Abstract
AIM: To determine the association of fasting plasma glucose (FPG) level within normal range and the risk of prediabetes and type 2 diabetes in an Iranian population. METHODS: A total of 806 first-degree relatives (FDRs) of patients with type 2 diabetes who had FPG levels less than 5.6 mmol/l (100 mg/dl) in 2003 to 2005, and who did not have diabetes or impaired fasting glucose (IFG), were followed through 2010 for the occurrence of prediabetes or type 2 diabetes. At baseline and through follow-ups, participants underwent a standard 75 g 2-hour oral glucose tolerance test (OGTT). RESULTS: The incidence of type 2 diabetes, impaired glucose tolerance (IGT), and IFG was 9.6 (95% confidence interval (CI): 6.8-12.4), 28.7 (23.8-33.6), and 33.0 (27.7-38.2) per 1,000 person-years based on 4,489 person-years of follow-up, respectively. FPG was associated with the incidence of diabetes, IGT, and IFG. The multivariate-adjusted hazard ratios (95% CI) for diabetes, IGT, and IFG were 1.36 (1.01-1.84), 1.45 (1.10-1.91) and 1.31 (1.00-1.71), for the highest quintile of FPG compared with the lowest quintile, respectively. CONCLUSIONS: An increase in FPG in the normal range is associated with an increase in the incidence of IGT, IFG, and type 2 diabetes. These results prove FPG in the normal range to be useful in identifying apparently healthy FDRs of patients with type 2 diabetes at risk of developing prediabetes and diabetes.
Keywords: prediabetes, type 2 diabetes, impaired fasting glucose, impaired glucose tolerance, first-degree relative
Abbreviations: ADA - American Diabetes Association; ANOVA - analysis of variance; BMI - body mass index; BP - blood pressure; CI - confidence interval; DBP - diastolic blood pressure; DCCT - Diabetes Control and Complications Trial; FDR - first-degree relatives; FPG - fasting plasma glucose; HbA1c - glycated hemoglobin; HDL - high-density lipoprotein; HR - hazard rate; IFG - impaired fasting glucose; IGT - impaired glucose tolerance; IPDS - Isfahan Diabetes Prevention Study; LDL - low-density lipoprotein; NGT - normal glucose tolerance; OGTT - oral glucose tolerance test; PG - plasma glucose; ROC - receiver-operating characteristic; SD - standard deviation; SE - standard error; WC - waist circumference; WHR - waist-to-hip ratio
Introduction
Fasting plasma glucose (FPG) is the most widely used diagnostic and screening test for the detection of diabetes. Previously, we have shown that FPG has more discriminatory power to distinguish between individuals at diabetes risk and those not at risk than post-load glucose values during oral glucose tolerance test (OGTT) and HbA1c [1].
In 2003, the American Diabetes Association (ADA) Expert Committee recommended lowering the diagnostic cut-off value for impaired fasting glucose (IFG) from 6.1 mmol/l (110 mg/dl) to 5.6 mmol/l (100 mg/dl), since subjects with a FPG between 5.6 mmol/l and 6.1 mmol/l were found to have a greater risk of developing diabetes and its complications than subjects with a FPG below 5.6 mmol/l [2-7]. In fact, lowering the criterion for IFG was suggested primarily to balance the population risk of developing diabetes between IFG and impaired glucose states [3, 6]. Recent studies suggested that even a lower FPG level within the considered normoglycemic range (i.e. <5.6 mmol/l) could account for an increased risk for type 2 diabetes [7-10]. However, considerable controversy exists regarding the advantage and economic feasibility of this approach [11-13].
The association between FPG levels in the normal range and type 2 diabetes has been described in a few studies from developed countries. However, the incidence and relative risk of diabetes using repeat standard OGTT in individuals grouped by different baseline FPG levels and comprehensive data based on standard OGTT for developing countries and prediabetes has not been examined so far. Therefore, at ethnological and etiological levels, the study contributes by characterizing the occurrence of prediabetes and diabetes in a specific population.
Glucose metabolism risk factors are determined by genetic and early environmental influences [14-16]. First-degree relatives (FDRs) of patients with type 2 diabetes have a common genetic basis, and are at increased risk of glucose intolerance and diabetes. Therefore, this population is appropriate to test the hypothesis whether individuals with FPG levels in the normal range can also be at risk of developing diabetes, similar to those with elevated FPG levels of above 5.6 mmol/l (100 mg/dl). This test was included in the ongoing Isfahan Diabetes Prevention Study (IDPS). Another important feature of IDPS is that it includes a large cohort of more than 3,000 FDRs who were followed up for a long period of more than 10 years. In particular, we intended to determine whether a higher FPG level, still within normal range, may independently constitute a risk factor for prediabetes and type 2 diabetes.
Subjects and methods
The IDPS is being conducted in Isfahan, a large urban area situated in central Iran, with a population of almost four and half million in 2006 (2,335,399 men, 2,223,857 women). IDPS is an ongoing cohort study to assess the efficacy of intensive diet and exercise to prevent or delay the onset of type 2 diabetes in FDRs of patients with type 2 diabetes.
Participants and data collection
The study was performed between 2003 and 2005. 3176 FDRs (826 men and 2350 women) of a consecutive sample of type 2 diabetes patients attending the clinics at the Isfahan Endocrine and Metabolism Research Center were evaluated for inclusion in the study. The participants completed laboratory tests including standard 75 g 2-hour OGTT, HbA1c, and a questionnaire on their health status and on various potential risk factors for diabetes. The participants received follow-up tests according to a medical care standard in diabetes [17] to update the information on demographic, anthropometric, and lifestyle factors and on newly diagnosed diabetes. Accordingly, if OGTT was normal at baseline, repeated testing was carried out at least at 3-year intervals. Otherwise, repeat testing was carried out annually. The IDPS baseline methods have been described in detail elsewhere [18]. The participants included siblings and children. The tenants of the Declaration of Helsinki were followed. The institutional review board of the Isfahan University of Medical Sciences approved the study, and informed consent was obtained from every participant.
Ascertainment of prediabetes and diabetes
Cases of prediabetes and diabetes were identified by baseline and follow-up OGTT according to ADA criteria [6]. Pregnant women were excluded. The study included data of 806 FDRs (184 men and 622 women) who had the following characteristics:
- FPG levels less than 5.6 mmol/l (100 mg/dl) at registration
- Absence of diabetes or impaired fasting glucose (IFG)
- At least one subsequent review in mean (SD) follow-up period of 5.6 (1.3) years
- Aged 30 years and over (Figure 1)
Figure 1. Schematic diagram of the study population and course of investigation.
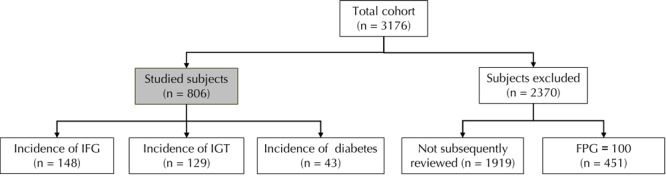
Of the 3176 first-degree relatives (FDRs) of type 2 diabetes patients, followed between 2003 and 2010, 806 individuals fulfilled the inclusion criteria (FPG ≤ 5.6 mmol/l, absence of IFG or diabetes, age ≥ 30 yr, and at least one review in follow-up period of 5.6 yr), and were included in the study to test for diabetes development. 2370 individuals were exceluded because they did not fulfill the inclusion chriteria or because of pregnancy. 1919 of these did not have a clinical visit in follow up, 451 had FPG ≥ 100. Of the 806 included subjects, 148 developed IFG, 129 IGT, and 43 diabetes. The other 486 FDRs remained without signs of diabetes development. FPG: fasting plasma glucose. IFG: impaired fasting glucose. IGT: impaired glucose tolerance.
2370 subjects were excluded because of diviating characteristics. The number of screenings and follow-up visits ranged from 2 to 7 times. Overall, 72.6% of participants were screened three times or more, 41.5% were screened four to seven times. The total follow-up was 4,489 person-years. Attendees at follow-up visits did not differ significantly from non-attendees with respect to most baseline characteristics, including gender, height, hip circumference, HbA1c, triglycerides, and blood pressure (BP). However, non-attendees had:
- Higher waist circumference (WC) (89.1 cm versus 88.1 cm, p = 0.011)
- Higher body mass index (BMI) (29.0 vs. 28.6, p = 0.025)
- Higher FPG (5.4 mmol/l vs. 4.9 mmol/l, p = 0.001)
- Higher plasma glucose at 30 min (8.0 mmol/l versus 7.6 mmol/l, (p = 0.001) and 60 min (8.2 mmol/l vs. 7.8 mmol/l, p = 0.001)
- Higher cholesterol (5.2 mmol/l vs. 4.9 mmol/l, p < 0.01)
- Higher high density lipoprotein (HDL) cholesterol (1.19 mmol/l vs. 1.16 mmol/l, p = 0.011)
- Higher low density lipoprotein (LDL) cholesterol (3.1 mmol/l vs. 3.0 mmol/l, p = 0.001)
Also non-attendees were older (43.4 years vs. 42.2 years, p = 0.001).
Procedures
The participants reported to the clinic in the morning after overnight fast. Patients were asked to abstain from vigorous exercise on the evening before and in the morning of the investigation. Smokers were encouraged to abstain from smoking in the morning of the investigation.
On arrival at the clinic, the information given by the participants in the questionnaire on family history was verified. Subsequently, height, weight, waist, and hip circumference were measured using standard techniques, with the patients in light clothes and without shoes. Weight was measured to the nearest 0.1 kg. Height, waist, and hip circumference were measured to the nearest 0.5 cm. Waist was measured midway between the lower rib margin and the iliac-crest at the end of a gentle expiration. Hip circumference was measured over the greater trochanters directly over the underwear. BMI (calculated as weight in kg, divided by height in meters squared) was used as a measure of overall obesity. Resting BP was measured after the subjects had been seated for 10 minutes, using standard techniques.
FPG was measured using the glucose oxidase method. All subjects underwent a standard OGTT (75 g 2-hour glucose), including FPG assessment, at baseline and follow-up. Venous blood was sampled at fasting, 30, 60, and 120 min. after oral glucose administration. Plasma samples obtained after centrifugation were analyzed the same day.
HbA1c (measured by ion-exchange chromatography), total cholesterol, triglyceride, HDL, and LDL cholesterol (calculated by Friedewald equation, provided total triglycerides did not exceed 4.5 mmol/l [19]) were assessed at the baseline and through follow-ups. Original HbA1c values were converted to Diabetes Control and Complications Trial (DCCT)-aligned HbA1c. All blood sample procedures were performed in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using the enzyme-linked method. The same methodology was used at baseline and follow-ups.
Definitions
Based on the data at last follow-up, participants were classified as normoglycemic, prediabetic, or diabetic according to ADA criteria [6]. Diabetes was defined as:
1. FPG ≥7.0 mmol/l (≥126 mg/dl), or
2. 2-hour plasma glucose of ≥11.1 mmol/l (≥200 mg/dl).
Prediabetes was defined as:
1. IFG (FPG: 5.6-6.9 mmol/l (100-125 mg/dl), and
2. 2-hours plasma glucose <7.8 mmol/l (<140 mg/dl)), or
3. IGT (FPG <7.0 mmol/l (<126 mg/dl), but with 2-hour plasma glucose concentration of ≥7.8 mmol/l (≥140 mg/dl) and <11.1 mmol/l (<200 mg/dl)).
Whereas, normal glucose tolerance (NGT) was present if FPG was below 5.6 mmol/l and 2-hours plasma glucose was less than 7.8 mmol/l [6, 17].
Determination of prediabetes and diabetes incidence
The incidence of prediabetes and diabetes was expressed as the number of IFG, IGT, or type 2 diabetes cases per 1,000 person-years of follow-up. As relevant period was considered the date of completion of the baseline examination between 2003 and 2005 either until:
1. the occurrence of prediabetes or diabetes,
2. the date of the last completed follow-up,
3. death, or
4. end of follow-up on December 31, 2010,
whatever came first.
Statistical analysis
Statistical methods used included Student's t-test, chi squared test, analysis of variance, Kruskal-Walis test for normally or non-normally distributed continuous variables respectively, and Cox’s proportional hazards model. Univariate and multivariate Cox's proportional hazards models were matched to identify predictors of new-onset prediabetes or diabetes using SPSS version 18 for Windows (SPSS Inc., Chicago, IL, USA).
Variables age, BMI, WC, triglyceride, LDL, HDL, total cholesterol, and systolic BP were entered into multivariate-adjusted analyses as continuous variables, while gender and quintiles of FPG were categorical. Adjustment for age and gender was examined in separate models. Age-adjusted means were calculated and compared using general linear models. The ability of FPG <5.6 mmol/l (<100 mg/dl) to predict the incidence of prediabetes or diabetes was examined by receiver-operating characteristic (ROC) curves and their respective areas under the curve, with sensitivity plotted as a function of 1-specificity. Areas under the ROC curves were compared by the algorithm developed by DeLong et al. [20]. In the analyses, men and women were combined to increase statistical power and to simplify the presentation. All tests for statistical significance were two-tailed. Confidence intervals (CI) were set at 95%. P < 0.05 was considered significant.
Results
Characteristics
The study participants are classified into five groups (quintiles) depending on their baseline FPG. Their baseline characteristics of the study participants by quintiles of FPG are shown in Table 1. In age-adjusted comparisons of variables at baseline, age, WC, waist-to-hip ratio (WHR), FPG, plasma glucose at 30, 60, and 120 min, cholesterol LDL, triglyceride, and systolic BP were more likely to increase and follow-up duration more likely to decrease across the quintiles of FPG. The mean (SD) age of participants was 42.2 (6.2) years, 77.2% were women.
Table 1. Age, age-adjusted and proportional characteristics of first-degree relatives of type 2 diabetes patients grouped by quintile of fasting plasma glucose at baseline in the Isfahan Diabetes Prevention Study (IDPS), 2003-2010.
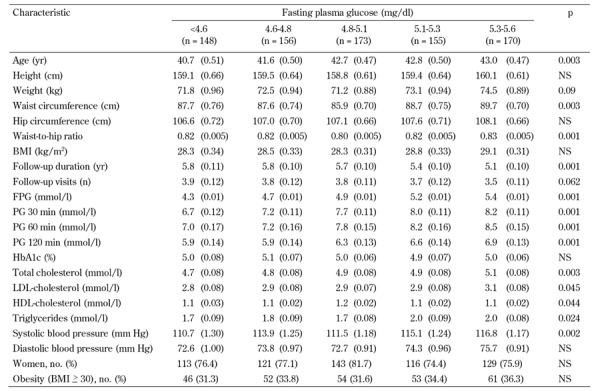
Legend: Age-adjusted means were calculated using general linear models. Data are expressed as mean (SE) or number (%). P-values represent comparisons across all five quintile groups using ANOVA. BMI: body mass index. FPG: fasting plasma glucose. HbA1c: glycated hemoglobin. HDL: high-density lipoprotein. LDL: low-density lipoprotein. PG: plasma glucose. NS: not significant.
The analysis showed that:
- 484 (60.3%) participants had NGT,
- 147 (18.3%) developed IFG,
- 128 (16.0%) developed IGT, and
- 43 (5.4%) developed diabetes.
Baseline characteristics of these patients are shown in Table 2. As expected, those who developed diabetes were older and had higher age-adjusted mean weight, BMI, WC, hip circumference, WHR, FPG, and plasma glucose at 30, 60, and 120 min, HbA1c, triglyceride, and cholesterol, but lower HDL, and proportionally more frequently obese and female.
Table 2. Age, age-adjusted and proportional characteristics of first-degree relatives of type 2 diabetes patients grouped by diabetes status in the Isfahan Diabetes Prevention Study (IDPS), 2003-2010.
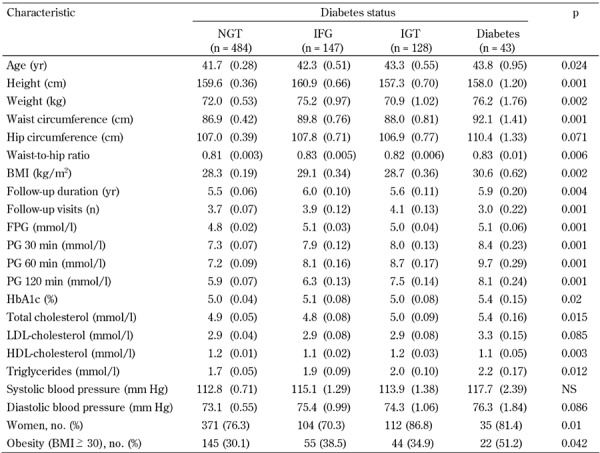
Legend: Age-adjusted means were calculated using general linear models. Data are expressed as mean (SE) or number (%). P-values represent comparisons across all four groups using ANOVA. BMI: body mass index. FPG: fasting plasma glucose. HbA1c: glycated hemoglobin. HDL: high-density lipoprotein. LDL: low-density lipoprotein. PG: plasma glucose. NS: not significant.
The overall incidence of subsequent diabetes was 9.6 (95% CI: 6.8-12.4) per 1,000 person-years. The incidence of IGT and IFG was 28.7 (23.8-33.6) and 33.0 (27.7-38.2) per 1,000 person-years. Compared with participants with FPG < 4.6 mmol/l (bottom quintile), age-adjusted risk of diabetes, IGT, and IFG was 43%, 47%, and 36% higher in those with FPG ≥5.3 mmol/l (top quintile) at baseline in age-adjusted models.
Controlling for age and gender did not alter the relationship between diabetes, IGT, and IFG, compared to the model adjusted for age alone. The additional adjustment for other time-dependent covariates slightly reduced the relationship between diabetes, IFG and IGT, and FPG compared to the model adjusted for age and gender. Over 32% of incident cases of diabetes and IFG, and over 25% cases of IGT, arose among subjects with a baseline FPG between 5.3 to 5.6 mmol/l (top quintile) (Table 3).
Table 3. Incidence rates and relative risks of prediabetes and type 2 diabetes by quintiles of fasting plasma glucose in the Isfahan Diabetes Prevention Study (IDPS), 2003-2010.
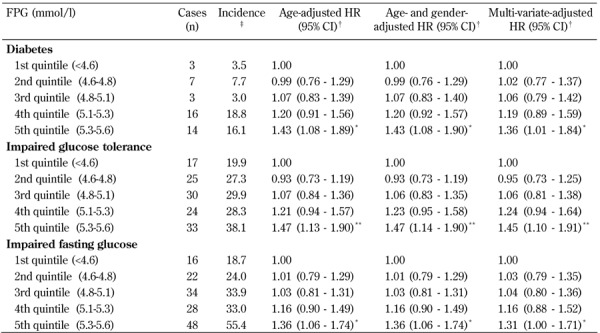
Legend: † Hazard ratios (95% CI) calculated by Cox's proportional hazards model, adjusted for age, gender, body mass index, waist circumference, triglyceride, LDL, HDL, total cholesterol, and blood pressure. ‡ Per 1000 person-years. * p < 0.05. ** p < 0.01. CI: confidence interval. FPG: fasting plasma glucose. HR: hazard ratio.
Compared with individuals with FPG levels below 4.6 mmol/l, those in the 4.6 to 5.1 mmol/l category were not at significantly greater risk of diabetes, IGT, and IFG, after adjustment for other risk factors. However, those in the 5.1 to 5.3 mmol/l group had a 19% greater risk of diabetes relative to the individuals in the less than 4.6 mmol/l group (HR 1.19; 95% CI: 0.89-1.59).
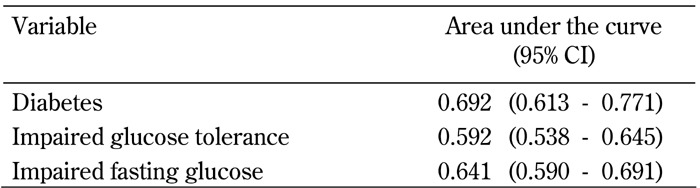
The areas under the ROC curves for incidence of type 2 diabetes, IGT, and IFG were 0.692 (95% CI: 0.613-0.771), 0.592 (95% CI: 0.538-0.645), and 0.641 (95% CI: 0.590-0.691) for FPG < 5.6 mmol/l, respectively (Figure 2 and Table 4). Fasting plasma glucose <5.6 mmol/l were significant predictors for future risk of type 2 diabetes, IGT, and IFG (p < 0.001). The areas under the curves for FPG < 5.6 mmol/l for type 2 diabetes was slightly, but not significantly, greater than that of IGT or IFG.
Figure 2.
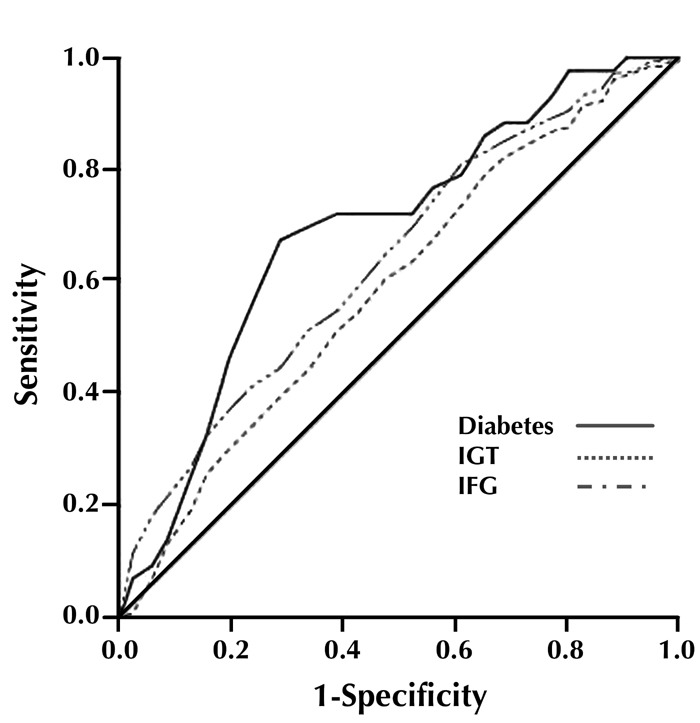
Receiver operating characteristic (ROC) curves for normal fasting plasma glucose for prediction of IGT, IFG, and type 2 diabetes in first-degree relatives of type 2 diabetes patients, with baseline FPG levels <5.6 mmol/l. The estimates of the area under the ROC curves and their 95% confidence intervals are shown in Table 4.
Table 4. Area under the curve (95% confidence interval).
Discussion
In this follow-up study, we found an increased risk of prediabetes and diabetes across quintiles of FPG level within the normal range. These associations remained significant even after adjusting for a wide range of patient characteristics.
Some studies have assessed the risks of diabetes with FPG levels within the normoglycemic range [2, 7-10]. However, none of these studies have examined the incidence and relative risk of diabetes using repeat standard OGTT in individuals defined by different baseline FPG levels. Similar to our findings, all of these studies have shown that FPG levels within the normoglycemic range are a risk factor for type 2 diabetes. In an Israeli study, Tirosh et al. adjusted analysis for a number of diabetes risk factors and showed that FPG levels greater than 4.8 mmol/l (87 mg/dl) significantly increased diabetes risk among young men with FPG level <5.6 mmol/l (<100 mg/dl) [8]. In a study conducted on the island of Mauritius, Shaw et al. found that the risk of diabetes start to increase at a FPG level of greater than 5.2 mmol/l (94 mg/dl) [2]. In another study conducted in Italy, Brabbilla et al. found an increased risk of type 2 diabetes for FPG levels between 5.0 and 5.5 mmol/l (91 and 99 mg/dl) [7]. In a cohort analysis of 46,578 community-based health maintenance organization subjects, Nichols et al. found a strong association between normal FPG levels and diabetes incidence after controlling for a large number of known risk factors [9]. In their data, a FPG level of 5.0 to 5.2 mmol/l (90 to 94 mg/dl) conferred a 49% greater risk of developing diabetes compared to a level less than 4.7 mmol/l (85 mg/dl). In healthy Japanese workers, Hayashino et al. found that an increased FPG level within the normal range was associated with the risk of diabetes, with a threshold level of 94 mg/dl (fourth quartile) above which the risk of diabetes was significantly increased [10].
The present study shows that a relation exists between the level of FPG in the normal range and diabetes development. The lower the FPG levels at baseline the lower the risk of progression to diabetes, IGT, and IFG. The FPG level of 5.3 mmol/l (96 mg/dl) is largely consistent with the suggested FPG level of 5.2 mmol/l (94 mg/dl) as an optimal point of specificity and sensitivity for predicting type 2 diabetes [21, 22]. Other studies have even suggested a lower threshold [7-9]. Furthermore, the HR of 1.19 in the group with baseline FPG levels between 5.1 mmol/l and 5.3 mmol/l, despite not statistically significant, suggests that the upper portion of this range may also carry some risk. However, those who progressed to diabetes, IFG, and IGT had other adverse characteristics that may help to identify their increased risk, namely high obesity and poor lipid profiles. FDRs of patients with type 2 diabetes are at high risk of glucose intolerance. They would likely benefit from lifestyle modifications that are known to reduce diabetes risk [23-25].
The mechanism by which higher normal FPG levels reflect negative effects on diabetes risk is not entirely clear. Putative mechanisms include increased hepatic insulin resistance [26, 27], impaired early insulin response [28], and decrease non-insulin-dependent glucose clearance [29]. Progressive beta-cell failure is the principal factor responsible for the development of prediabetes and diabetes [30].
The strengths of the present study include the prospective cohort design with a large pool of long-term followed up FDRs of diabetes patients, the sample consisting of both men and women of a wide age range from an Iranian population, and the reliable method of diabetes diagnosis based on both repeat standard OGTT and information on potential determinants of diabetes. The multiple examinations with OGTTs make the progression rates very accurate. Another important aspects of the study is that anthropometric variables were collected using direct measurement rather than self-report. Selection and information bias is considered unlikely by virtue of the prospective design.
Our study was addressed to the identification of individuals at increased risk of developing type 2 diabetes. We accompanied FDRs of patients with the disease during a long follow-up enabling us to investigate the features of diabetes development in the clinic. The study included more than 800 participants who were thoroughly examined and followed up, and the follow-up period was 5.7 years. Due to the still conflicting results in assessing diabetes prediction, an even longer follow-up in a large cohort could contribute to a clarification of the question. We did not assess the use of drugs known to affect glucose levels as covariates. Losses to follow-up are the major source of bias in longitudinal studies. At follow-up, non-attendees of the entire population did not differ from attendees by major risk factors for progression to diabetes, although a difference too small to explain the high progression rate to diabetes in our study was seen in the mean levels of lipid profile, WC, BMI, plasma glucose, and age.
In conclusion, our study indicates that an increase in FPG in the normal range is associated with an increase in the incidence of IGT, IFG, and type 2 diabetes. These findings may prove FPG in the normal range to be useful in identifying apparently healthy individuals at risk of developing prediabetes and diabetes.
Disclosure: The authors report no conflict of interests.
Authors contributions: MJ conceived and designed the study, analyzed the data and wrote the manuscript. MA recruited samples, contributed to discussion and revision of the manuscript, and obtained funding for the IDPS.
Acknowledgments
This work was supported by grants from the Isfahan Endocrine and Metabolism Research Center, Iran. The authors are grateful to Mr. Majid Abyar for technical computer assistance.
References
- 1.Janghorbani M, Amini M. Comparison of fasting glucose with post-load glucose values and glycated hemoglobin for prediction of type 2 diabetes: the Isfahan diabetes prevention study. Rev Diabet Stud. 2009;6:117–123. doi: 10.1900/RDS.2009.6.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw JE, Zimmet PZ, de Courten M, Dowse GK, Chitson P, Gareeboo H, Hemraj F, Fareed D, Tuomilehto J, Alberti KG. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care. 1999;22(3):399–402. doi: 10.2337/diacare.22.3.399. [DOI] [PubMed] [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, Yazdi H, Booker L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–312. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Janghorbani M, Amini M. Progression to impaired glucose metabolism in first-degree relatives of patients with type 2 diabetes in Isfahan, Iran. Diabetes Metab Res Rev. 2009;25:748–755. doi: 10.1002/dmrr.1038. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brambilla P, La Valle E, Falbo R, Limonta G, Signorini S, Cappellini F, Mocarelli P. Normal fasting plasma glucose and risk of type 2 diabetes. Diabetes Care. 2011;34(6):1372–1374. doi: 10.2337/dc10-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353(14):1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 9.Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519–524. doi: 10.1016/j.amjmed.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H. Normal fasting plasma glucose levels and type 2 diabetes: the high-risk and population strategy for occupational health promotion (HIPOP-OHP) study. Acta Diabetol. 2007;44:164–166. doi: 10.1007/s00592-007-0258-2. [DOI] [PubMed] [Google Scholar]
- 11.Vaccaro O, Riccardi G. Changing the definition of impaired fasting glucose: impact on the classification of individuals and risk definition. Diabetes Care. 2005;28:1786–1788. doi: 10.2337/diacare.28.7.1786. [DOI] [PubMed] [Google Scholar]
- 12.Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26:3329–3330. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- 13.Schriger DL, Lorber B. Lowering the cut point for impaired fasting glucose: where is the evidence? Where is the logic? Diabetes Care. 2004;27:592–601. doi: 10.2337/diacare.27.2.592. [DOI] [PubMed] [Google Scholar]
- 14.Park HS, Yim KS, Cho SI. Gender differences in familial aggregation of obesity-related phenotypes and dietary intake pattern in Korean families. Ann Epidemiol. 2004;14:486–491. doi: 10.1016/j.annepidem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, Cockram CS, Chan JC. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006;22(1):46–52. doi: 10.1002/dmrr.577. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Cosegregation of obesity with familial aggregation of type 2 diabetes mellitus. Diabetes Obes Metab. 2000;2:149–154. doi: 10.1046/j.1463-1326.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 17.Executive summary. Standard of medical care in diabetes 2008. Diabetes Care. 2008;31:S5–S11. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 18.Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first degree relatives of patients with type 2 diabetes in Isfahan, Iran: Prevalence and risk factors. Rev Diabet Stud. 2007;4(3):169–176. doi: 10.1900/RDS.2007.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23(8):1108–1112. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 22.Shaw JE, Zimmet PZ, Hodge AM, de Courten M, Dowse GK, Chitson P, Tuomilehto J, Alberti KG. Impaired fasting glucose: how low should it go? Diabetes Care. 2000;23(1):34–39. doi: 10.2337/diacare.23.1.34. [DOI] [PubMed] [Google Scholar]
- 23.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB. et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 24.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M. et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 25.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with life-style intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul-Ghani MA, Jenkinson C, Richardson D, DeFronzo RA. Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study (VEGAS) Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 27.Abdul-Ghani MA, Matsuda M, DeFronzo RA. Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med. 2008;25:1289–1294. doi: 10.1111/j.1464-5491.2008.02597.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanat M, Norton L, Winnier D, Jenkinson C, Defronzo RA, Abdul-Ghani MA. Impaired early but not late phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. 2011;48:209–217. doi: 10.1007/s00592-011-0285-x. [DOI] [PubMed] [Google Scholar]
- 29.Jani R, Molina M, Matsuda M, Balas B, Chavez A, DeFronzo RA, Abdul-Ghani M. Decreased non-insulin-dependent glucose clearance contributes to the rise in FPG in the non-diabetic range. Diabetes Care. 2008;31(2):311–315. doi: 10.2337/dc07-1593. [DOI] [PubMed] [Google Scholar]
- 30.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]


