Abstract
Differences in adult attachment may concord with differences in social perception. The present study aimed to measure neural activity associated with the presentation of visual social stimuli. In an affective oddball paradigm, event-related brain potentials were recorded while participants viewed negative, positive, and neutral images of people and categorized them according to valence. Brain response amplitudes were examined across valence categories and across attachment groups. Results revealed differences between anxious and avoidant groups in “emotion bias.” The avoidant group displayed a bias towards more neural activation in response to negative compared to positive images. The anxious group trended in the opposite direction. Results are discussed in terms of possible attachment-based differences in motivated attention to social stimuli.
Keywords: Attachment, Event-related brain potentials, Negativity bias, Late positive potential, Attention, Emotion, Social perception, Emotional images
1. Introduction
Perception is not reality. However, perceptions help to shape one’s own reality, in part by guiding behavior. For example, if others are habitually perceived as being hostile, then one is likely to shrink from them and live in relative isolation. By contrast, if others are habitually perceived as warm and inviting, one is likely to engage with them. Adult attachment theory provides a conceptual framework to aid in understanding these perceptual and behavioral differences. Attachment theorists have predicted that an individual’s attachment style affects the way in which incoming interpersonal information is automatically processed and encoded (e.g. Shaver & Mikulincer, 2003); interpersonal perceptions are an integral aspect of attachment behavior (Shaver & Mikulincer, 2002). Furthermore, the domain of interpersonal perceptions which are attachment-relevant may include more than just perceptions of close relationship partners. Shaver and Mikulincer (2003) posited that individual differences in attachment-system functioning that began with singular relationships in childhood can form the foundation upon which later, more global social appraisals are built. The authors posit that insecure attachment styles are associated with biased automatic encoding of incoming information. This results in social appraisals that are biased towards conforming with expectations that are congruent with one’s attachment style (Dykas & Cassidy, 2011; Shaver & Mikulincer, 2003). Brumbaugh and Fraley (2007) found indirect support for that notion in the relative consistency of attachment patterns transferred onto novel others that resembled attachment partners. Since the novel others were mere hypothetical constructs, it is interesting to note that attachment feelings were immediately transferred onto them. It is therefore important to consider what immediate effects attachment styles might have on the neurological processing of novel others. Neurological processing is of course immensely complex, but insight can be gained by narrowing the investigation to comparing two basic motivational categories: positive and negative.
The two categories of positive and negative are basic and vital in motivated behavior (see e.g. Norris, Gollan, Berntson, & Cacioppo, 2010, for an overview). Most often, one is likely to approach what is perceived as positive and withdraw from what is perceived as negative. Such phenomena can be extended to include approach and withdrawal behaviors in the social domain (see e.g. Gable & Berkman, 2008). It is important to note that situations and people are rarely objectively positive or negative. The answer to why some people approach and others avoid may lie in the strength of subjective perceptual processing of positive versus negative motivational cues. In this vein, an exploration of the role of the negativity bias in attachment behavior may be important. The negativity bias refers to the general tendency for negative information to outweigh positive information. According to Rozin and Royzman (2001), “the principle, which we call negativity bias, is that in most situations, negative events are more salient, potent, dominant in combinations, and generally efficacious than positive events” (p. 297). Evidence for the general accuracy of this principle comes from a variety of domains as diverse as impression formation (Peeters, 1971), politics (Jordan, 1965), risk-taking (Kahneman & Tversky, 1979), emotional reactivity (Taylor, 1991), person perception (Rothbart & Park, 1986), and attentional capture (Pratto & John, 1991), to name a few (for reviews see Baumeister, Bratslasky, Finkenauer, & Vohs, 2001; Rozin & Royzman, 2001).
Despite this overall trend for a negative bias, different populations have been shown to vary in the extent and even the direction of bias displayed. For example, researchers have discovered an attenuation or reversal of the negativity bias in older adults (e.g. Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Wood & Kisley, 2006). In contrast, an increase in the negativity bias has been noted in other groups such as those with clinical depression and anxiety. For example, negative attributional styles (Abramson, Metalsky, & Alloy, 1989), and negative schemas (Beck, 1987) which give greater weight to negative aspects of one’s self and environment have both been associated with depression. Affective anxiety has been consistently associated with greater attentional biases towards threat-related information (for a meta-analysis see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). Additionally, excessively negative interpretive biases have been noted in a range of anxiety disorders such as social anxiety (Amir & Foa, 2001), PTSD (Foa, Steketee, & Rothbaum, 1989), and panic disorder (Clark & Beck, 2010). It is important to note that these group-based differences in the negativity bias are not simply curious artifacts. In each of the aforementioned groups, the biases are thought to be generative factors in behavior. For example, the increased negative biases in anxiety and depressive disorders are thought to be active ingredients in the generation and maintenance of symptoms (e.g. Beck, 1987; Clark & Beck, 2010). These biases, as in social anxiety (Amir & Foa, 2001) can facilitate the avoidance of other people. From this perspective, examination of the relationship between the negativity bias and attachment behavior, especially attachment avoidance, may be useful.
An interpersonal negativity bias should be facilitative of general interpersonal distancing. Accordingly, avoidant attachment has been linked with more negative views of relationship partners (e.g. Collins, 1996; Feeney & Noller, 1991), a more negative view of others (Bartholomew & Horowitz, 1991), and negative views of human nature (Collins & Read, 1990). In contrast to avoidance-related biases, it might be logical to infer the existence of a social positivity bias in attachment anxiety because of their desire for proximity maintenance. Indeed, anxious attachment has been conceptualized as involving a positive view of others (Bartholomew & Horowitz, 1991). However, that idea does not seem consistent with the accumulated evidence. Mikulincer and Shaver (2007) offer instead a nuanced view of attachment anxiety, proposing that it is associated with complex views of others which involve hopes for proximity attainment, yet doubts about a partner’s ability to consistently provide it. Development for these individuals is associated with frustration at the hands of close others, yet also with enough gratification so that bids for proximity are intensified (Cassidy & Berlin, 1994). Therefore, a somewhat negative view of others is adopted because important others have not provided optimal security (Mikulincer & Shaver, 2007). However, a totally negative view is not adopted because that would imply proximity seeking is hopeless. There is experimental evidence that supports these assessments.
For example, using the Implicit Association Test, DeWitte and De Houwer (2008a) found avoidance to be related to interpersonal distancing themes. Two experiments were conducted; one contrasted goal-related words (I want; I don’t want) with proximity and distance-related words. In the second experiment, the researchers replaced the goal-related words with valenced words (positive; negative). Attachment avoidance was related to the desire for distancing in the first experiment and the positivity of distancing in the second. Thus, avoidance was related to apparently greater neural accessibility to distancing information as connected to valence and goals. In contrast, attachment anxiety was found to be related to proximity goals, but only on explicit paper and pencil tasks. Such mixed results regarding anxiety and proximity goals have also been encountered elsewhere (e.g. Mikulincer, Shaver, Bar-On, & Ein-Dor, 2010), and may reflect the ambivalence associated with anxiety as described above (Mikulincer et al., 2010; Simpson, Rholes, & Nelligan, 1992).
Zhang and Hazan (2002) also provided some support for the notion of attachment-based differences in the negativity bias. Their experimental design was similar to the one used by Rothbart and Park (1986) which revealed a negativity bias in social judgments in a general sample. Rothbart and Park found that, overall, participants required more information to confirm others’ positive traits than to confirm negative ones. The converse was true when asked to disconfirm traits: Participants required more information to disconfirm negative traits than to disconfirm positive ones. Thus, Rothbart and Park found a negativity bias both in confirmation and disconfirmation of others’ traits. Zhang and Hazan applied the paradigm to adult attachment and found these judgments to vary with attachment dimensions. Although the authors did not discuss findings in terms of the negativity bias, their results indicated the following differences: High scores on the avoidance dimension were associated with a negativity bias that was similar to Rothbart and Parks’ overall findings. However, unlike the avoidance dimension, the anxiety dimension did not display a negativity bias.
Studies that employ facial images may be important, both because of the general adaptive relevance of facial expressions (Darwin, 1872/1965) and because of the importance of facial expressions in the child-caregiver relationship (Trevarthen, 1985). In a series of experiments, Fraley, Niedenthal, Marks, Brumbaugh, and Vicary (2006) employed a morph-movie paradigm in which facial expressions slowly shifted into and out of emotional expressions. Participants were asked to judge the point at which expressions began and ended. Highly anxious participants judged both the onset and offset of emotion more quickly than their less anxious counterparts. In contrast, no significant results were found for avoidance. The results therefore indicate that attachment anxiety is associated with a perceptual vigilance to changes in emotional expression. No negative or positive biases were discovered: the hyper-vigilance was equally evident across valence categories. However, these experiments were designed to measure perceptual vigilance to changes in emotional expressions; not the relative motivational significance of each emotional category itself.
More direct investigations of attentional deployment may bring additional insightbecause of the link between motivation and attention (e.g. Lang, Bradley, & Cuthbert, 1990). Dewitte and De Houwer (2008b) performed such an investigation using an emotional variation of Posner’s (1980) exogenous cueing task. Attentional biases were calculated as a function of reaction times on trials in which happy, angry, and neutral faces were presented as cues. Results revealed a main effect of attachment anxiety in which increases in anxiety were related to attention away from both angry and happy facial images. Interactions revealed that high scores on both anxiety and avoidance (fearful avoidance) best predicted these effects. However, Cooper, Rowe, Penton-Voak, and Ludwig (2009) failed to replicate these results. Additionally, their own results across three studies were so inconsistent that they questioned the validity of the emotional variation of Posner’s paradigm. Therefore, investigations using other measures of attention may be more appropriate.
A promising method for further examination of attachment-related social information processing uses scalp-recorded event-related potentials; the late-positive potential (LPP) waveform component may be particularly useful. The LPP is sensitive to emotional content of stimuli and has successfully demonstrated the existence of a negativity bias in general samples (Ito, Larsen, Smith, & Cacioppo, 1998). Furthermore, it is sensitive to group-based differences, indicating the elimination of the negativity bias in older adults (Wood & Kisley, 2006). Most importantly, it is thought to be a measure of motivated attention that reflects the amount of “motivational relevance” perceived in a stimulus (Schupp et al., 2000). This is because LPP amplitudes have been shown to covary with self-reports of affective valence and arousal, with increases in amplitudes covarying with increases in subjective ratings of extremity (Cacioppo, Crites, Gardner, & Berntson, 1994). This is true for both increasing positivity and increasing negativity: regardless of the direction of valence, the further from “neutral” a stimulus is perceived to be, the larger the resulting LPP amplitude. Accordingly, increases in the motivational relevance of stimuli engender more motivated attention and are reflected in larger LPP amplitudes. As regards the present study, attachment-based variation in social motivation may therefore be discernable.
The LPP has been employed in a previous study of attachment. Zilber, Goldstein, and Mikulincer (2007) examined LPP amplitudes in response to affective images, and found attachment-based differences in neural responses to negative images (as calculated by subtracting evoked amplitudes of neutral images). The difference in LPP amplitudes between negative and neutral targets was found to be greatest for individuals scoring above the mean on the anxiety axis of the Experiences in Close Relationships Scale (ECR). Thus, a kind of negativity bias was found for anxious individuals. However, the authors did not discuss the findings in terms of a negativity bias, nor did they conceive of their study in that fashion. Additionally, the negativity bias found by these researchers was not of the form as generally conceived, which is a comparison between positive and negative valence categories (Baumeister, Bratslasky, Finkenauer, & Vohs, 2001; Ito, Larsen, Smith, & Cacioppo, 1998; Rozin & Royzman, 2001). Furthermore, it is important to note that the emotion bias we are investigating regards social stimuli. The stimuli employed by Zilber et al. (2007) were a mixture of images, some with people and some without. Brain responses can differ in response to these two categories. A recent fMRI study of discovered that an area in the medial prefrontal cortex, among others, appears to be attuned to variations in social valence, but not in object valence (Harris, McClure, Van Den Bos, Cohen, & Fiske, 2007). Additionally, Ito and Cacioppo (2000) found that LPP amplitudes were higher for images with people than without. It is important to note that the existence of these differences does not detract from the validity of the results from Zilber et al. because their participants were exposed to each category equally. However, it is important in regards to the present research question which involves social perception. In this context it is important to differentiate between object and valence categories because there may be attachment-based variation between the two. For example, avoidant individuals may have large responses to positive images of objects but not to positive images of people; this could differ in the opposite way for attachment anxiety. The present study aims to avoid such difficulties and to extend the results of Zilber et al. by employing only target images of people and calculating the emotion bias by comparing amplitudes between negative and positive valence categories.
The present study was based on an affective oddball paradigm, similar to the one employed by by Ito, Larsen, Smith, and Cacioppo (1998), in which rarely-appearing affective target images were embedded in a context of commonly-appearing affectively neutral images. Continuous electroencephalographic recordings were taken and event-related potential waveforms were later calculated for each valence category (positive, negative, and neutral), using the late-positive potential (LPP) waveform. The emotion bias was calculated by subtracting the LPP amplitudes evoked for negative images from LPP amplitudes evoked for positive images. It was predicted that the emotion bias would significantly differ between the three groups, with the avoidant group displaying the most prominent negative bias. Notably, Dykas and Cassidy (2011) argued for negatively biased social information processing for both anxiety and avoidance in their recent review. However, because of the ambivalence associated with attachment anxiety (e.g. Mikulincer, Shaver, Bar-On, & Ein-Dor, 2010; Mikulincer & Shaver, 2007), a prediction was not made for that group. Similarly, results for the secure group were difficult to predict because of the paucity of previous social emotion bias research for that group. Therefore, no specific predictions were made for the anxious and secure groups other than their being different from the avoidant group.
2. Method
2.1 Participants
Participants were screened and excluded a priori for use of psychotropic medications or exceeding the age limit of 28 years. It had been determined a priori that those over 28 years of age would be excluded from the analysis to limit age-related variation in LPP amplitudes. This is because affectively elicited LPP amplitudes have been shown to decline with age (Kisley, Wood, & Burrows, 2007). Eighty-two undergraduate students underwent electrophysiological recording in exchange for extra credit or $20 cash. Individuals were excluded from the analysis because of equipment failure n = 4, not completing the questionnaires n = 4, SAM (Self-Assessment Manikin, described below) ratings diametrically inconsistent with image category (i.e. rating negative images as positive or positive images as negative) n = 4. After the above exclusions, the sample consisted of 70 individuals to be sorted into groups. Individuals scoring higher than 1 SD above the mean on the ECR Avoidance scale and simultaneously scoring below that point on the ECR Anxiety scale were categorized as avoidant. Those scoring higher than 1 SD above the mean on the Anxiety scale and below that point on the Avoidance scale were categorized as anxious. Those scoring lower than the mean on both scales were categorized as secure. In this sample the mean and standard deviations for these scales were as follows: Avoidance Scale, M = 3.12, SD = 1.02; Anxiety Scale, M = 3.82, SD = 1.15. Forty-two participants met criteria and were categorized as follows: 14 avoidant (3 male), ages ranging from 18–28 years (M = 20.36, SD = 2.82), 12 anxious (2 male), ages ranging from 18–21 (M = 19.42, SD = 1.17), and 16 secure (4 male), ages ranging from 18–28 (M = 21.00, SD = 3.25). One-way ANOVAs were carried out to test for differences in age and education level. Groups did not significantly differ on years of education F(2, 39) = 1.09, p > .05. The homogeneity of variance assumption was violated for age, so a Welch’s test was used, indicating no between-group differences in age F(2, 23.42) = 1.95, p > .05. Overall ethnic identification was 76.2% White, 14.3% Hispanic, 4.8% Asian, and 4.8% Black.
2.2 Materials
Attachment scale
The Experiences in Close Relationships scale (ECR) is a 36 item self-report questionnaire that measures adult attachment styles. Participants use a 7-point Likert scale to rate the extent to which they agree or disagree with each item. According to Mikulincer and Shaver (2007), this measure has been used in hundreds of studies with consistently high reliability; alpha coefficients are generally near .90 and test-retest coefficients generally range from .50 to .75. The scale is scored along two dimensions, attachment anxiety and attachment avoidance. Scores that are high on the anxiety and low on the avoidance dimension are representative of people with anxious attachment styles. Scores low on the anxiety and high on the avoidance dimension are representative of people with avoidant attachment styles. Scores that are low on both dimensions are representative of people with secure attachment styles. For the present sample, Cronbach’s alphas were .94 and .95 for the avoidance and anxiety scales, respectively.
Images
All images were drawn from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005). IAPS is a database of color photographs that have been normatively evaluated for affective valence and arousal. A total of forty-five images were chosen and utilized in this study. Of these, three affectively negative (#’s 2703, 6250, 9050), three positive (4599, 8470, 8490), and three neutral images (2190, 2397, 2595) were utilized as target images from which LPP waveforms were drawn. The negative images showed people in distress or threatening images: starving children, a man pointing a gun directly at the viewer, and traumatized people at a plane crash site respectively. The positive images showed excited and happy people: romantic couple, victorious gymnast, people on a rollercoaster. The people in neutral images showed little facial expression: expressionless man, two sleeping businessmen on a subway, two women in conversation. The remaining 36 non-target images (6150, 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7025, 7030, 7034, 7035, 7036, 7037, 7038, 7040, 7041, 7050, 7052, 7053, 7056, 7059, 7090, 7150, 7161, 7175, 7186, 7211, 7217, 7233, 7235, 7491, 7500, 7705, 7950) were all affectively neutral, used to create a neutral context in which target images appeared. Importantly, all of the target images were of a person or people, whereas all of the context images were of objects or scenes without people.
Subjective ratings
After the recording paradigm was completed, participants were instructed to rate the nine target images in terms of subjective valence (1 = most negative, 5 = neutral, 9 = most positive) and arousal (1 = least arousing, 5 = neutral, 9 = most arousing) using the Self Assessment Manikin instrument (SAM; Lang, Bradley, & Cuthbert, 2005). Because positive and negative valence ratings are anchored on opposite ends of a nine-point scale, valence comparisons were made by subtracting the scale’s mean point (5) from the rating and taking the absolute value of the difference. No between-group differences were found for subjective valence ratings. Furthermore, it was of interest to test whether the valence categories themselves differed from one another. Therefore, a repeated-measures ANOVA was undertaken, revealing a main effect of valence, F(2, 38) = 127.23, p < .05. Post-hoc Bonferonni pairwise comparisons indicated that subjective valence ratings for neutral images (M = 0.36, SD = 1.18) were less extreme than the ratings for positive images (M = 3.17, SD = 1.01) which in turn were less extreme than ratings for negative images (M = 3.63, SD = 0.58). Subjective ratings of arousal were also compared between groups; again, no between-group differences were found. Furthermore, the categories (positive, negative, neutral) were again compared against one another. Repeated-measures analysis of subjective arousal revealed a main effect of valence, F(2, 38) = 144.17, p < .05. Post-hoc Bonferonni pairwise comparisons revealed that arousal ratings for neutral images (M = 2.46, SD = 1.33) were lower than the ratings for the negative images (M = 6.52, SD = 1.65) and the positive images (M = 6.28, SD = 1.76). Arousal ratings were not significantly different for positive versus negative images, which accords with previous findings (Ito, Larsen, Smith, & Cacioppo, 1998).
2.3 Procedure
Laboratory procedure
Once in the laboratory, participants read and signed the informed consent form, which included a brief description of the procedure. Participants were then prepared for recording using self-adhering, disposable silver/silver chloride electrodes attached at the following locations of the international 10–20 system: Fz, Cz, Pz, and left and right mastoids. We and others have found this limited set of channels to be sufficient for tracking effects of valence and individual difference variables on LPP amplitude (Kisley, Wood, & Burrows, 2007; Ito et al., 1998). Eye movements were tracked with electrodes placed above and beside the left eye, with the ground electrode attached to the participant’s forehead. All electrode impedances were maintained below 5 kΩ. Participants were then read instructions from a script that informed them how to engage in the behavioral task. After completing the paradigm, which took approximately 25 min, participants subjectively rated the target images on valence and arousal using SAMs. Then participants were debriefed and the session ended. Participants were in the laboratory for a total duration of 1.5 hrs on average.
Behavioral task
Participants’ electroencephalograms were recorded while they sat in an easy chair and engaged in the behavioral task of categorizing the images that appeared on the screen before them. Images were presented on a 17-in. liquid-crystal display (LCD) 2.5 ft from the participants. The pictures were arranged in an affective oddball paradigm, with infrequent negative, positive, and neutral target images embedded in a context of frequent neutral context pictures. Participants used a mouse with three buttons to categorize target pictures as being positive, negative, or neutral. The images appeared on the screen for 1 s and then disappeared to be replaced with a black screen and words in white letters reminding them of their three available response choices. Participants were instructed to wait until the image disappeared and was replaced by the response screen before making their response. After a mouse response was made, 1.2 s elapsed before the next image appeared. Images were shown in blocks of five, with one target image appearing in each block. The target pictures were counterbalanced to appear in either the third, fourth, or fifth position an equal number of times for each valence category. The blocks were shown in random sequence; participants saw each block 10 times. After each block, there was a pause. Participants were instructed to pause for as long as they needed and then to proceed by clicking the mouse.
Waveform analysis
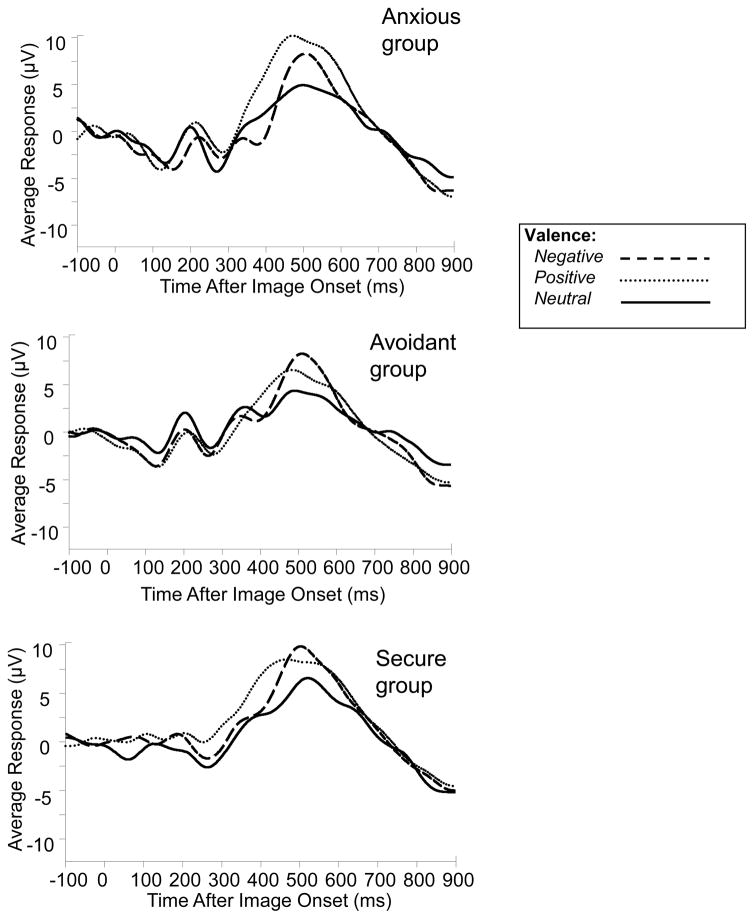
Offline, average waveforms were computed for each subject, for each target valence category. Waveforms were computed from electroencephalographic activity that occurred during each target presentation period, or epoch. Epochs began 100 ms before and ended 900 ms after image presentation, were re-referenced to left and right mastoid average voltage, and were baseline corrected to mean pre-stimulus voltage. Epochs were subject to artifact rejection such that any epoch with voltage ± 100 μV was excluded. Overall, 25% of positive, 24% of negative, and 24% of neutral trials were rejected due to artifacts. Furthermore, using one-way ANOVAs, attachment groups were compared on the percentage of trials accepted for each valence category; no significant differences were found. When computing average waveforms, categorization accuracy (i.e., “positive” response to positive image, “negative” to negative, etc.) was not used as a criterion for selection of single trials. However, as presented in the Results section, behavioral response accuracy was high (about 95%) for valenced images. Averages for each target category (positive, negative, and neutral) were computed from the remaining trials, and were low-pass filtered at 9 Hz (zero-phase shift, 24 dB octave). There were three images per target category, and each target image was seen 10 times. Therefore, average waveforms were computed for each participant from up to 30 positive, 30 negative, and 30 neutral target image viewing trials. The criterion for a participant’s waveforms to be included in the analysis was the existence at least five artifact-free trials per valence category to increase reliability of the waveforms. All participants met this criterion. For each waveform, peak amplitudes and latencies were recorded from the largest peak occurring between 400–900 ms after image onset (Kisley, Wood, & Burrows, 2007). This analysis was performed at electrode Pz because it has been shown that the LPP in response to emotional images is maximal at this electrode site (Cacioppo et al., 1994). Emotion bias was calculated by subtracting the amplitude of the negative from the positive waveform for each individual. Composite waveforms representing each of the three valence categories were created for each attachment group and are displayed in Figure 1.
Figure 1.
Across-subjects average ERP waveforms recorded from site Pz, separated by image valence (neutral, positive, negative) and group. The LPP is the prominent waveform component reaching maximal amplitude near 500 ms after the image onset (at time = 0 ms). Note that these “grand-averaged” waveforms are for illustrative purposes and were not analyzed here.
3. Results
3.1 Late positive potential
A one-way between-groups ANOVA was carried out to test for differences in emotion bias, which revealed significant between-groups differences F(2, 39) = 3.39, p < .05. Post hoc analyses using Tukey’s HSD revealed that the bias was significantly different between the avoidant group (M = − 1.79, SD = 2.23) and the anxious group (M = 1.67, SD = 3.54). Note that the negative sign in front of the avoidant group’s mean value indicates a larger response amplitude for negative than for positive images in that group; the reverse is true for the anxious group, for which the sign was positive. Emotion bias for the secure group (M = 0.54, SD = 4.27) was not significantly different from either group. In order to determine whether the emotion bias (the sum of subtracting negative from positive amplitudes) was significantly different from zero within each group, one-sample t-tests were carried out for each group. It was revealed that the emotion bias was significantly different from zero in the avoidant group t (13) = −3.00, p < .05, but not in the anxious t (11) = 1.63, p > .05, and not in the secure group t (15) = 0.50, p > .05. A one-sample t-test was also carried out for the combined sample to examine whether there was an emotion bias overall. Results revealed no overall emotion bias t (41) = 0.14, p > .05, which is in accordance with the findings of a previous study that compared LPP responses to positive and negative images of people (Ito & Cacioppo, 2000). No significant effects of valence or group membership were found for peak LPP latencies.
Because the emotion bias variable is a derivative of two other variables, it was of interest to determine which of those two had a stronger impact on emotion bias variability. In other words, was variation in responding to positive or responding to negative images more influential in determining the presence and direction of emotional bias? Therefore, relationships between the emotion bias variable and its two contributors were examined for the overall sample. Those variables are LPP amplitude in response to positive images (LPP for positive images) and LPP amplitude in response to negative images (LPP for negative images). Emotion bias was significantly correlated with LPP for positive images r = .58, n = 41, p < .05. Overall, as LPP for positive images increased, the emotion bias increased in positivity. In contrast, relationships between emotion bias and LPP for negative images did not reach statistical significance. Therefore, it appears that variability in the emotion bias was driven mainly by variability in LPP for positive images.
3.2 Behavioral Responses
A series of one-way ANOVAs were carried out to test for between-group differences in the valence categorizations made via mouse clicks after each slide presentation during the task. Group differences were not statistically significant for response times overall or when analyzed by valence category. Overall mean response time was 946 ms (SD = 385) for the avoidant group, 992 ms (SD = 662) for anxious, and 920 ms (SD = 442) for secure. In addition to response times, responses were also examined for accuracy. Groups did not significantly differ in response accuracy for any valence category. Overall, participants categorized 94.6% of positive targets as positive and 96.6% of negative targets as negative. However, only 47.6% of neutral targets were categorized as being neutral. Therefore, alternate responses to neutral images were examined. Groups were compared for differences in the number of negative responses to neutral targets and in the number of positive responses to neutral targets. Groups were not significantly different in the number of negative responses to neutral targets, but did significantly differ in the number of positive responses to neutral targets F(2, 40) = 5.79, p < .05. The secure group categorized on average 51.7% (SD = 31.0%) of neutral targets as being positive, whereas the proportions for the anxious and avoidant groups were 16.7% (SD = 21.9%) and 32.1% (SD = 26.6%), respectively. Post hoc analyses using Tukey’s HSD revealed that the proportion of positive responses to neutral targets was higher for the secure group than for the anxious group. Positive responses to neutral targets in the avoidant group did not significantly differ from those of either group. It was of interest to know if the tendency to respond positively to neutral images resulted in higher LPP amplitudes for neutral images and/or substantially different subjective experiences of valence and arousal for neutral images. To test for this possibility, correlational analyses were performed between positive categorization responses to neutral targets and the neutral dependent variables: peak LPP amplitude in response to the same neutral targets, and subjective ratings of valence and arousal collected with the SAMs instrument after the categorization task was completed. Results indicated that the number of positive responses to neutral images was correlated with subsequent SAM valence ratings for neutral images, r = .60, n = 41, p < .05, but not with SAM arousal ratings for neutral images, and not with LPP amplitudes in response to neutral images.
4. Discussion
As predicted, brain responses to images differed as a function of image valence and also as a function of attachment style. The avoidant group displayed larger neural responses to negative than to positive images, a tendency that was diametrically opposite that of the anxious group. Although the emotion bias was not significantly different from zero within the anxious group, it was significantly different from that of the avoidant group. The results for these two groups indicate differing patterns of LPP amplitudes across negative and positive valence categories. In effect, the avoidant group displayed a negative bias which contrasted with a tendency towards a positive bias in the anxious group. In comparison, the secure group did not favor either positive or negative categories.
These findings suggest that, in the time window of around 500 ms after stimulus presentation, differently valenced social stimuli engender differing degrees of motivational relevance among attachment styles. Avoidant attachment appears to be associated with an asymmetry towards the greater motivational relevance of negative interpersonal stimuli. Conversely, anxious attachment appears to be associated with an asymmetry towards the greater motivational relevance of positive interpersonal stimuli. No such asymmetries, as reflected in LPP amplitudes, were found to be associated with secure attachment. In interpreting these results, it should be noted that subjective importance appears to be the vital element in motivational relevance.
Evidence for the importance of subjective motivation on LPP amplitudes comes from a study by Stockburger, Schmalzle, Flaisch, Bublatsky, and Schupp (2009). Their participants underwent ERP recordings while viewing images of flowers and of food. The same participants were recorded at two different times when they were in two different physiological states: once when hungry and once when satiated. LPP amplitudes for food images were higher and amplitudes to flower images were lower when participants were hungry than when satiated. Therefore, the effect of picture valence on LPP amplitudes was at least somewhat dependent on the internal motivational states of participants. In regards to socially-oriented images, the import of the subjective element on LPP amplitudes was demonstrated in a study by Langeslag, Jansma, Franken, and Van Strien (2007). These researchers presented participants with images of their romantic partners, of a friend (to control for familiarity effects), and of an unknown beautiful person. LPP amplitudes were largest in response to images of the romantic partner. The results therefore demonstrate that subjective variations in motivational relevance are strong contributors to variations in LPP amplitudes.
With this in mind, it’s possible that the balance of motivational relevance can account for the observed differences in emotion bias between the avoidant and anxious groups. From this standpoint, the motivational balance favors negative over positive social stimuli in avoidant attachment. This formulation makes sense in regards to the behavior commonly associated with this attachment style: relative interpersonal withdrawal. Perhaps this behavior reflects a higher weight given to negative (or a lower weight given to positive) social stimuli in these individuals. Conversely, the behavior associated with anxious attachment (striving for interpersonal closeness) is perhaps reflective of a motivational balance favoring positive social stimuli in these individuals. The positive images in the present study contained social cues that indicate the possibility for closeness (e.g., smiles, excitement). Such cues should be motivationally relevant for individuals with anxious attachment styles. Their thoughts and feelings seem to center on whether their partner is available and responsive (Mikulincer & Shaver, 2003), and the above cues are indicative of availability and responsivity. Although the pictures were not of their attachment partners, such cues are probably reinforcing and thus generalizable. Enhanced neural responsivity to such cues could therefore likely be present, at least in the time window examined in this study (around 500 ms after stimulus onset).
The notion that anxious and avoidant attachment are differentially associated with sensitivity to rewarding interpersonal cues has some empirical support. Picardi, Caroppo, Toni, Bitetti, and Di Maria (2005) found attachment anxiety to be positively correlated and avoidance to be negatively correlated with reward dependence, a dimension on the Temperament and Character Inventory (TCI). The TCI is based on a psychobiological model of personality, which argues for the existence of specific neurological differences behind each personality dimension (Cloninger, Svrakic, & Przybeck, 1993). High scores on reward dependence are purported to reflect “enhanced learning from reward signals, persistence in repeating actions associated with rewards, high sociability and reliance on social approval” (Cohen, Schoene-Bake, Elger, & Weber, 2008, p. 33); there is evidence that higher scores on this dimension are associated with stronger neural connections in the striatum (Cohen et al., 2008). The striatum is strongly implicated in motivated behavior and reward (e.g. Ikemoto & Panskeep, 1999; Palmiter, 2008). However, if the anxious group does find positive interpersonal cues rewarding, these rewards may not always result in behavioral approach. There is evidence that the short the period between stimulus processing and response execution is enough time to produce ambivalence (Mikulincer, Shaver, Bar-On, & Ein-Dor, 2010). Perhaps this is because the activation of one goal-state (e.g. approach), quickly activates its opposite (avoidance) in the minds of anxious persons due to their insecurities (Mikulincer et al., 2010).
In contrast to anxious individuals, it appears that avoidant individuals find little potential reward in social interactions. Indeed, Troisi, Alcini, Coviello, Nanni, and Siracusanu (2010) found that social anhedonia was associated with avoidant attachment and not with anxious attachment in a non-psychiatric sample. Mikulincer and Shaver (2007) describe a similar phenomenon that operates in close relationships, observing that avoidance involves the diversion of attention away from positive attachment-related information:
As a result, genuine signals of a partner’s support and love can be missed and, even when noted, can be processed only shallowly, be easily forgotten, and remain inaccessible when later appraisals of relationship partners are made. This dismissal of positive information about relationship partners sustains avoidant people’s negative and critical images of others. (p. 169)
The idea that attachment avoidance involves the dismissal of positive information which serves to sustain avoidance is interesting because it has parallels in the realm of attitude formation. Shook, Fazio, and Vasey (2007) investigated the mechanisms involved in the formation of attitudes to novel stimuli. These researchers employed a computer game in which participants were to learn whether novel “beans” were good or bad. Participants gained points for approaching good beans and lost points for approaching bad ones. Approach was required to discover whether a bean was bad or good. Findings revealed an overall negativity bias: participants categorized more negative than positive beans correctly in a test at the end of the game. Interestingly, systematic variation was discovered in the extent of this bias. Those who made fewer approaches knew less than others about the positive beans, but not about the negative beans; thus they had larger negativity biases that were dependent on lower scores in the positive valence category. A possible role for the positive valence category in attachment avoidance is therefore intriguing.
The present results appear to be in contradiction to those of Zilber, Goldstein, and Mikulincer (2007) which indicated greater LPP amplitudes in response to negative stimuli (as subtracted from LPP amplitudes to neutral stimuli) in anxious attachment. However, perhaps the discrepancy can be explained by differences in target images. As mentioned above, Zilber, Goldstein, and Mikulincer used a mixture of target images: the majority of them did not show people. A person or people were in 40% of their positive images, 40% of negative images, and 15% of neutral images. Therefore, it is possible that anxious individuals respond more negatively to negative object images than to negative people images, and vice-versa for avoidance. Indeed, it is possible that the LPP results for the avoidant group are reflective of negativity specifically in regards to other people. Accordingly, Collins and Read (1990) found a relationship between avoidance and negative views of human nature. Additionally, diary studies that assess the quality of daily interactions with partners have found that, compared with secure attachment, avoidant attachment is associated with higher levels of negative and lower levels of positive emotions during interactions (as reviewed in Mikulincer & Shaver, 2007).
One limitation of the present study was the relatively small sample size. The sample size was reduced from the original group here because we decided to categorize individuals as “anxious” and “avoidant” (using a 1 SD criterion) rather than investigating the relationship between brain responses and attachment variables along continuous dimensions (e.g. Mikulincer & Shaver, 2007)1. The previous investigation into LPP responses and attachment had employed a similar categorical approach (Zilber, Goldstein, & Mikulincer, 2007). Another limitation was the fact that trait anxiety was not controlled for. Trait anxiety was not predictive of results in the LPP study by Zilber, Goldstein, and Mikulincer (2007), nor was it predictive of a negativity bias in a pilot study from our lab (Satrom, 2007), so we decided not to add an instrument for assessing it here. However, because trait anxiety can be confounded with attachment anxiety (Shaver & Brennan, 1992), future studies should include such a measure. Another helpful change would involve the content of the target images. Although all the target images used in the present study were of people, they did not all represent attachment themes. Perhaps more insight into specific attachment-based differences could be derived from including only attachment-themed images as targets (e.g. interpersonal affiliation, discord and rejection). Another potential problem with the targets used in the present study is that they were complex images containing many visual elements. Individual nuances in the attributions of personal relevance to overall image themes or their individual elements are potential confounds. In a similar vein, while “negative” and “positive” are primary, over-arching categories that are important for survival (e.g. Norris, Gollan, Berntson & Cacioppo, 2010; Rozin & Royzmann, 2001), they are also most likely too inclusive to fully understand attachment-based nuances. Therefore, future studies should benefit from using simpler, more exclusive social stimuli such as faces with varying emotional expressions.
One finding in the present study that was not predicted, and may be informative, concerns responses to neutral targets. Using the mouse to categorize pictures during the task, the secure group rated neutral target images as being positive more often than the other groups did. This finding might be due to the fairly ambiguous nature of facial expressions in the neutral images. This ambiguity left room for differences in interpretation; thus individuals in the secure group interpreted the people and scenes as being positive more than the other groups. Perhaps this is reflective of the positive expectations and ideas about others that are more often associated with secure attachment than with other attachment styles (see, e.g. Mikulincer & Shaver, 2007). Results concerning neutral targets may be important to consider in future investigations, as the ambiguous nature of the images might provide additional insight into any attachment-based propensities to interpret stimuli more positively or negatively.
Acknowledgments
The authors would like to extend their heartfelt gratitude to Michelle Privratsky, Mindy Kasper, and David Callaway for assistance with data collection. Financial support was provided to the second author by the National Institute on Aging (1 R15 AG037393-01).
Footnotes
The pattern of results in this type of research might depend on the analytical approach taken (dimensional vs. categorical), especially if the underlying relationship between variables is not linear. For example, using an linear correlation approach with the present study sample, and including all individuals with usable electrophysiological data (n = 70), we found a trend for a significant relationship between the ECR anxiety dimension and the emotion bias in the LPP waveform responses, in the direction expected from the results presented above (i.e., more positive brain response bias associated with higher levels of anxious attachment), r = 0.23, p = .054, but the relationship between the ECR avoidance dimension and the emotion bias of the LPP was not significant, r=−0.15, p=0.20.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson LY, Metalsky FI, Alloy LB. Hopelessness depression: A theory based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Amir N, Foa EB. Cognitive biases in social phobia. In: Hoffman SG, DiBartolo PM, editors. From social anxiety to social phobia: Multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001. pp. 254–267. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bartholomew K, Horowitz LM. Attachment styles among young adults: Test of a four-category model. Journal of Personality and Social Psychology. 1991;61:226–244. doi: 10.1037//0022-3514.61.2.226. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratlavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Brumbaugh CC, Fraley RC. Transference of attachment patterns: How important relationships influence feelings toward novel people. Personal Relationships. 2007;14:513–530. [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorization: I. A late positive brain potential that varies as a function of trait negativity and extremity. Journal of Personality and Social Psychology. 1994;67:115–125. doi: 10.1037//0022-3514.67.1.115. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;4:644–655. [PubMed] [Google Scholar]
- Cassidy J, Berlin LJ. The insecure/ambivalent pattern of attachment: Theory and research. Child Development. 1994;65:971–981. [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive therapy of anxiety disorders: Science and practice. New York: Guilford Press; 2010. [Google Scholar]
- Cloninger RC, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake J-C, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nature Neuroscience. 2008;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Collins NL. Working models of attachment: Implications for explanation, emotion, and behavior. Journal of Personality and Social Psychology. 1996;71:810–832. doi: 10.1037//0022-3514.71.4.810. [DOI] [PubMed] [Google Scholar]
- Collins NL, Read SJ. Adult attachment, working models, and relationship quality in dating couples. Journal of Personality and Social Psychology. 1990;58:644–663. doi: 10.1037//0022-3514.58.4.644. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Rowe AC, Penton-Voak IS, Ludwig C. No reliable effects of emotional facial expression, adult attachment orientation, or anxiety on the allocation of visual attention in the spatial cueing paradigm. Journal of Research in Personality. 2009;43:643–652. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. Chicago: University of Chicago; 1965. (Original work published 1872) [Google Scholar]
- Dewitte M, De Houwer J. Proximity and distance goals in attachment. European Journal of Personality. 2008a;22:675–694. [Google Scholar]
- Dewitte M, De Houwer J. Adult attachment and attention to positive and negative emotional face expressions. Journal of Research in Personality. 2008b;42:498–505. [Google Scholar]
- Dykas MJ, Cassidy J. Attachment and the processing of social information: Theory and evidence. Psychological Bulletin. 2011;137:19–46. doi: 10.1037/a0021367. [DOI] [PubMed] [Google Scholar]
- Feeney JA, Noller P. Attachment style and verbal descriptions of romantic partners. Journal of Social and Personal Relationships. 1991;8:187–215. [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy. 1989;20:155–176. [Google Scholar]
- Fraley RC, Niedenthal PM, Marks M, Brumbaugh C, Vicary A. Adult attachment and the perception of emotional expressions: Probing the hyperactivating strategies underlying anxious attachment. Journal of Personality. 2006;74:1163–1190. doi: 10.1111/j.1467-6494.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Gable SL, Berkman ET. Making connections and avoiding loneliness: Approach and avoidance social motives and goals. In: Elliot AJ, editor. Handbook of approach and avoidance motivation. New York: Psychology Press; 2008. pp. 203–216. [Google Scholar]
- Harris LT, McClure SM, Van Den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ito TA, Cacioppo JT. Electrophysiological evidence of implicit and explicit categorization processes. Journal of Experimental Social Psychology. 2000;36:660–676. [Google Scholar]
- Ito TA, Larsen JT, Smith K, Cacioppo JT. Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Jordan N. The asymmetry of liking and disliking. A phenomenon meriting further reflection and research. Public Opinion Quarterly. 1965;29:315–322. [Google Scholar]
- Kahneman D, Tyversky A. Prospect theory: An analysis of decisions under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Kisley MA, Wood S, Burrows CL. Looking at the sunny side of life: Age-related change in an event-related potential measure of the negativity bias. Psychological Science. 2007;18:838–843. doi: 10.1111/j.1467-9280.2007.01988.x. [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, Jansma BM, Franken IHA, Van Strien JW. Event-related potential responses to love-related facial stimuli. Biological Psychology. 2007;76:109–115. doi: 10.1016/j.biopsycho.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. New York: The Guilford Press; 2007. [Google Scholar]
- Mikulincer M, Shaver PR, Bar-On N, Ein-Dor T. The pushes and pulls of close relationships: Attachment insecurities and relational ambivalence. Journal of Personality and Social Psychology. 2010;98:450–468. doi: 10.1037/a0017366. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson CG, Cacioppo JT. The current status of research on the structure of evaluative space. Biological Psychology. 2010;84:422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Annals of the New York Academy of Sciences. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters G. The positive-negative asymmetry: On cognitive consistency and positivity bias. European Journal of Social Psychology. 1971;1:455–474. [Google Scholar]
- Picardi A, Caroppo E, Toni A, Bitetti D, Di Maria G. Stability of attachment-related anxiety and avoidance and their relationships with the five-factor model and the psychobiological model of personality. Psychology and Psychotherapy: Theory, Research and Practice. 2005;78:327–345. doi: 10.1348/147608305X26882. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pratto L, John OP. Automatic vigilance: The attention-grabbing power of negative social information. Journal of Personality and Social Psychology. 1991;81:380–391. doi: 10.1037//0022-3514.61.3.380. [DOI] [PubMed] [Google Scholar]
- Rothbart M, Park B. On the confirmability and disconfirmability of trait concepts. Journal of Personality and Social Psychology. 1986;50:131–142. doi: 10.1037/0022-3514.92.3.542. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Satrom S. Unpublished Master’s Thesis. University of Colorado; Colorado Springs: 2007. Personality correlates of the negativity bias. [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Shaver PR, Brennan KA. Attachment style and the big five personality traits: Their connection with romantic relationship outcomes. Personality and Social Psychology Bulletin. 1992;18:536–545. [Google Scholar]
- Shaver PR, Mikulincer M. Attachment-related psychodynamics. Attachment & Human Development. 2002;4:133–161. doi: 10.1080/14616730210154171. [DOI] [PubMed] [Google Scholar]
- Shaver PR, Mikulincer M. The psychodynamics of social judgments: An attachment theory perspective. In: Forgas JP, Williams KD, Von Hippel W, editors. Social judgments: Implicit and explicit processes. Cambridge: Cambridge University Press; 2003. pp. 85–114. [Google Scholar]
- Shook NJ, Fazio RH, Vasey MW. Negativity bias in attitude learning: A possible indicator of vulnerability to emotional disorders? Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:144–155. doi: 10.1016/j.jbtep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Rholes WS, Nelligan JS. Support-seeking and support giving within couples in an anxiety-provoking situation: The role of attachment styles. Journal of Personality and Social Psychology. 1992;62:434–446. [Google Scholar]
- Stockburger J, Schmalze R, Flaisch T, Bublatsky F, Schupp HT. The impact of hunger on food cue processing: An event-related brain potential study. Neuroimage. 2009;47:1819–1829. doi: 10.1016/j.neuroimage.2009.04.071. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Facial expressions of emotion in mother-infant interaction. Human Neurobiology. 1985;4:21–32. [PubMed] [Google Scholar]
- Troisi A, Alcini S, Coviello M, Nanni RC, Siracusano A. Adult attachment style and social anhedonia in healthy volunteers. Personality and Individual Differences. 2010;48:640–643. [Google Scholar]
- Wood S, Kisley MA. The negativity bias is eliminated in older adults: Age-related reduction in event-related brain potentials associated with evaluative categorization. Psychology and Aging. 2006;21:815–820. doi: 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hazan C. Working models of attachment and person perception processes. Personal Relationships. 2002;9:225–235. [Google Scholar]
- Zilber A, Goldstein A, Mikulincer M. Adult attachment orientations and the processing of emotional pictures – ERP correlates. Personality and Individual Differences. 2007;43:1898–1907. [Google Scholar]