingly. For example, if we were to test all pairwise SNP–SNP interactions for all imputed SNPs in this study, we would be increasing the multiple testing factor by around 100 000. We would also be reducing the sample size for each test, particularly for combinations of rare variants, making it difficult, if not impossible, to reach statistical significance. Hypothesis-driven tests derived from functional studies and model systems may prove more productive than data mining in this case.
Whatever the true effect of the factors described above, it seems likely that we will need to consider new study designs and new statistical techniques to further investigate the effect of DISC1 variation on schizophrenia and other psychiatric illnesses.
Acknowledgments
Iain Mathieson and Jonathan Flint are supported by the Wellcome Trust. We thank all the researchers who provided data for this study.
The authors declare no conflict of interest.
References
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:413–433. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait—evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- Jacobs PA, Brunton M, Frackiewicz A, Newton M, Cook PJL, Robson EB. Studies on a family with three cytogenetic markers. Ann Hum Genet. 1970;33:325–336. [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Chen QY, Chen Q, Feng GY, Lindpaintner K, Wang LJ, Chen ZX, et al. Case-control association study of Disrupted-in-Schizophrenia-1 (DISC1) gene and schizophrenia in the Chinese population. J Psychiat Res. 2007;41:428–434. doi: 10.1016/j.jpsychires.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Hennah W, Hiekkalinna T, Parker A, Meyer J, Lönnqvist J, et al. Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol Psychiatry. 2004;9:1037–1041. doi: 10.1038/sj.mp.4001536. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14:865–873. doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- Hennah W, Varilo T, Kestilä M, Paunio T, Arajärvi R, Haukka J, et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12:3151–3159. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Tang F, Yue W, Ruan Y, Lu T, Liu Z, et al. Positive association of the Disrupted-in-Schizophrenia-1 gene (DISC1) with schizophrenia in the Chinese Han population. Am J Med Genet B. 2007;144B:266–270. doi: 10.1002/ajmg.b.30322. [DOI] [PubMed] [Google Scholar]
- Rastogi A, Zai C, Likhodi O, Kennedy JL, Wong AH. Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res. 2009;114:39–49. doi: 10.1016/j.schres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Saetre P, Agartz I, De Franciscis A, Lundmark P, Djurovic S, Kãhler A, et al. Association between a disrupted-in-schizophrenia 1 (DISC1) single nucleotide polymorphism and schizophrenia in a combined Scandinavian case–control sample. Schizophr Res. 2008;106:237–241. doi: 10.1016/j.schres.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Laje G, Abou Jamra R, Becker T, M°hleisen TW, Vasilescu C, et al. The DISC locus and schizophrenia: evidence from an association study in a central European sample and from a meta-analysis across different European populations. Hum Mol Genet. 2009;18:2719–2727. doi: 10.1093/hmg/ddp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson PA, Wray NR, Millar JK, Evans KL, Hellard SL, Condie A, et al. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population Mol Psychiatry 200510657–668.616. [DOI] [PubMed] [Google Scholar]
- Zhang F, Sarginson J, Crombie C, Walker N, St Clair D, Shaw D. Genetic association between schizophrenia and the DISC1 gene in the Scottish population. Am J Med Genet B. 2006;141B:155–159. doi: 10.1002/ajmg.b.30274. [DOI] [PubMed] [Google Scholar]
- Song W, Li W, Feng J, Heston LL, Scaringe WA, Sommer SS. Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem Bioph Res Co. 2008;367:700–706. doi: 10.1016/j.bbrc.2007.12.117. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Handford EJ, Schurov I, Rain JC, Pelling M, Duran-Jimeniz B, et al. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin RM, Abecasis GR, Altshuler DL, Auton A, Brooks LD, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Database of Single Nucleotide Polymorphisms (dbSNP) Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine(dbSNP Build ID: 131). http://www.ncbi.nlm.nih.gov/SNP/ (accessed 27th June 2010).
- Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Stephens M. Using linear predictors to impute allele frequencies from summary or pooled genotype data. Ann Appl Stat. 2010;4:1158–1182. doi: 10.1214/10-aoas338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Wakefield J. Bayes factors for genome-wide association studies: comparison with P-values. Genet Epidemiol. 2009;33:79–86. doi: 10.1002/gepi.20359. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index. Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Kavvoura FK, Ioannidis JPA. Methods for meta-analysis in genetic association studies: a review of their potential and pitfalls. Hum Genet. 2008;123:1–14. doi: 10.1007/s00439-007-0445-9. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Hodgkinson CA, Harrison PJ. DISC-1 Leu607Phe alleles differentially affect centrosomal PCM1 localization and neurotransmitter release. Mol Psychiatry. 2009;14:556–557. doi: 10.1038/mp.2009.13. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Walker M, Hyde TM, Kleinman JE, Harrison PJ. The DISC1 Ser704Cys substitution affects centrosomal localization of its binding partner PCM1 in glia in human brain. Hum Mol Genet. 2010;19:2487–2496. doi: 10.1093/hmg/ddq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci USA. 2009;106:15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan CX, Morita M, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Li Y, Vinckenbosch N, Tian G, Huerta-Sanchez E, Jiang T, Jiang H, et al. Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding variants. Nat Genet. 2010;42:969–972. doi: 10.1038/ng.680. [DOI] [PubMed] [Google Scholar]
- Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40:584–591. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Visscher PM. Narrowing the boundaries of the genetic architecture of schizophrenia. Schizophr Bull. 2010;36:14–23. doi: 10.1093/schbul/sbp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JA, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Porteous DJ. Rethinking the genetic architecture of schizophrenia. Schizophr Res. 2010;117:222. doi: 10.1017/S003329171000070X. [DOI] [PubMed] [Google Scholar]
- Devon RS, Anderson S, Teague PW, Burgess P, Kipari TM, Semple CA, et al. Identification of polymorphisms within Disrupted in Schizophrenia 1 and Disrupted in Schizophrenia 2, and an investigation of their association with schizophrenia and bipolar affective disorder. Psychiatr Genet. 2001;11:71–78. doi: 10.1097/00041444-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Wood LS, Pickering EH, Dechairo BM. Significant support for DAO as a schizophrenia susceptibility locus: examination of five genes putatively associated with schizophrenia. Biol Psychiatry. 2007;61:1195–1199. doi: 10.1016/j.biopsych.2006.07.005. [DOI] [PubMed] [Google Scholar]
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Betcheva ET, Mushiroda T, Takahashi A, Kubo M, Karachanak SK, Zaharieva IT, et al. Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J Hum Genet. 2009;54:98–107. doi: 10.1038/jhg.2008.14. [DOI] [PubMed] [Google Scholar]
- Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, et al. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009;5:e1000373. doi: 10.1371/journal.pgen.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiu L, Mattingsdal M, Kähler AK, Brown A, Gustafsson O, Agartz I, et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiat Res. 2010;44:748–753. doi: 10.1016/j.jpsychires.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Morgan TV, Athanasiou M, Dain B, Reed CR, Kane JM, et al. Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Mol Psychiatry. 2007;12:572–580. doi: 10.1038/sj.mp.4001983. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, et al. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]